Cell Bio unit 2
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
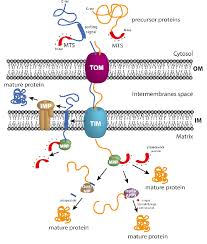
How are mitochondrial proteins translocated?
Synthesized in the cytosol as precursor proteins
Post-translationally translocated into mitochondria
This is the main pathway for most mitochondrial proteins
Import of Nuclear-Encoded Mitochondrial Proteins
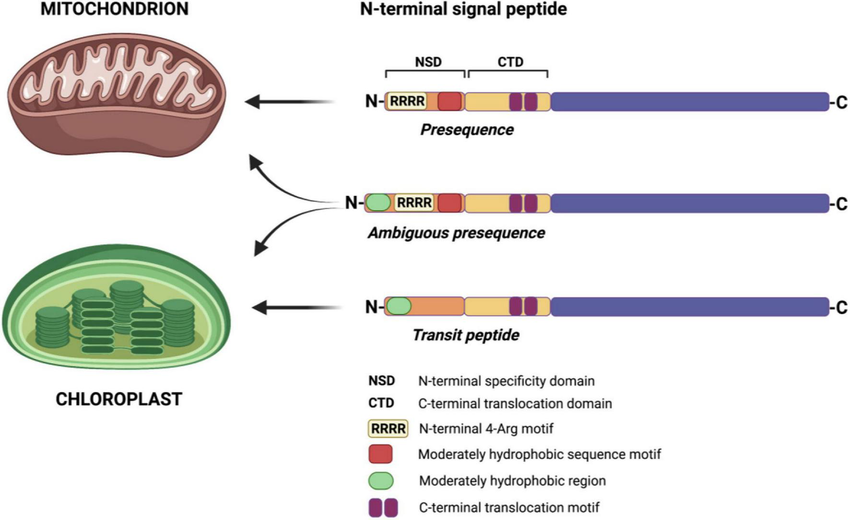
Most proteins use an N-terminal mitochondrial signal sequence:
α-helical, ~15–55 amino acids
Positively charged residues on one side
Hydrophobic residues on the other
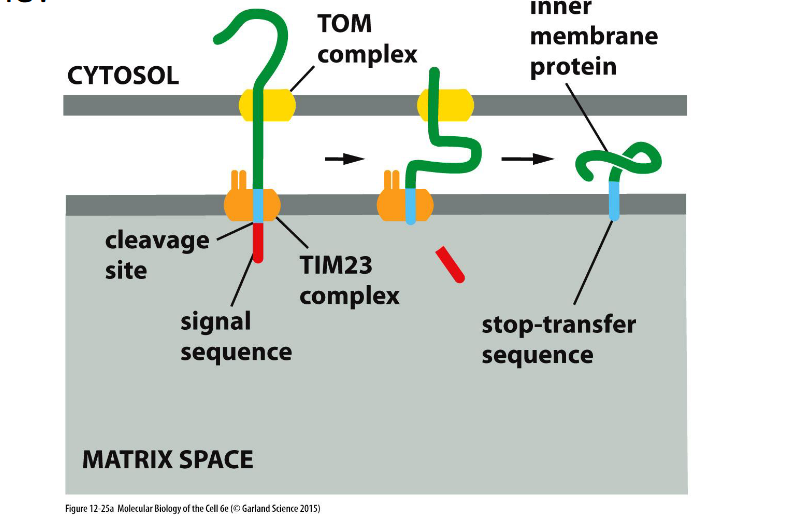
Some proteins (especially in the outer membrane) use an internal signal sequence that is not cleaved.
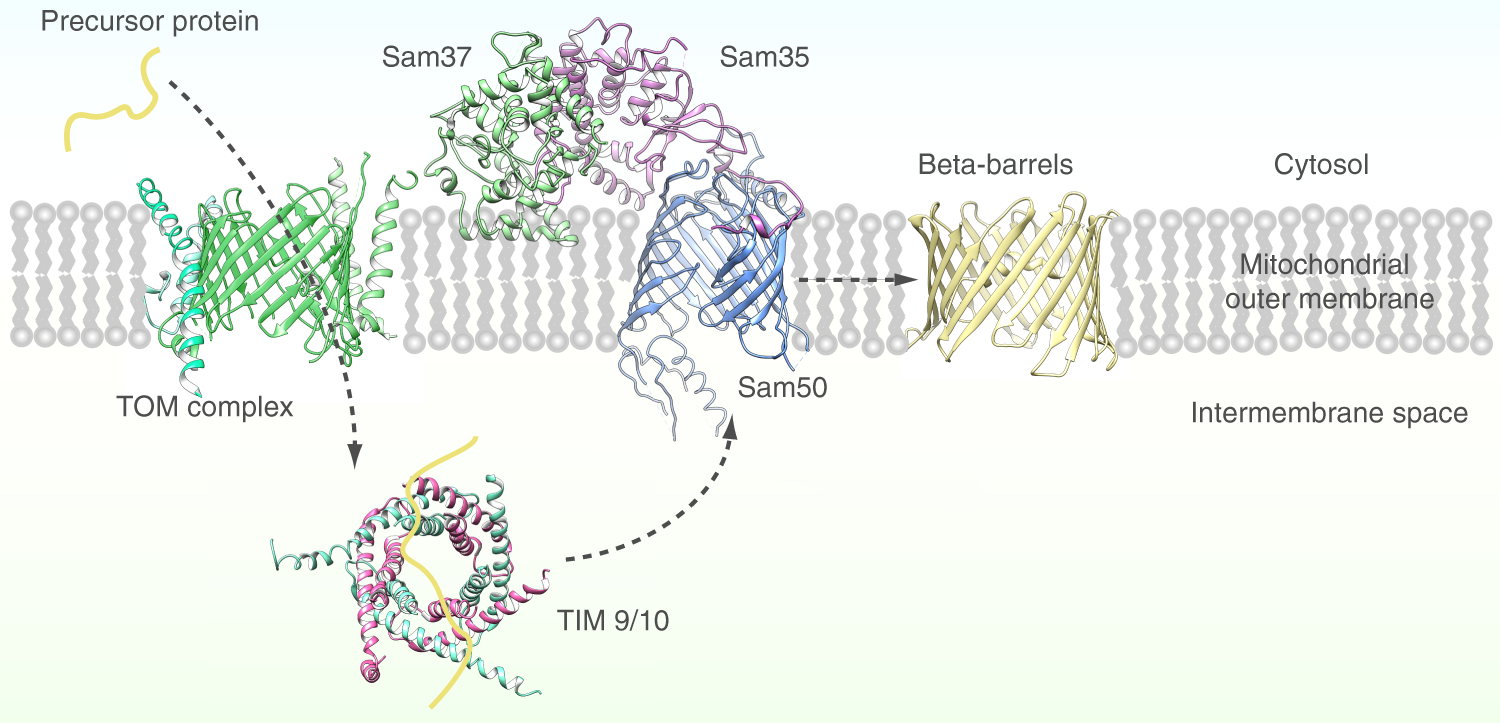
SAM (sorting & assembly machinery)
Insertion of beta barrels into outer membrane
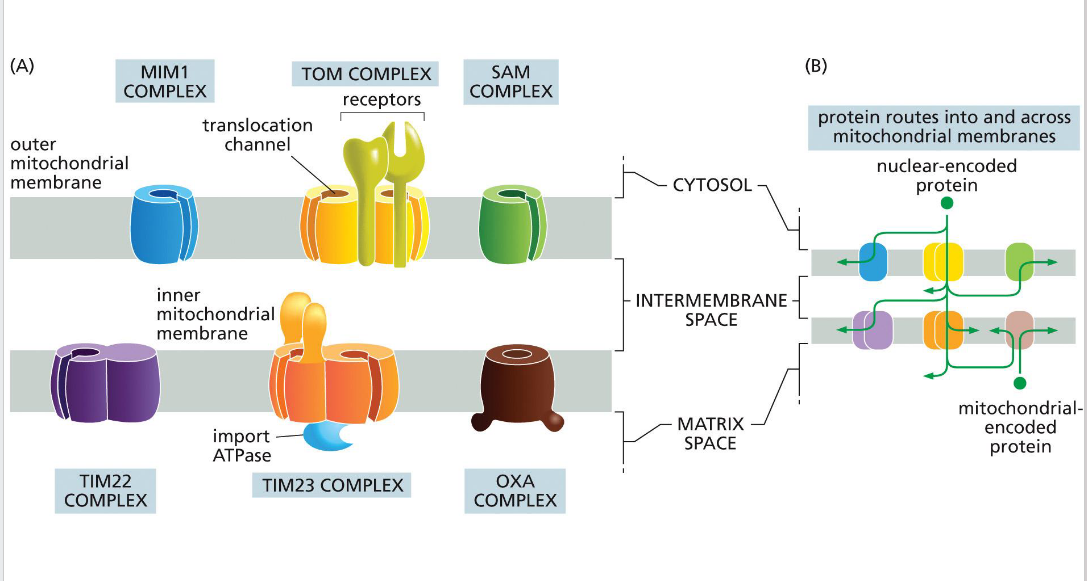
OXA (cytochrome oxidase activity)
Facilitates final insertion of mitochondrial or imported proteins into the inner membrane from the matrix side.
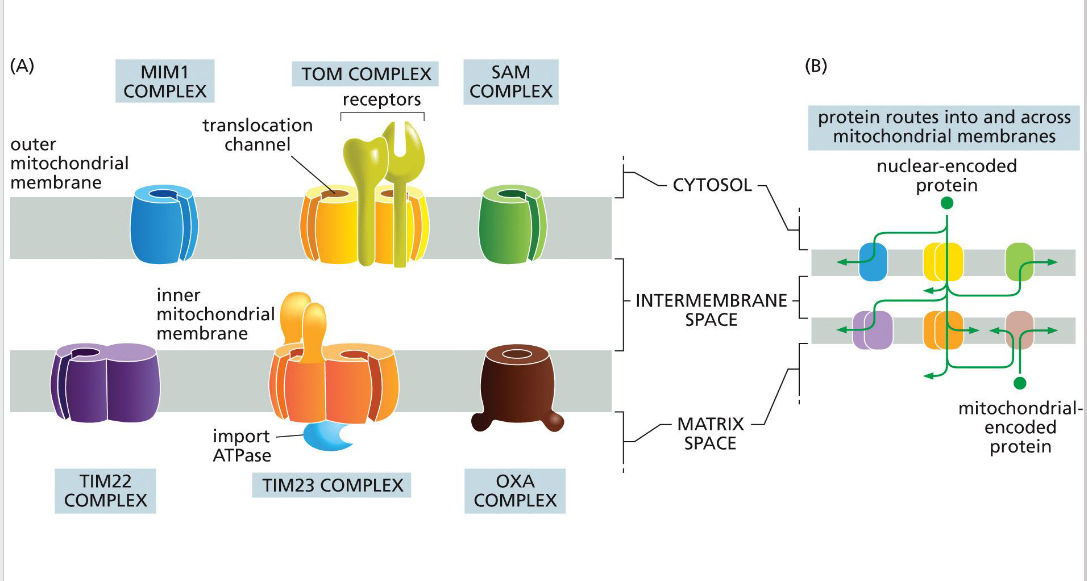
TIM (translocator of the inner mitochondrial membrane)
TIM23
• Transports some proteins into matrix as well as the inner membrane and some transmembrane proteins
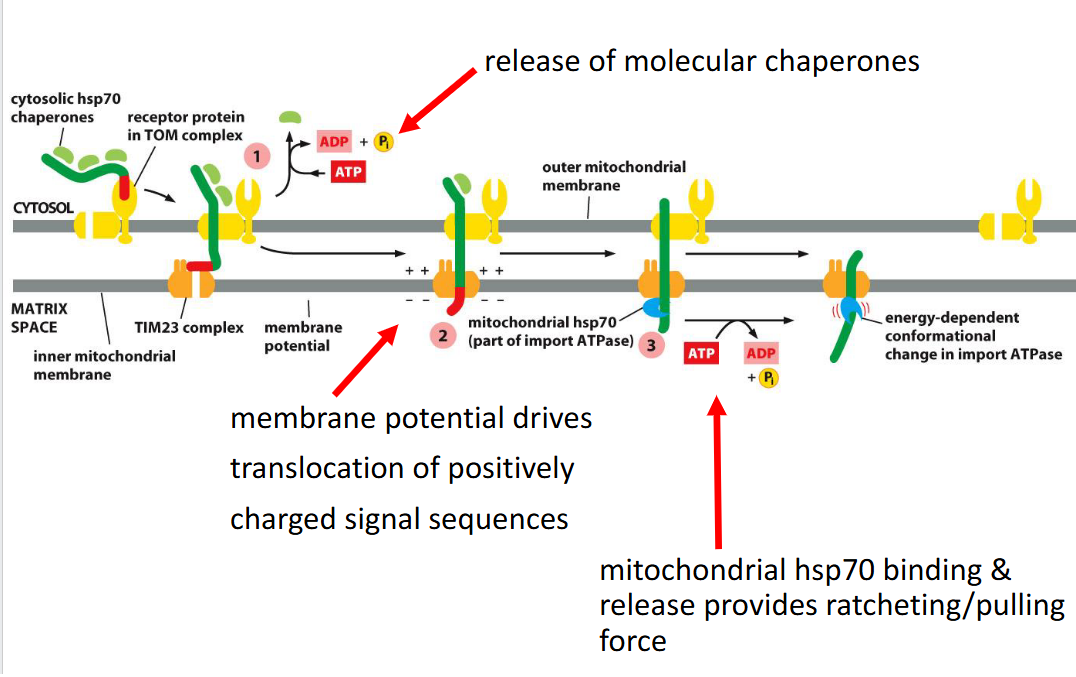
- relies on the inner membrane potential to drive translocation of positively charged signal sequences into the matrix (negatively charged).
• TIM22
-Transports some transmembrane proteins
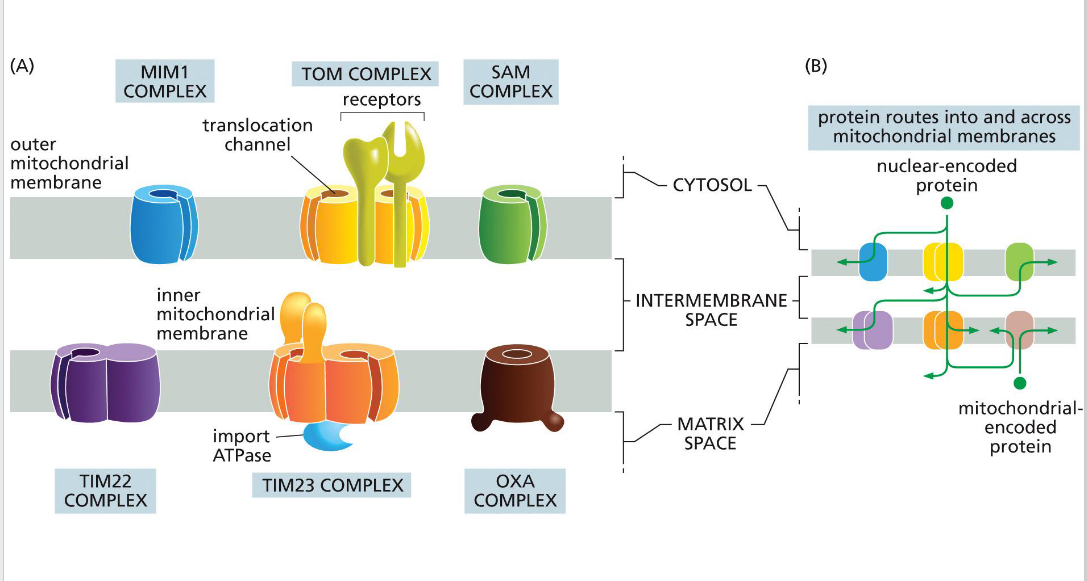
TOM (translocator of the outer
membrane)
A protein complex in the outer mitochondrial membrane that serves as the main entry portal for nearly all nuclear-encoded mitochondrial proteins. It recognizes targeting signals and translocates precursor proteins into the intermembrane space, where they may be further processed or handed off to TIM complexes for deeper import.
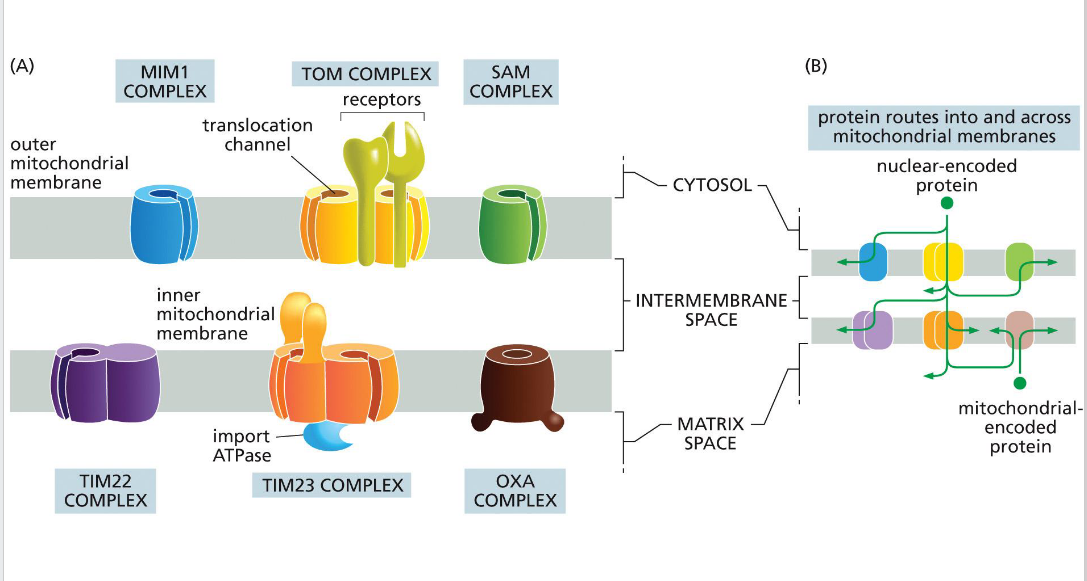
MIM (mitochondrial import machinery)
Facilitates insertion of single-pass transmembrane helices and tail-anchored proteins into the outer mitochondrial membrane.
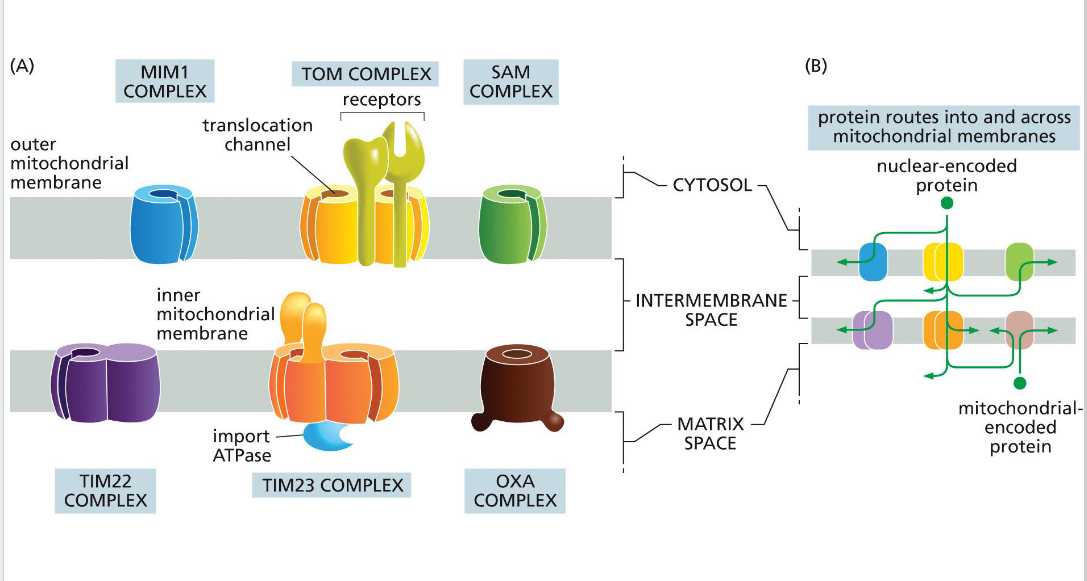
Directional transport requires energy
Targeting Proteins to the Inner Mitochondrial Membrane
Many proteins have a hydrophobic segment just after the N-terminal signal sequence, which acts as a stop-transfer sequence—halting translocation and anchoring the protein in the inner membrane.
Mitochondrial Targeting Sequence (MTS)
N-terminal amphipathic alpha-helix that directs precursor proteins to mitochondria; cleaved after import.
Chaperones (e.g., Hsp70)
Cytosolic and mitochondrial proteins that maintain precursor proteins in an unfolded state and assist in translocation
Membrane Potential
Electrochemical gradient across the inner membrane (negative) required for translocation through TIM complexes (matrix is negative as well)
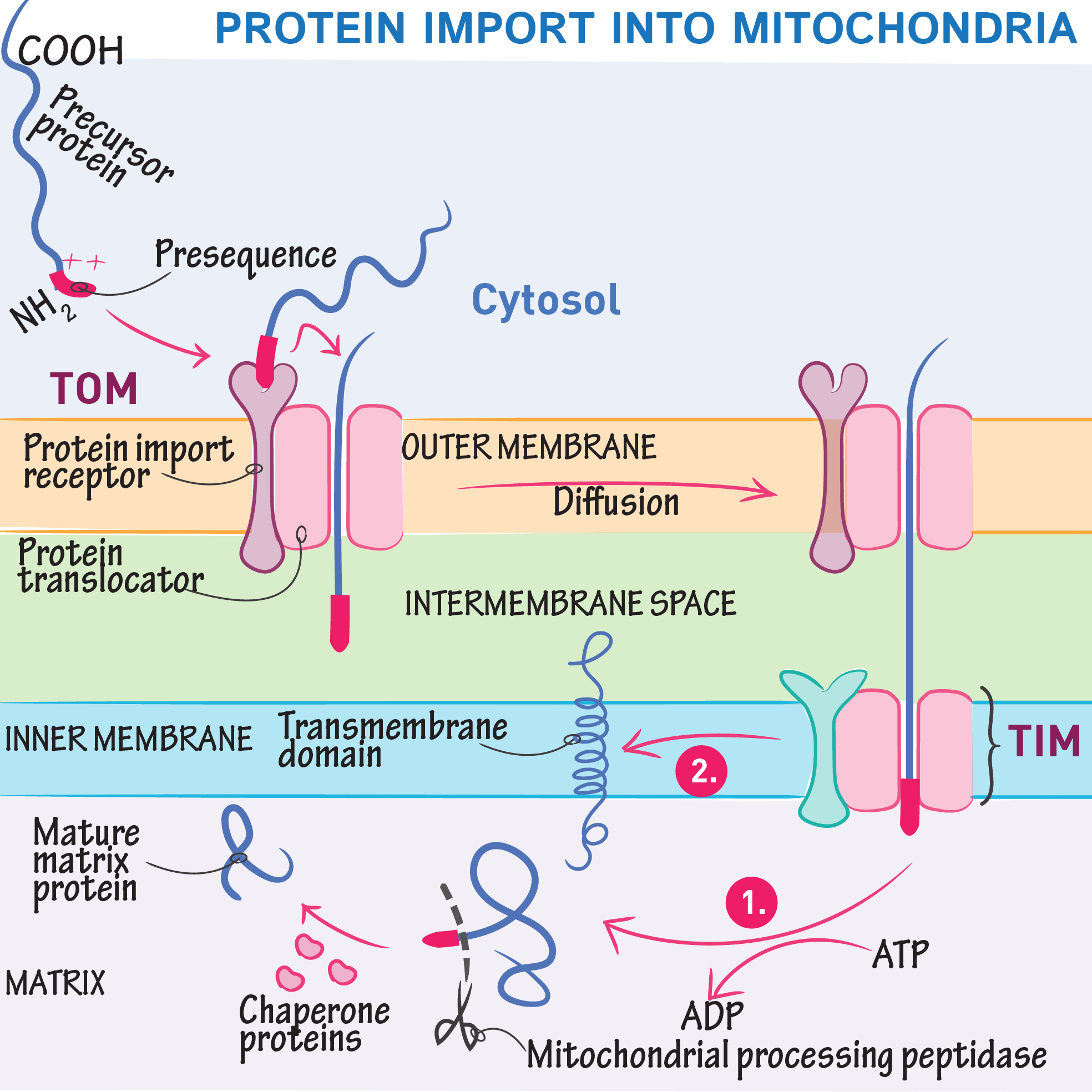
Presequence Targeting Signal
Matrix-bound proteins have an N-terminal presequence: an amphipathic α-helix with positively charged residues
This signal is electrostatically attracted to the negative matrix potential, helping guide the protein inward
TOM and TIM Complexes
Proteins first pass through the TOM complex in the outer membrane
Then engage the TIM23 complex, which spans the inner membrane and connects to the matrix
Molecular Pulling by mtHsp70
Inside the matrix, mtHsp70 (a mitochondrial chaperone) binds the incoming polypeptide
Uses ATP hydrolysis to ratchet the protein inward, overcoming any electrostatic resistance.
mtHsp70
Mitochondrial Hsp70 chaperone that pulls proteins into the matrix using ATP hydrolysis.
OXA Complex
Inserts proteins synthesized in the mitochondria into the inner membrane; also re-inserts some imported proteins.
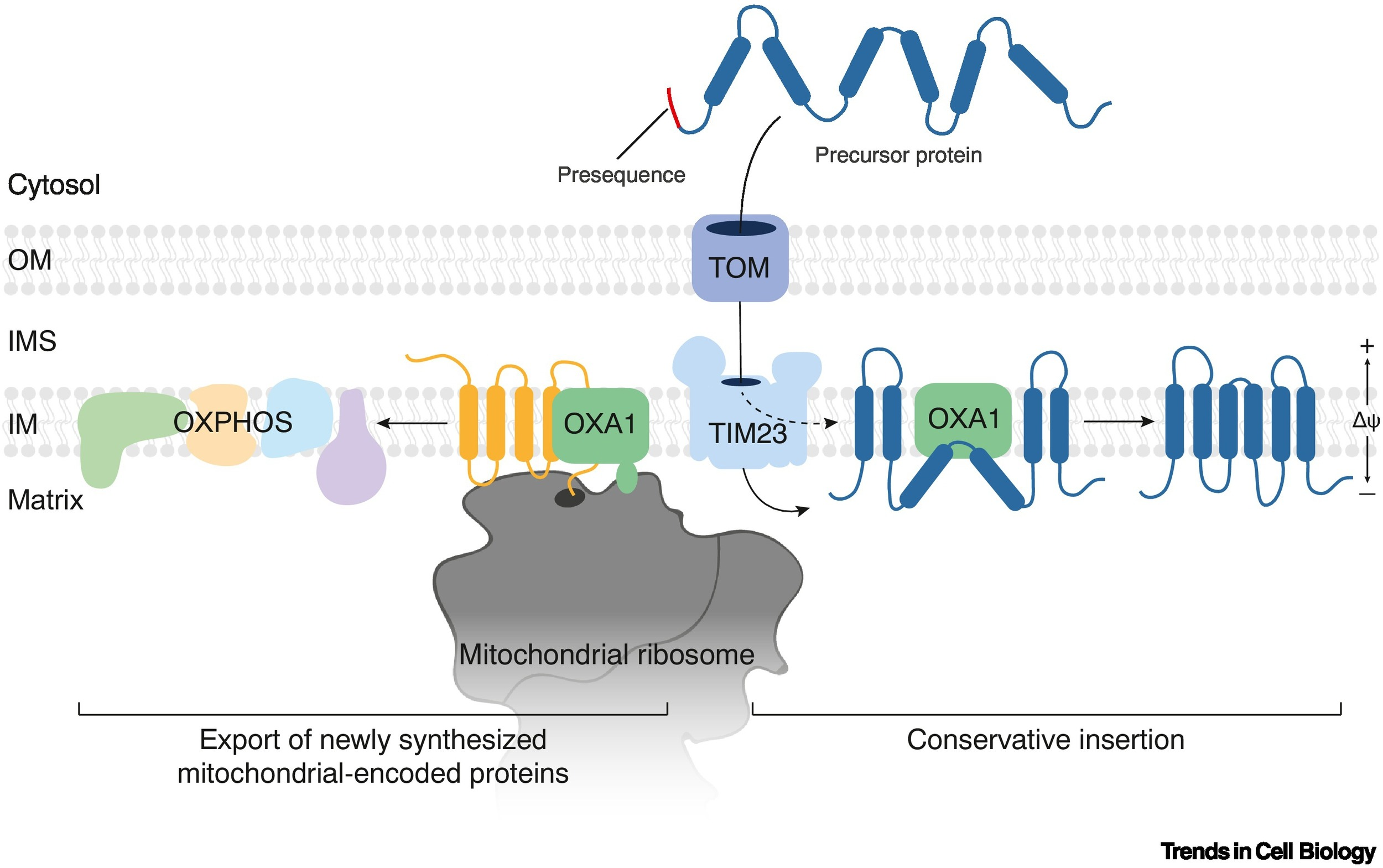
Mitochondrial Protein Import
The process by which nuclear-encoded proteins are synthesized in the cytosol and transported into mitochondria using specialized translocases and targeting signals.
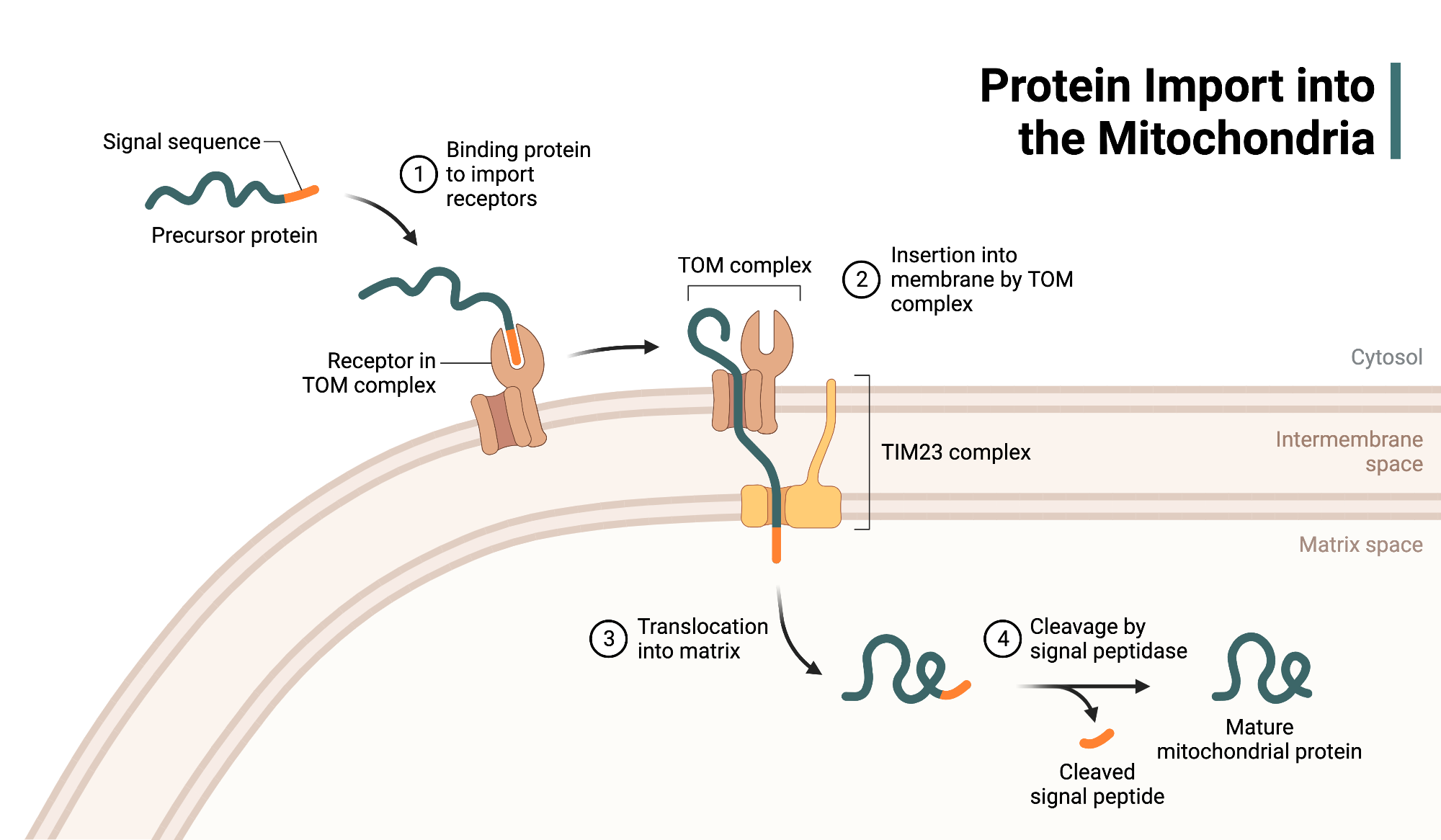
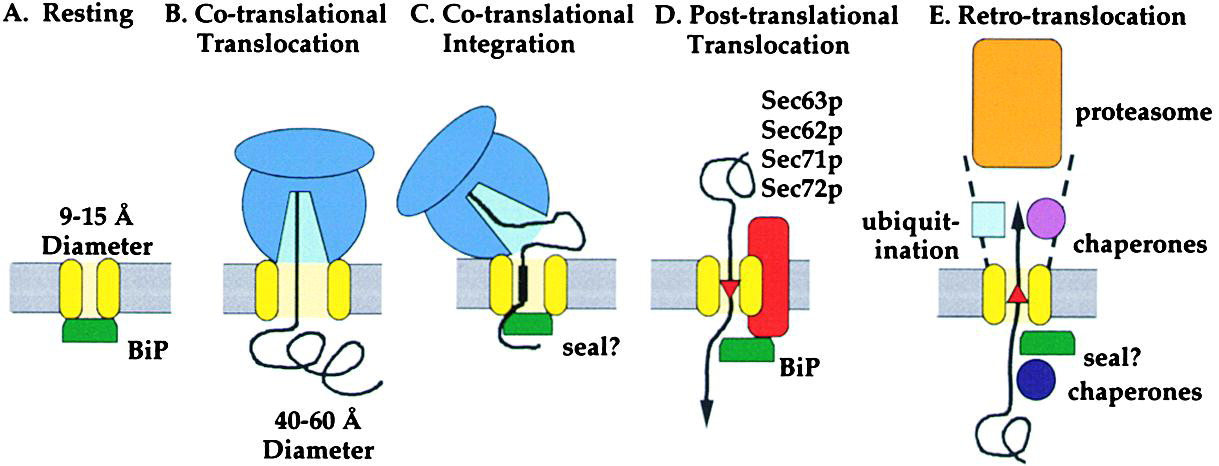
Post-Translational Import
Most mitochondrial proteins are imported after translation is complete, requiring chaperones to maintain them in an unfolded state.
β-Barrel Protein Import
TOM → small TIM chaperones → SAM complex → insertion into outer membrane.
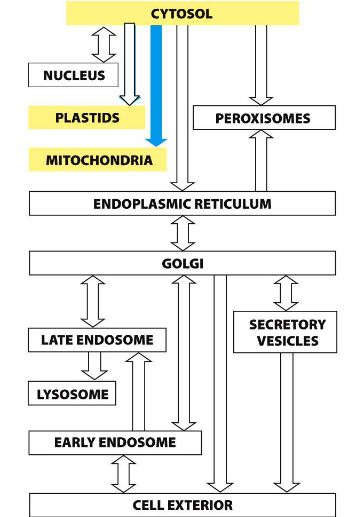
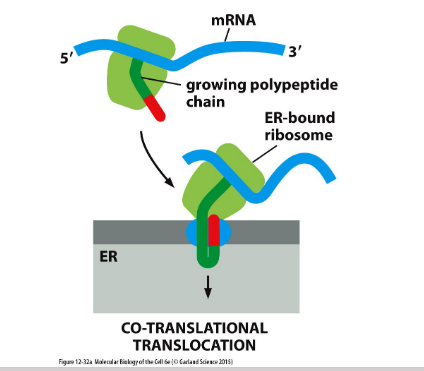
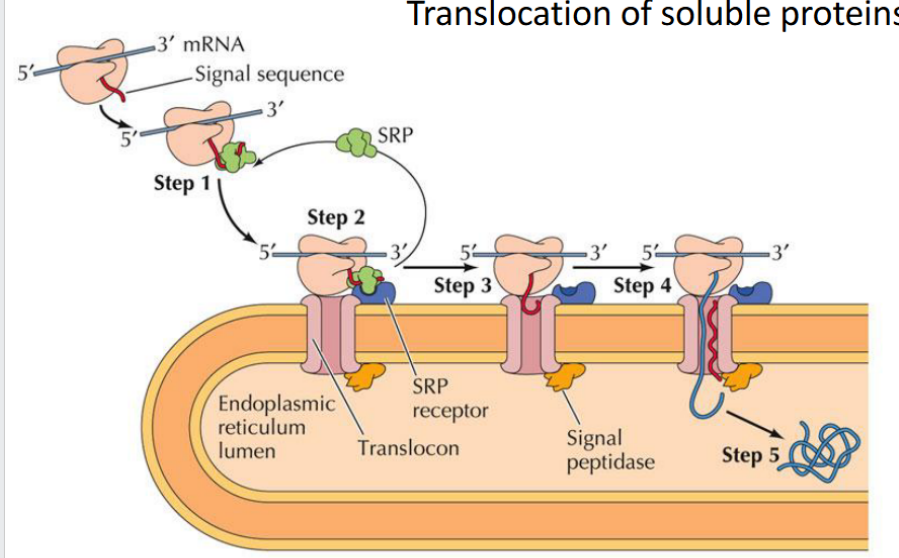
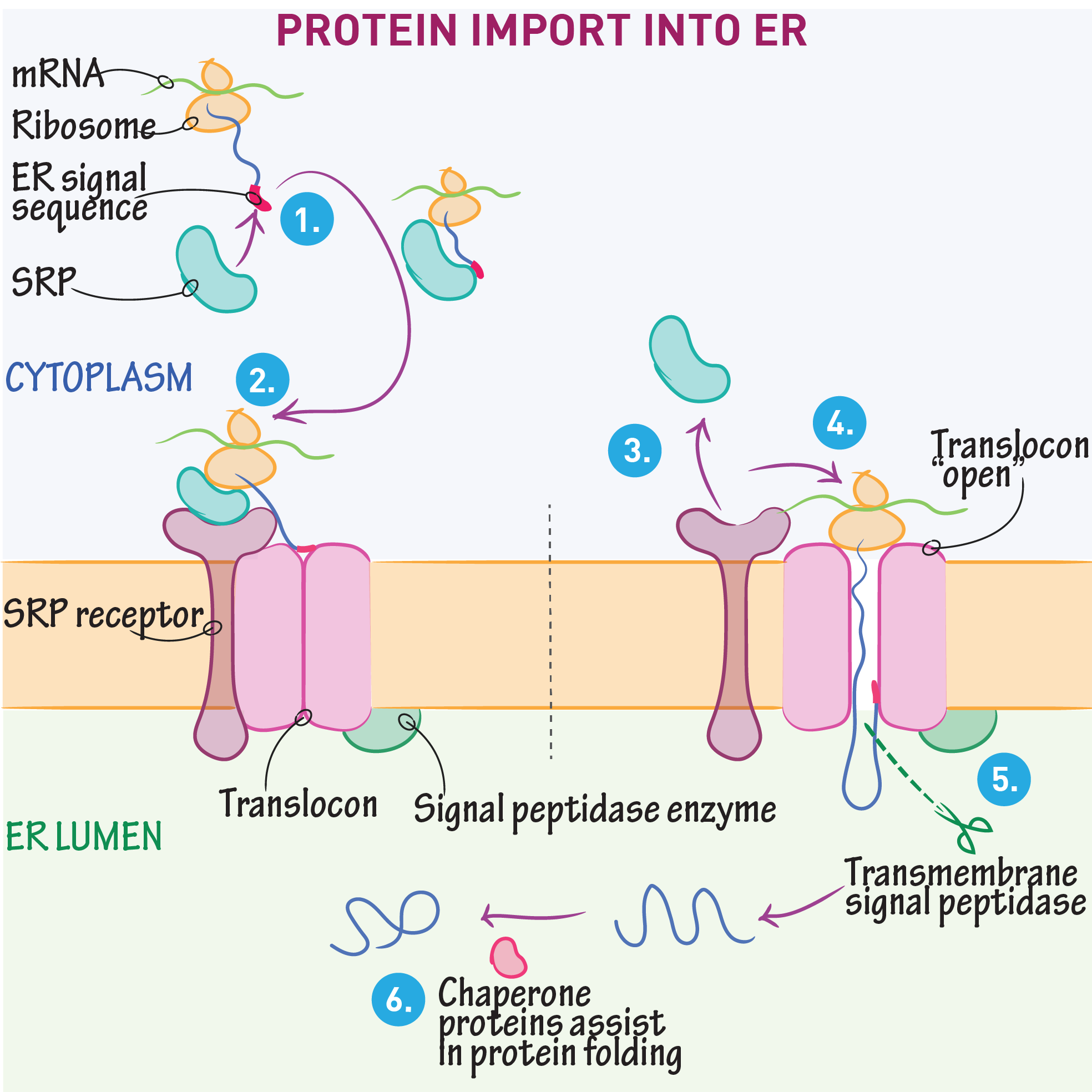
Protein translocation-ER
The endoplasmic reticulum (ER) makes up over half of a eukaryotic cell’s membrane system.
Rough ER handles co-translational translocation, where proteins enter the ER during synthesis.
Import into the ER
(1) Soluble proteins enter the ER lumen for folding and processing
(2) Transmembrane proteins (not soluble) are inserted into the ER membrane for later targeting to organelle or plasma membranes
Targeting the ER (Cotranslational Translocation)
Key components involved in guiding a protein into the ER during translation:
Signal Sequence – Directs the ribosome to the ER
SRP (Signal Recognition Particle) – Binds the signal sequence and pauses translation
SRP Receptor – Anchors the SRP–ribosome complex to the ER membrane
Translocon – Channel that threads the growing polypeptide into the ER lumen
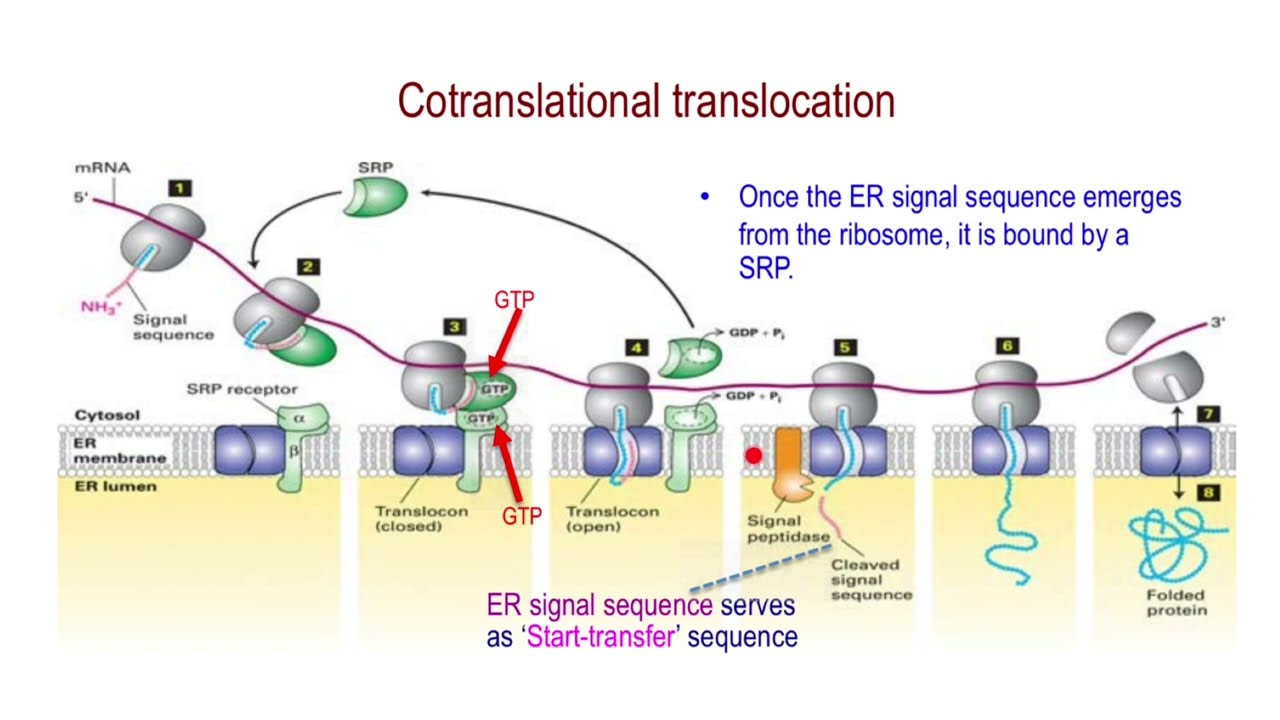
ER Signal sequence
Sequences vary
• Each has 7 or more nonpolar amino acids at its center
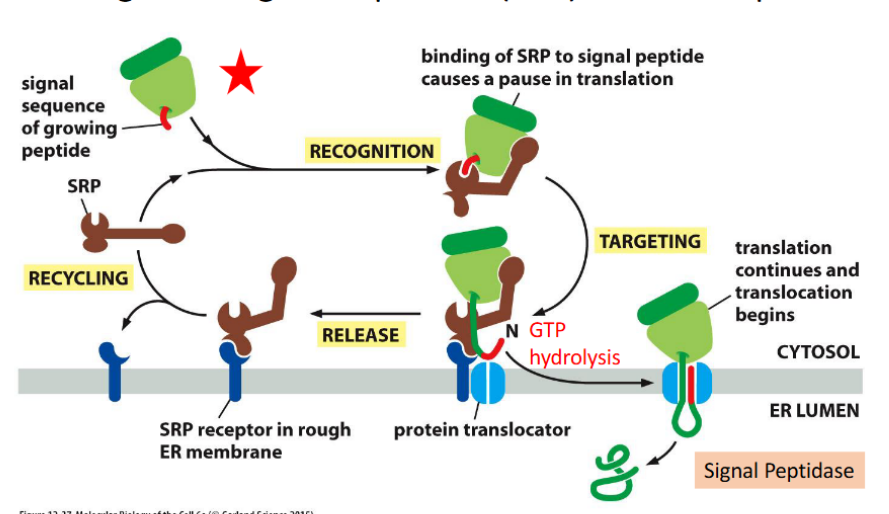
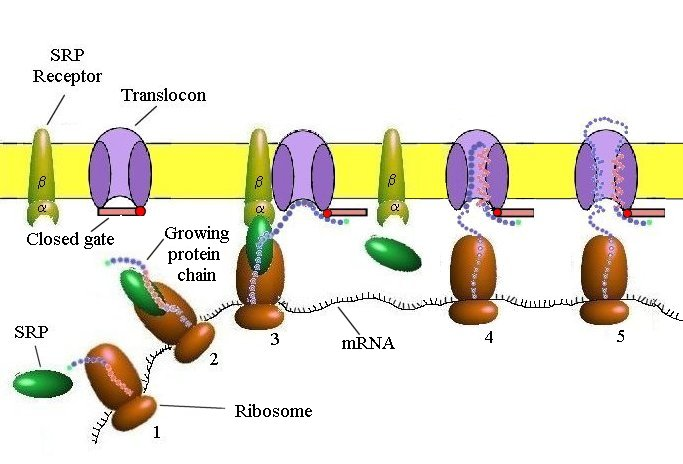
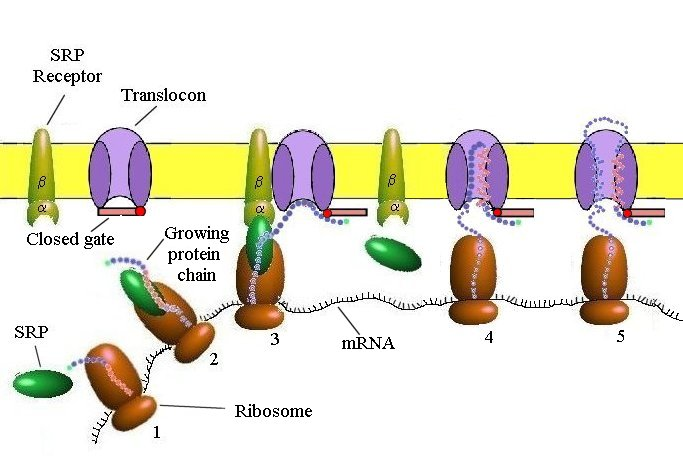
SRP & SRP Receptor: Translation Pausing and Targeting to ER
SRP (Signal Recognition Particle)
Has a flexible hydrophobic groove (lined with methionine) that binds diverse signal sequences
Binding blocks the elongation factor site → pauses translation
Contains a GTPase domain that interacts with the SRP receptor
Composed of 6 proteins + RNA in animal cells
➡ SRP–SRP Receptor Interaction
Each GTPase domain has low GTP affinity alone
Their interaction boosts GTP binding and hydrolysis
This triggers conformational changes → SRP release and protein handoff to translocon
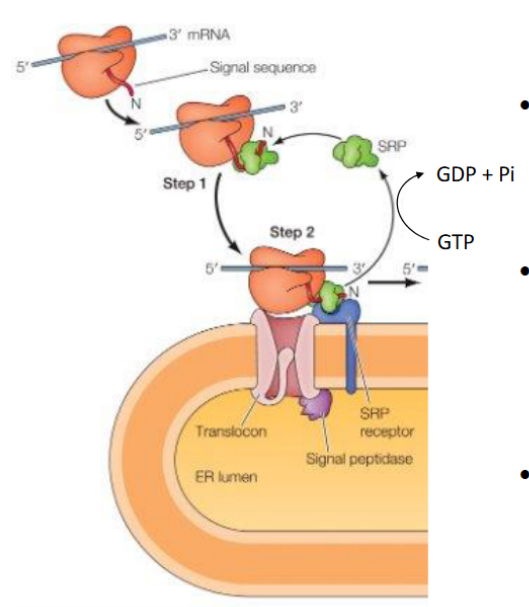
ER signal sequence (N-terminus) is guided by at least two
components:
Signal recognition particle (SRP) & SRP receptor
Protein translocator: Sec61 complex
a protein-conducting channel in the ER membrane that facilitates co-translational translocation of nascent polypeptides into the ER lumen or membrane.
🔹 Key Features
Aqueous Channel
Forms a hydrophilic pore that allows unfolded polypeptides to pass through
Gated Architecture
Contains a plug domain that seals the channel when inactive
Opens upon signal sequence recognition
Lateral Seam/Gate
Allows hydrophobic transmembrane segments to exit sideways into the lipid bilayer
Critical for membrane protein insertion
Driven by Translation
Ribosome docks directly onto Sec61
Protein synthesis pushes the growing chain through the channel
Co-Translational Translocation
Translocation occurs simultaneously with translation, ensuring efficient ER entry and folding
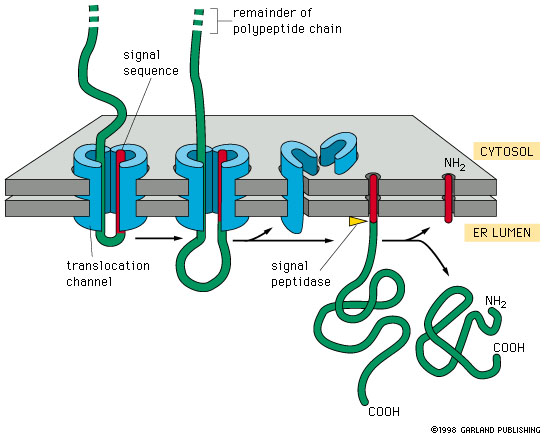
Translocation of soluble proteins
The signal sequence guides the protein into the ER, exits through the translocon’s lateral gate, and is then cleaved and degraded
The rest of the protein continues into the ER lumen, where it folds and may undergo modifications like glycosylation
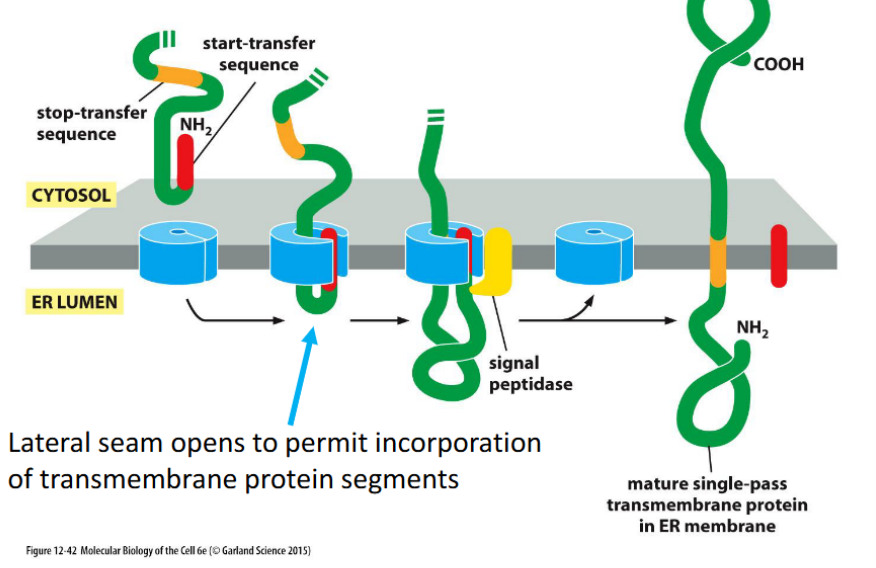
Integral Membrane Proteins: Single-Pass Insertion
Proteins with one transmembrane domain are inserted into the ER membrane via the translocon, using a start-transfer sequence and a stop-transfer sequence. The translocon’s lateral seam opens to embed the hydrophobic segment into the lipid bilayer.
Use one start-transfer sequence to initiate translocation into the ER membrane.
Use one stop-transfer sequence to halt translocation and anchor the protein.
This creates a single membrane-spanning domain.
Orientation (N-terminus in cytosol vs lumen) depends on the position and type of the signal sequence.
Signal sequence
Short amino acid sequence that directs a newly synthesized protein to the ER for translocation.
Signal recognition particle (SRP)
A cytosolic complex that binds to the signal sequence and pauses translation until the ribosome docks at the ER.
SRP Receptor
Located on the ER membrane; binds SRP and facilitates ribosome attachment to the translocon
Translocon (Sec61 Complex)
A protein-conducting channel in the ER membrane through which nascent polypeptides are threaded into the lumen.
protein complex facilitates translocation of proteins into the ER lumen
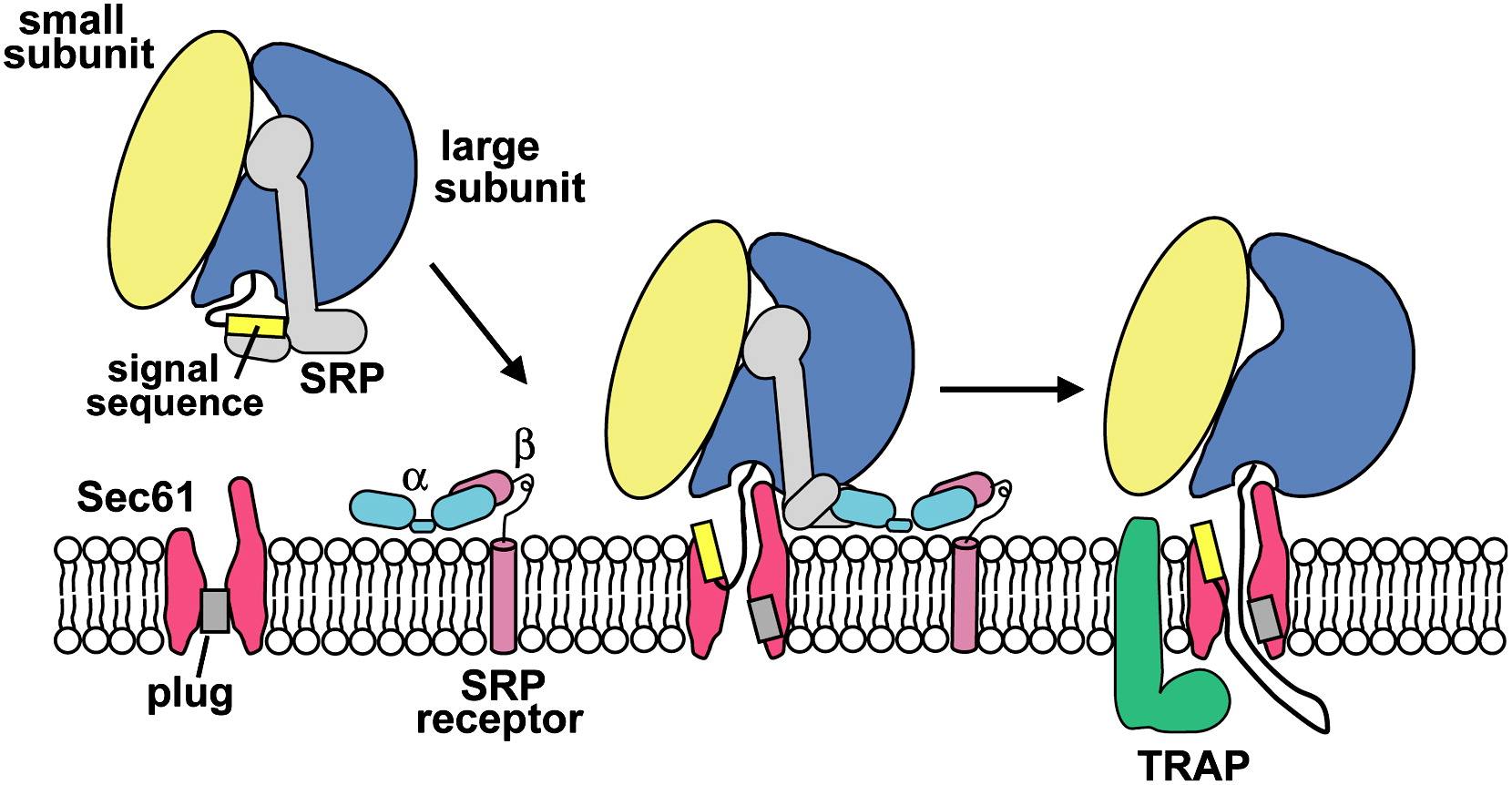
Co-translational Translocation
Process where a protein is translocated into the ER lumen while it is still being synthesized by the ribosome.
Post-translational Translocation
Process where a fully synthesized protein is imported into the ER, often assisted by chaperones.
Start-Transfer Sequence
A hydrophobic segment that initiates translocation through the translocon; can be cleaved or retained depending on the protein.
Stop-Transfer Sequence
A hydrophobic segment that halts translocation and anchors the protein in the membrane.
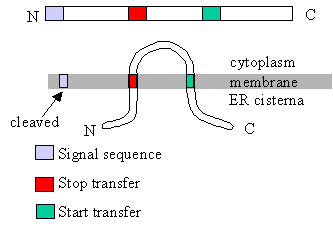
ER lumen
The internal space of the endoplasmic reticulum where soluble proteins are folded and modified.
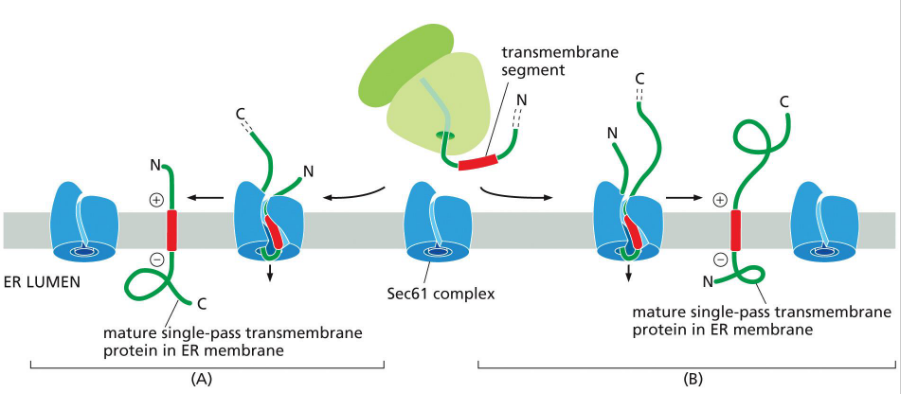
Insertion of a single pass transmembrane protein
*Not shown – SRP recognition and escort to ER membrane
Pre-Insertion (Not Shown in Diagram)
Signal Recognition Particle (SRP) binds the internal hydrophobic transmembrane segment (signal-anchor).
SRP escorts the ribosome–nascent chain complex to the SRP receptor on the ER membrane.
The ribosome docks onto the Sec61 translocon.
Pathway B: N-terminus in ER lumen, C-terminus in cytosol (N-terminal signal sequence)
Translation begins in the cytosol.
The internal signal-anchor sequence emerges and is recognized by SRP.
The ribosome docks at Sec61; the signal-anchor is inserted laterally into the membrane.
The N-terminal portion of the protein is translocated into the ER lumen.
The C-terminal portion remains in the cytosol.
Final orientation:
N-terminus → ER lumen
C-terminus → cytosol
Transmembrane segment → embedded in membrane
Pathway A: N-terminus in cytosol, C-terminus in ER lumen (internal transmembrane segment)
Translation starts in the cytosol.
The internal signal-anchor is recognized and inserted into Sec61.
The N-terminal portion stays in the cytosol.
The C-terminal portion is translocated into the ER lumen.
Final orientation:
N-terminus → cytosol
C-terminus → ER lumen
Transmembrane segment → embedded in membrane
🧲 Orientation Rule of Thumb
Positively charged residues flanking the transmembrane segment tend to stay cytosolic, due to attraction to negatively charged phospholipid heads.
This electrostatic bias helps determine which end stays cytosolic, but the full orientation mechanism is still under investigation.
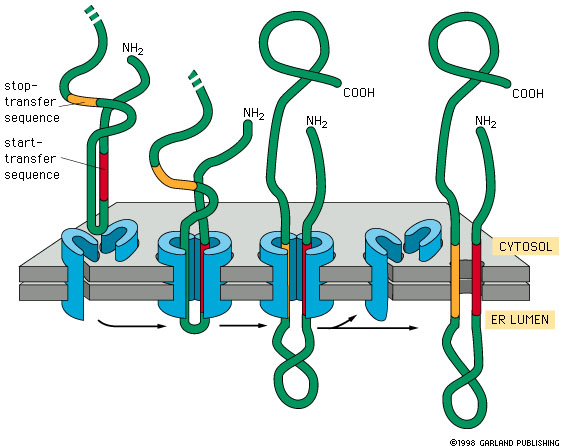
Insertion of transmembrane domains for a multipass protein
1. SRP Recognition and Docking (Not Shown)
The first hydrophobic segment (usually a start-transfer sequence) is recognized by the Signal Recognition Particle (SRP).
SRP escorts the ribosome–nascent chain complex to the ER membrane, docking at the Sec61 translocon.
This first segment sets the orientation of the protein and determines how subsequent segments will be threaded.
2. Sequential Insertion via Start/Stop Transfer Sequences
Each transmembrane domain is inserted using alternating start-transfer and stop-transfer signals:
🔹 First Segment (Start-Transfer)
Acts like an internal signal-anchor.
Initiates translocation of the downstream portion of the protein into the ER lumen.
The upstream portion remains in the cytosol.
🔹 Second Segment (Stop-Transfer)
Halts translocation through the translocon.
This segment is laterally released into the membrane.
The downstream portion stays in the cytosol.
3. Repeat for Additional Passes
Each start-transfer sequence resumes translocation into the ER lumen.
Each stop-transfer sequence halts it and embeds the next transmembrane domain.
This alternating pattern creates a zigzag threading of the protein through the membrane.
🧩 Final Result
The mature protein has multiple transmembrane domains embedded in the ER membrane.
The topology (which ends are cytosolic vs. luminal) is determined by:
The order of start/stop sequences
The charge distribution near each transmembrane segment
The initial orientation set by the first segment
occurs co-translationally
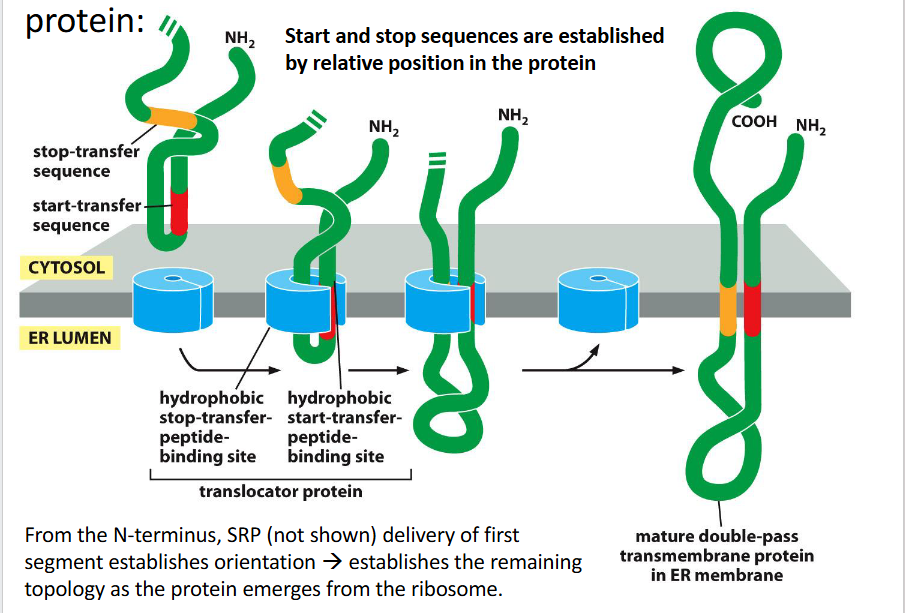
Transmembrane domain
-the hydrophobic segments of the protein that span across the ER membrane. They’re the parts that end up embedded in the lipid bilayer, anchoring the protein in place.
These sequences are hydrophobic stretches of amino acids that:
Start-transfer: initiates threading of the next portion of the protein into the ER lumen.
Stop-transfer: halts translocation and causes that segment to be released sideways into the membrane.
Each start/stop pair results in one transmembrane domain — meaning a portion of the protein that spans the membrane once.
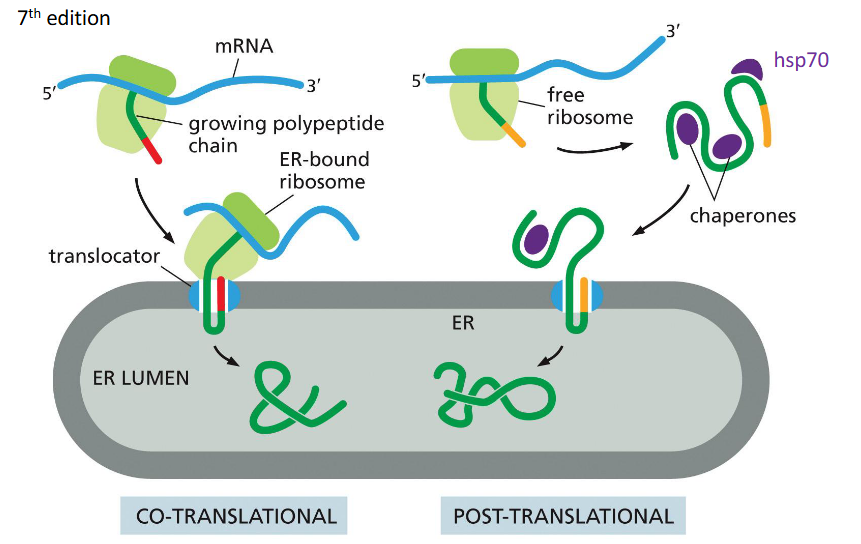
How is protein movement driven co-translationally?
Protein elongation during translation pushes the chain into the ER
Movement is coupled to ribosome activity
Uses the Sec61 translocon channel
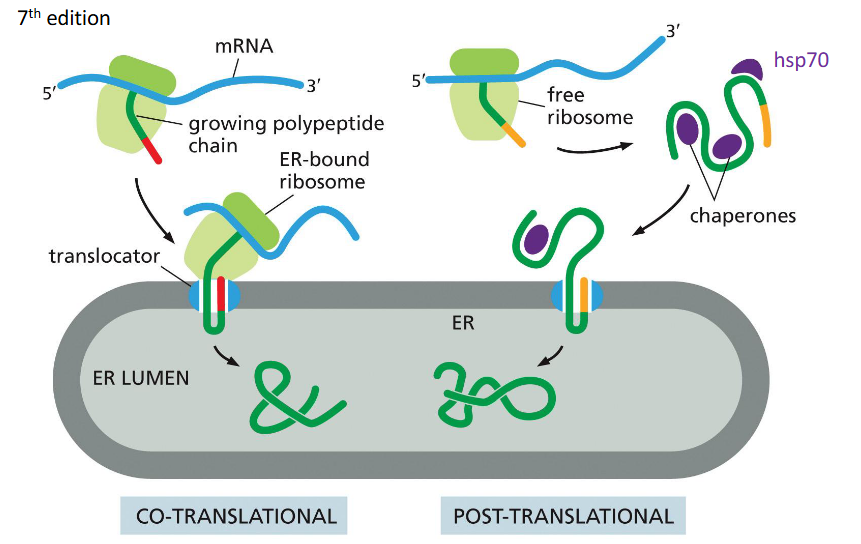
How is protein movement driven post-translationally?
Via “molecular ratcheting” by BiP, which pulls proteins into the ER lumen after synthesis.
ER resident proteins
Proteins that remain in the endoplasmic reticulum (ER) carry a C-terminal retention signal—typically a 4-amino-acid sequence—that prevents them from being secreted.
🔹 Key Examples
PDI (Protein Disulfide Isomerase)
Catalyzes oxidation of free sulfhydryl groups
Facilitates disulfide bond formation
Disulfide bonds stabilize extracellular proteins (e.g., surface-bound or secreted proteins)
BiP (Binding Immunoglobulin Protein)
ER chaperone
Pulls post-translationally translocated proteins into the ER lumen
Assists in protein folding
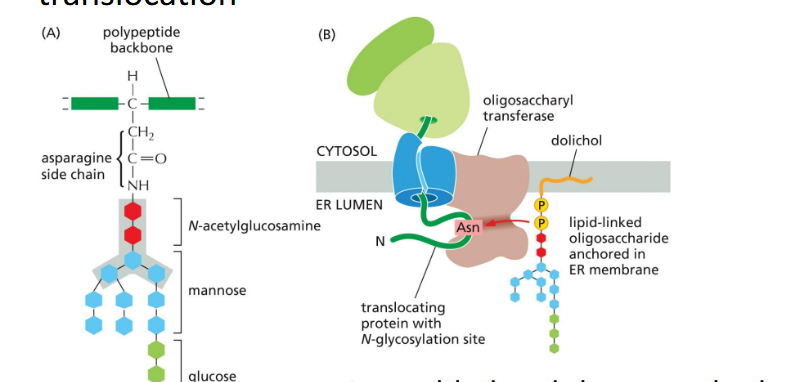
Glycosylation in the Rough ER
Definition:
a co-translational process where a preassembled oligosaccharide is transferred to nascent proteins, primarily through N-linked glycosylation.
🔹 Key Features
N-linked Glycosylation
Occurs on asparagine residues within the consensus sequence Asn-X-Ser/Thr
Happens during translocation into the ER lumen
En Bloc Transfer
A 14-sugar oligosaccharide is assembled on a lipid carrier (dolichol)
Transferred all at once to the protein
Initial Trimming in the ER
Three glucose residues and one mannose are commonly removed
Trimming helps regulate folding and ER quality control
Further Modification
Additional processing occurs in the Golgi, generating glycan diversity
ER Mannosidase
A slow-acting ___gradually trims mannose residues from glycoproteins. Once sufficient trimming occurs, the modified glycan is recognized by ER-luminal lectins (proteins that bind specifically to carbohydrates) associated with a retrotranslocator, marking the protein for export or degradation.
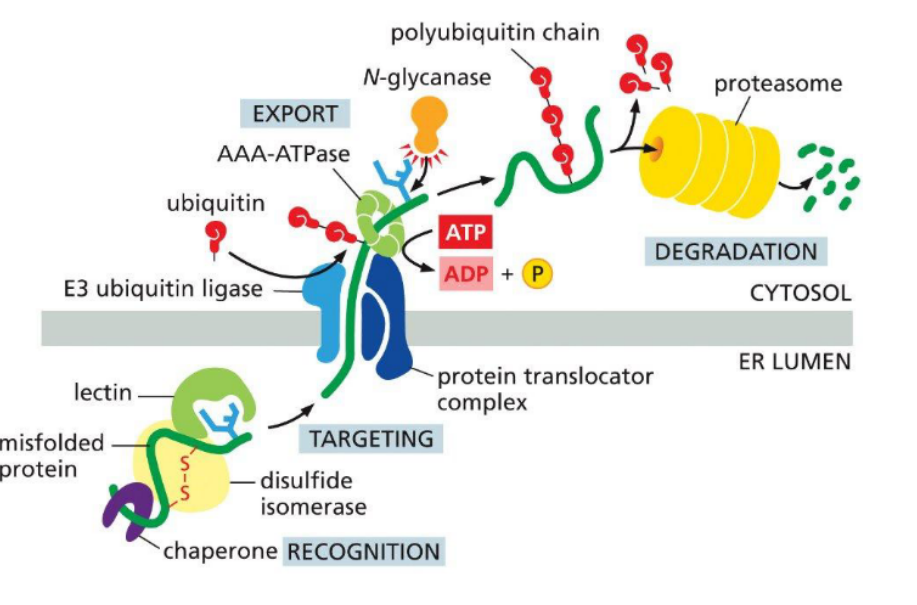
Retrotranslocation
the process by which misfolded or unassembled proteins are exported from the ER lumen back into the cytosol for degradation, typically as part of the ER-associated degradation (ERAD) pathway.
🔹 Key Requirements
Protein Unfolding
Substrates must be partially or fully unfolded to pass through the narrow translocon channel.
Translocator Complex
A protein-conducting channel (e.g., Derlin or Hrd1 complex) facilitates movement across the ER membrane.
Energy Source
ATP hydrolysis powers the process, often via cytosolic AAA-ATPases (e.g., p97/VCP)
These ATPases pull the protein through the translocator into the cytosol
ERAD = ER-associated degradation
-Prevents spread of aberrant protein to other cellular locations
-Deals with protein folding dynamics within the ER (many proteins fail to fold properly)
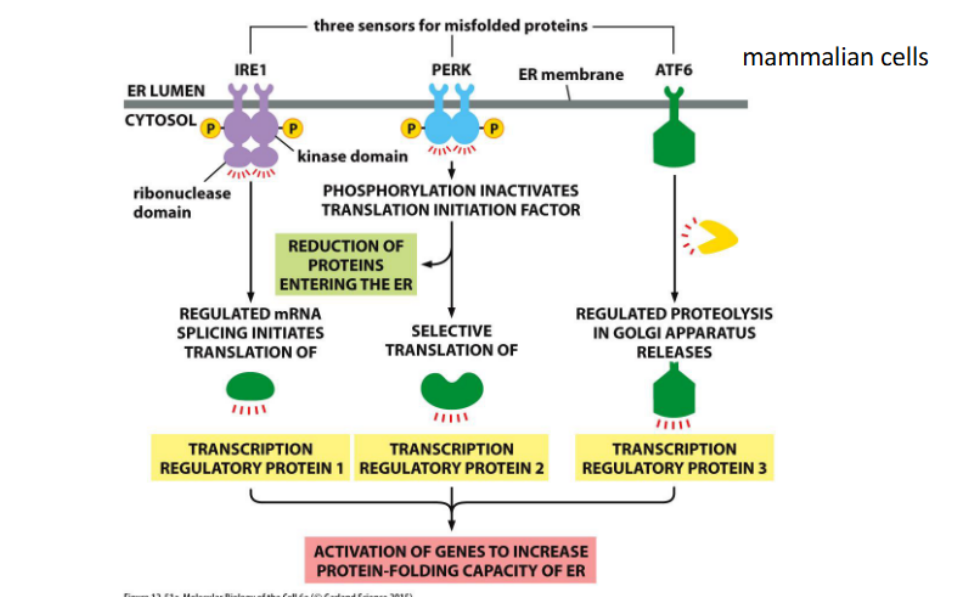
Unfolded protein response (UPR)
-in the ER
-Occurs when misfolded protein generation exceeds capacity for export to the cytoplasm
Cargo
may refer to
- membrane components or soluble lumenal molecules
Post-translational Translocation
Protein translocation into the ER after translation is complete. Movement is driven by ATP-dependent chaperones like BiP, which pull the unfolded protein through the translocon.
BiP (Binding Immunoglobulin Protein)
An ER-resident chaperone that facilitates post-translational translocation and assists in protein folding. It uses ATP hydrolysis to "ratchet" proteins into the ER lumen.
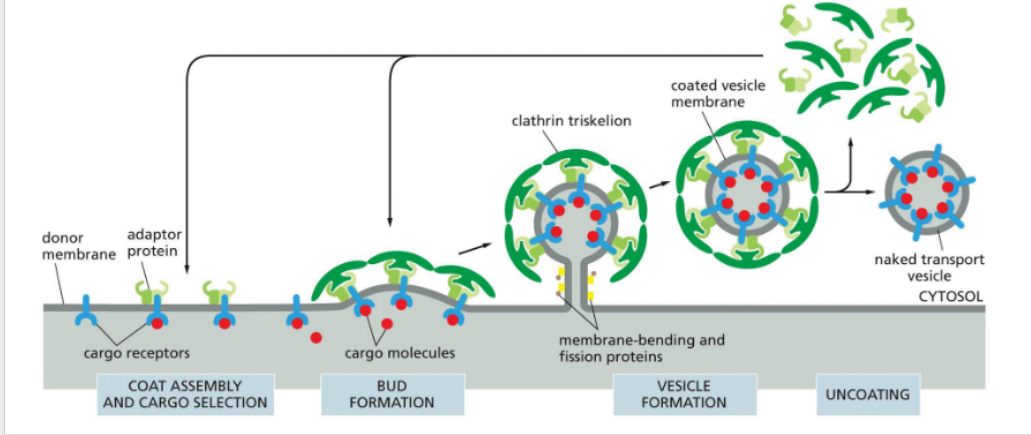
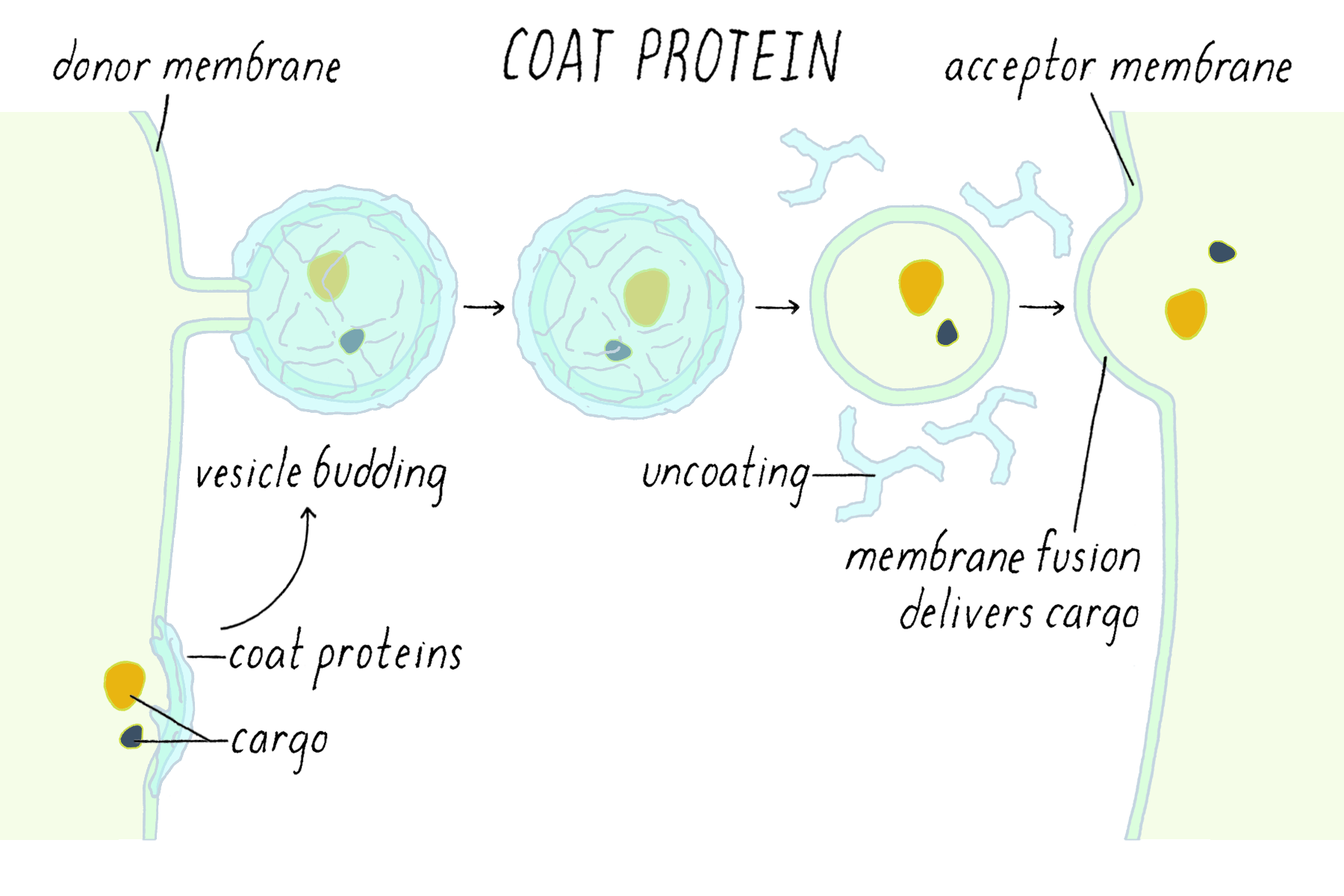
Vesicle budding
the process by which a small, membrane-bound sac (vesicle) forms by pinching off from a larger membrane, typically to transport cargo within or outside the cell.
initiated by the formation of a protein coat—a structured cage of proteins that assembles on the cytosolic side of the membrane, creating a coated vesicle.
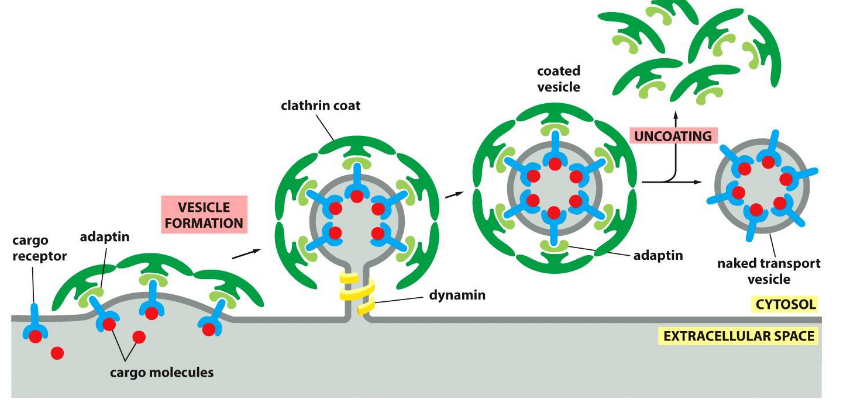
What is the purpose of the protein coat?
1. The coat helps shape the membrane into a bud
2. The coat helps concentrate and capture molecules for transport
Note – The coat must be discarded before the vesicle can fuse into the next membrane.
Transport vesicles
Definition: membrane-bound carriers that move cargo between organelles or to the cell surface. Their formation is spatially regulated and directionally specific.
🔹 Key Concepts
Directional Transport
Vesicles move cargo from donor to target membranes
Directionality is determined by coat type, Rab GTPases, and SNAREs
Specialized Membrane Regions
Vesicles bud from defined membrane domains (e.g., ER exit sites, trans-Golgi network)
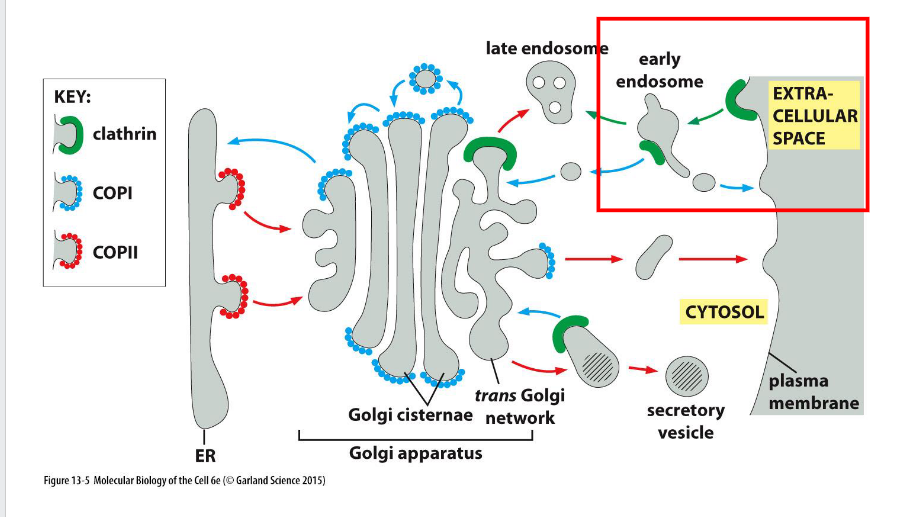
Four Major Vesicle Types
COPII (ER → Golgi), COPI (Golgi → ER), Clathrin (PM/TGN → Endosome), Retromer (Endosome → trans Golgi network/PM)
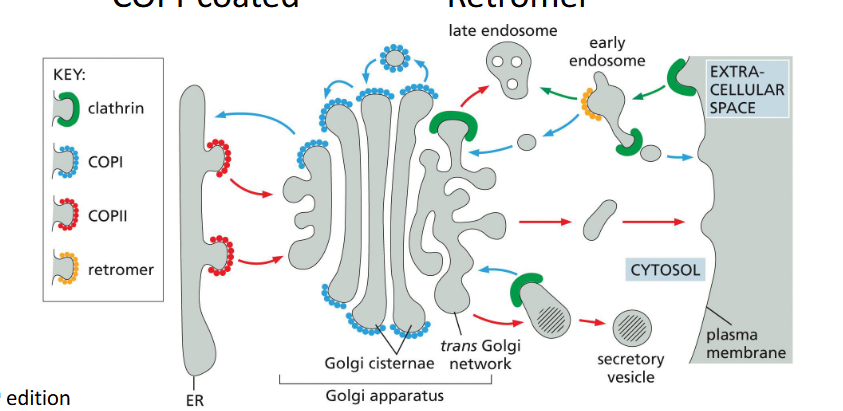
What components help with vesicular transport?
Definition:
Vesicular transport relies on a coordinated set of proteins that drive vesicle formation, targeting, and fusion.
🔹 Key Components
Coat Proteins
Drive vesicle budding and cargo selection (e.g., clathrin, COPI, COPII)
Adaptor Proteins
Link coat proteins to cargo receptors and membrane lipids (e.g., AP complexes)
Membrane Remodeling Proteins
Bend and pinch the membrane during budding (e.g., BAR domain proteins, dynamin)
Uncoating Mechanism
Removes coat proteins after vesicle formation to expose fusion machinery
Rab GTPases
Regulate vesicle targeting and docking by recruiting tethering factors
Tethering Proteins
Bridge vesicle and target membrane before fusion
SNAREs
Mediate membrane fusion by forming trans-SNARE complexes
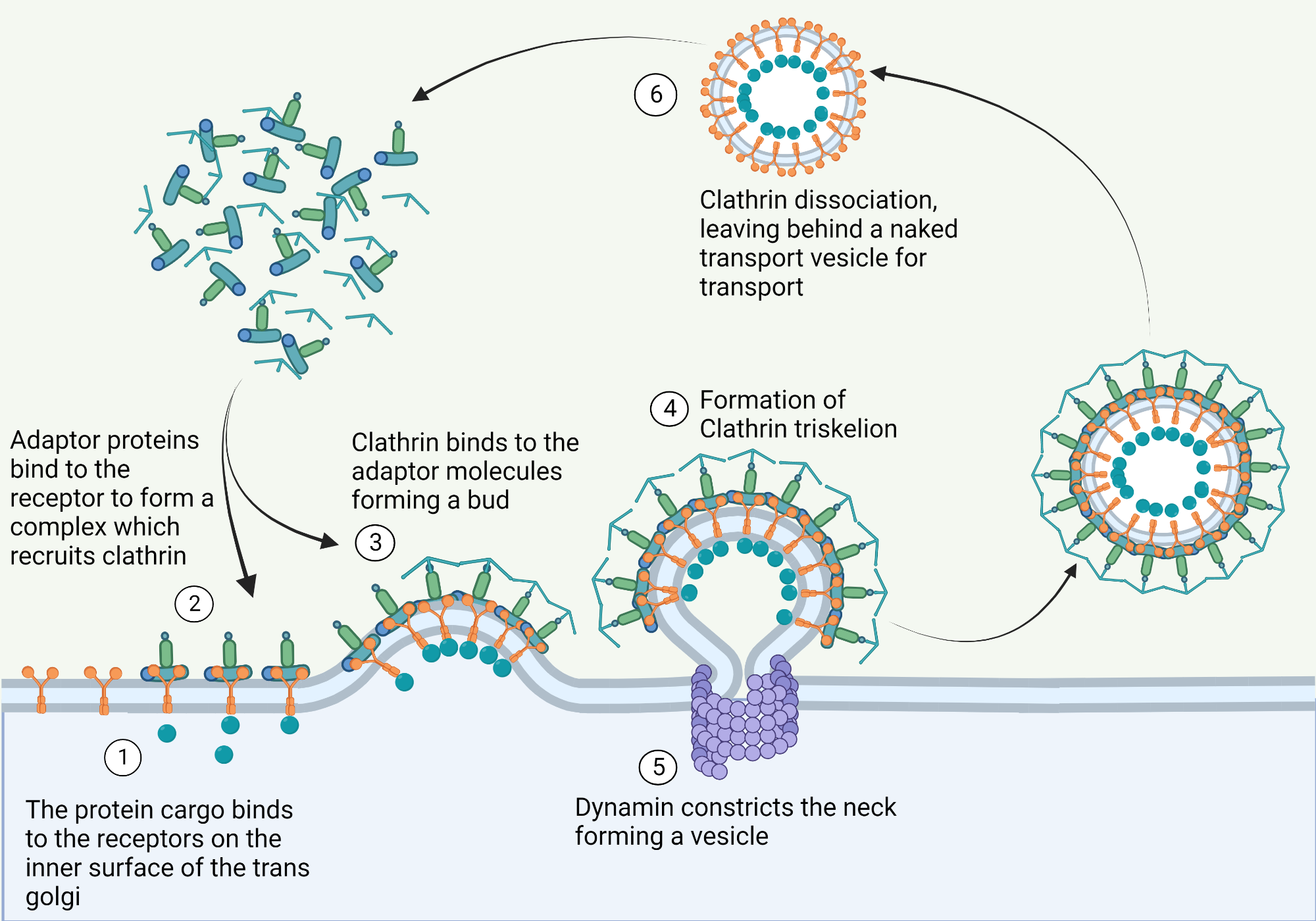
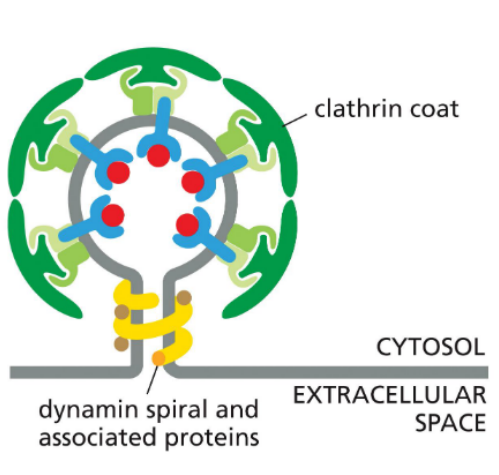
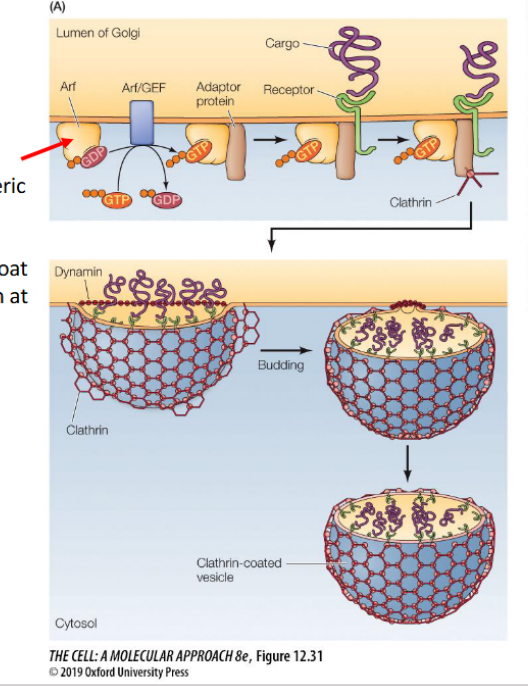
Clathrin
Definition:
a coat protein that drives vesicle formation at the plasma membrane by assembling into a structured lattice.
🔹 Key Features
Triskelion Structure
Each clathrin subunit contains three heavy chains and three light chains
Forms a three-legged triskelion shape
Lattice Assembly
Triskelions self-assemble into a polyhedral cage of hexagons and pentagons
Creates coated pits that curve the membrane
Membrane Focus
Primarily functions at the plasma membrane during endocytosis
Works with adaptor proteins (e.g., AP2) to select cargo and initiate vesicle budding
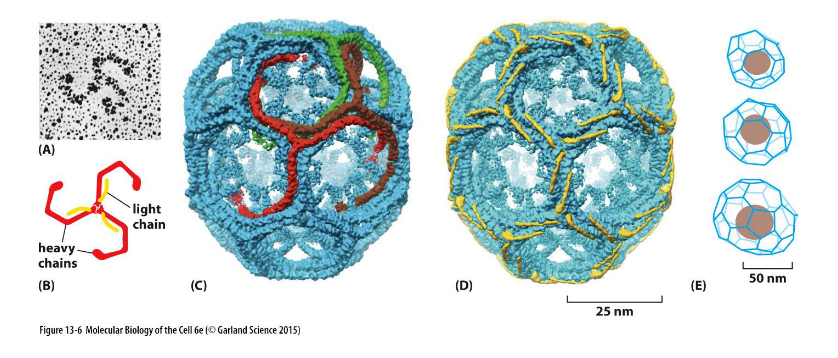
Clathrin coated vesicles
Budding from the membrane into endocytic pathway
Adaptins (adaptor proteins)
mediate cargo selection and recruit clathrin to initiate vesicle formation.
🔹 Key Functions
Cargo Selection
Bind to cargo receptors that recognize specific transport signals
Ensure only selected molecules are packaged into vesicles
Clathrin Recruitment
Anchor clathrin to the membrane, initiating coat assembly
Example: AP2 Complex
Recognizes phosphorylated phosphatidylinositol lipids in the membrane
Undergoes conformational change to expose cargo receptor binding sites
Facilitates membrane curvature and clathrin polymerization into a basket-like structure
Coincidence Detection
AP2 activation requires simultaneous recognition of both lipid and cargo signals
Ensures vesicle formation only occurs at the correct membrane domain
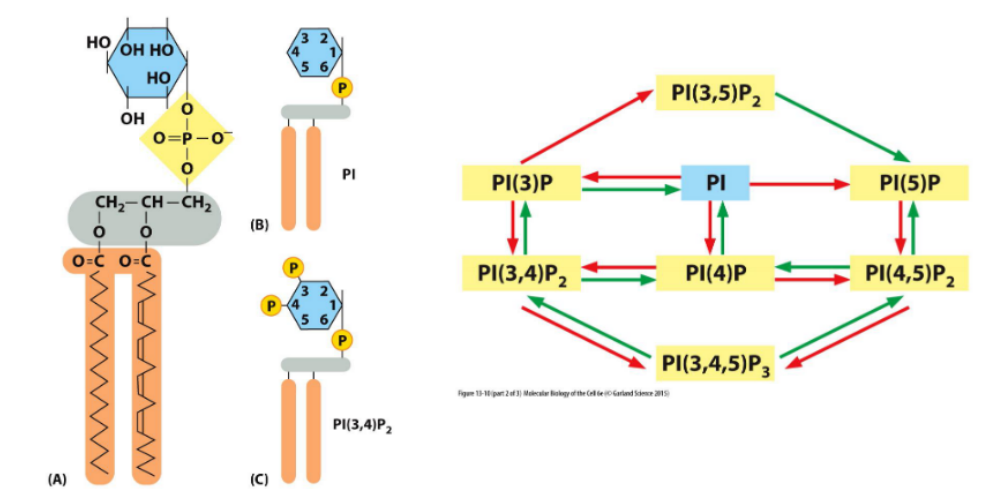
Phosphoinositides (PIs → PIPs)
Definition:
are membrane lipids whose inositol head groups can be phosphorylated at multiple positions, generating distinct PIP species that regulate membrane identity and trafficking.
🔹 Key Concepts
Phosphorylation Sites
Inositol ring can be phosphorylated at the 3′, 4′, and 5′ positions
Generates species like PI(3)P, PI(4,5)P₂, etc.
Enzyme Control
Kinases and phosphatases control PIP distribution
Their localization determines which PIPs are present in each membrane domain
Membrane Domain Identity
Specific PIPs define specialized membrane compartments (e.g., PI(3)P at early endosomes)
Vesicle Trafficking Roles
PIPs recruit trafficking proteins via specific binding to phosphorylated head groups
Regulate steps like budding, tethering, and fusion
Local Regulation
Tight spatial control of PIP-modifying enzymes ensures precise trafficking and signaling
Accessory molecules in vesicle formation
Beyond coat proteins, several accessory molecules assist in shaping and releasing vesicles during budding.
🔹 Key Contributors
BAR Domain Proteins
Bind curved membranes and promote bending
Help sculpt the vesicle bud
Actin Polymerization
Generates mechanical tension
Assists in membrane deformation and vesicle propulsion
Neck-Constricting Proteins (e.g., Dynamin)
Assemble at the bud neck
Use GTP hydrolysis to pinch off the vesicle
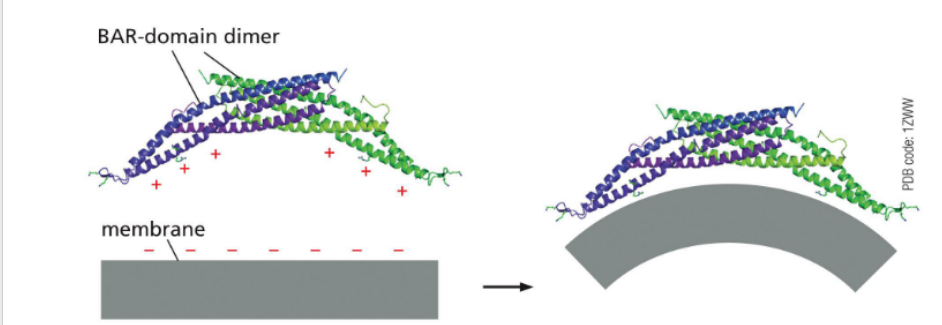
Dynamin
Has a PI-binding domain for membrane targeting
Contains a GTPase domain that powers membrane scission
May recruit lipid-modifying enzymes
Pinches off vesicles from the membrane during endocytosis
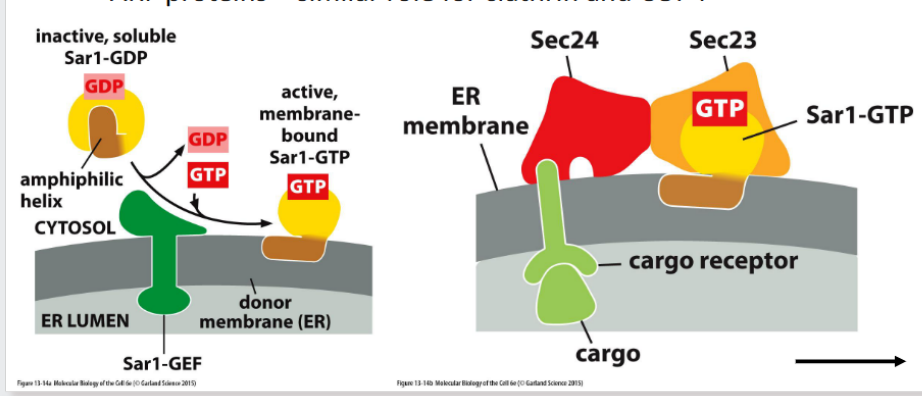
Coat-Recruitment GTPases
Definition:
regulate the timing and location of vesicle formation by controlling coat protein assembly.
🔹 Key GTPases
Sar1
Initiates COPII coat assembly at the ER
Activated Sar1 inserts into the membrane and recruits Sec23/24
ARF Proteins (ADP-Ribosylation Factors)
Regulate clathrin and COPI coat formation
Activated ARF binds the membrane and recruits adaptor proteins
🔍 Regulatory Role
Spatial Control: GTPase activation is restricted to specific membrane domains
Temporal Control: GTP hydrolysis triggers coat disassembly after vesicle formation
Arf
(monomeric GTPase) regulates clathrin coat formation at the Golgi
Targeting the Correct (Specific) Membrane
Definition:
Precise vesicle targeting and fusion rely on molecular address tags and recognition systems that ensure cargo reaches the correct destination.
🔹 Key Components
Rab Proteins (Monomeric GTPases)
Act as molecular zip codes for vesicles
Each Rab localizes to specific membranes (e.g., Rab5 on early endosomes, Rab7 on late endosomes)
In GTP-bound form, Rabs recruit Rab effectors (e.g., tethering proteins) to initiate docking
Rab Effectors
Large tethering complexes or motor adaptors
Mediate initial contact between vesicle and target membrane
Help position vesicles for SNARE-mediated fusion
SNARE Proteins
v-SNAREs on vesicles and t-SNAREs on target membranes
Form trans-SNARE complexes that pull membranes together for fusion
Provide specificity and force for membrane merging
SNARE Regulators
Include NSF and SNAPs, which disassemble SNARE complexes post-fusion
Ensure SNAREs are recycled and reset for future rounds
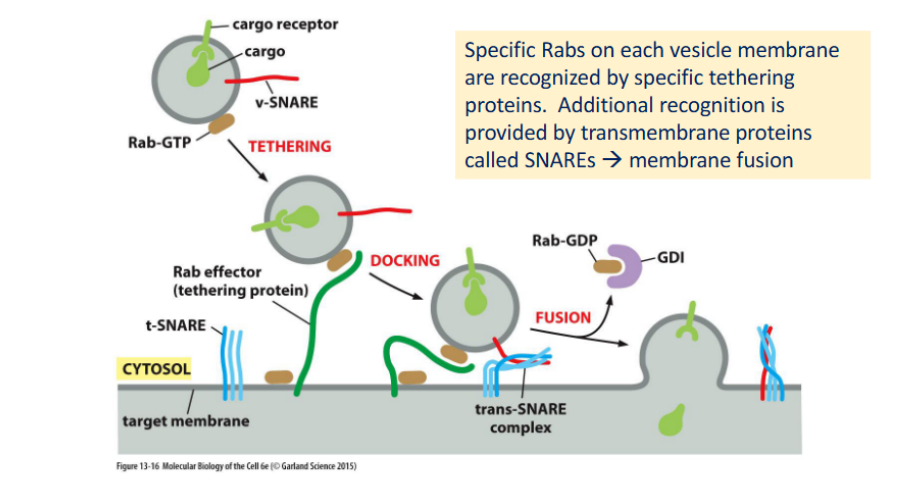
Rab proteins
monomeric (single subunit — it functions as an individual unit) GTPases that regulate vesicle targeting and fusion by cycling between active and inactive states.
🔁 Rab Activity Cycle
Active (GTP-bound):
Anchored to organelle or vesicle membranes via lipid modification
Interacts with Rab effectors to guide vesicle trafficking
Inactive (GDP-bound):
Bound to GDI (GDP Dissociation Inhibitor)
Remains soluble in the cytosol
Regulation:
The rate of GTP hydrolysis controls the concentration of active Rab on membranes and the recruitment of its effectors
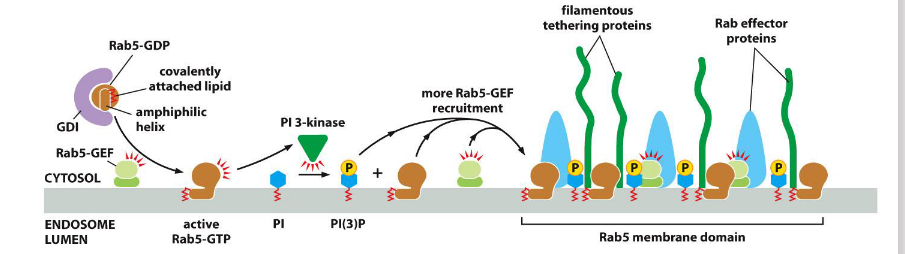
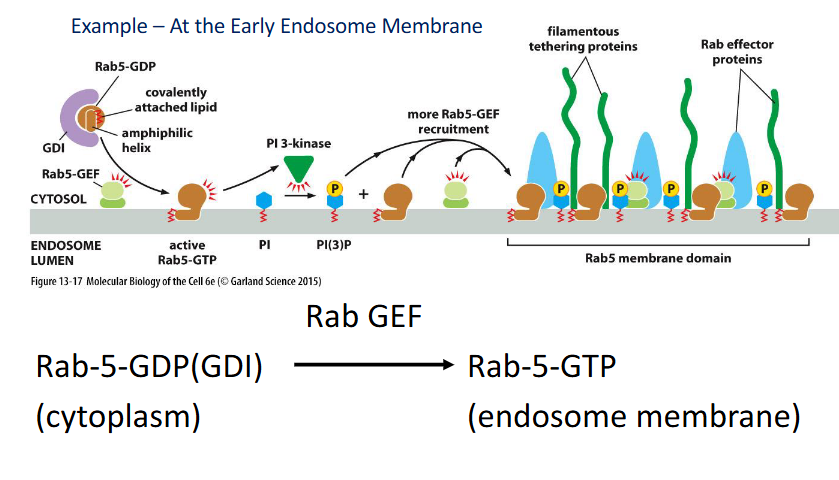
Active Rab5
helps establish specialized membrane domains by recruiting effectors and modifying lipids.
🔹 Key Functions
Positive Feedback Loop:
Recruits Rab-GEFs, which activate more Rab5-GTP locally
Lipid Modification:
Activates PI 3-kinase → converts PI to PI(3)P
PI(3)P recruits Rab effectors (e.g., tethering proteins) to the membrane
Example Site:
Early endosome membrane
Retrotranslocator
a protein complex in the endoplasmic reticulum (ER) membrane that moves misfolded proteins from the ER lumen or membrane back into the cytosol for degradation. This process is a key step in ER-associated degradation (ERAD).
Translocation
refers to the movement of a molecule—often a protein—across a membrane from one cellular compartment to another.
Characteristics of a single pass protein with an internal transmembrane segment
Internal Red Segment (Transmembrane Domain)
The red region is not at the N-terminus, but located internally in the polypeptide chain.
This is characteristic of a signal-anchor sequence, which both initiates translocation and anchors the protein in the membrane.
N-terminus in Cytosol / C-terminus in ER Lumen
The orientation shows the N-terminal end remaining in the cytosol, while the C-terminal end enters the ER lumen.
This is typical of proteins with internal signal-anchor sequences, which insert with this topology.
Sec61 Complex Facilitating Lateral Insertion
The red transmembrane segment is shown integrating laterally into the membrane via Sec61, confirming it’s a membrane anchor.
Single Transmembrane Segment
Only one red segment is shown crossing the membrane, indicating it’s a single-pass protein.
Coat proteins
shape the vesicle and help recruit adaptins
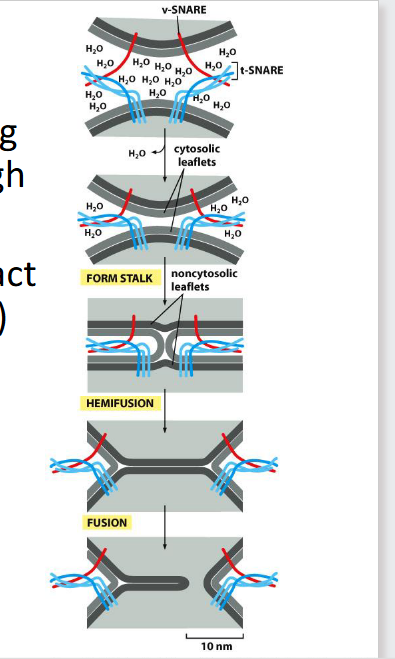
SNAREs
mediate vesicle docking & fusion
Help displace water and bring
membranes into close enough
proximity to merge
• v-SNAREs (single helix) interact
with t-SNAREs (three helices)
to form a bundle termed a
trans SNARE complex
Note: AAA proteins couple ATP hydrolysis with conformational changes
Previous example – AAA proteases are used to translocate proteins into the proteosome for degradation
Secretory Pathway
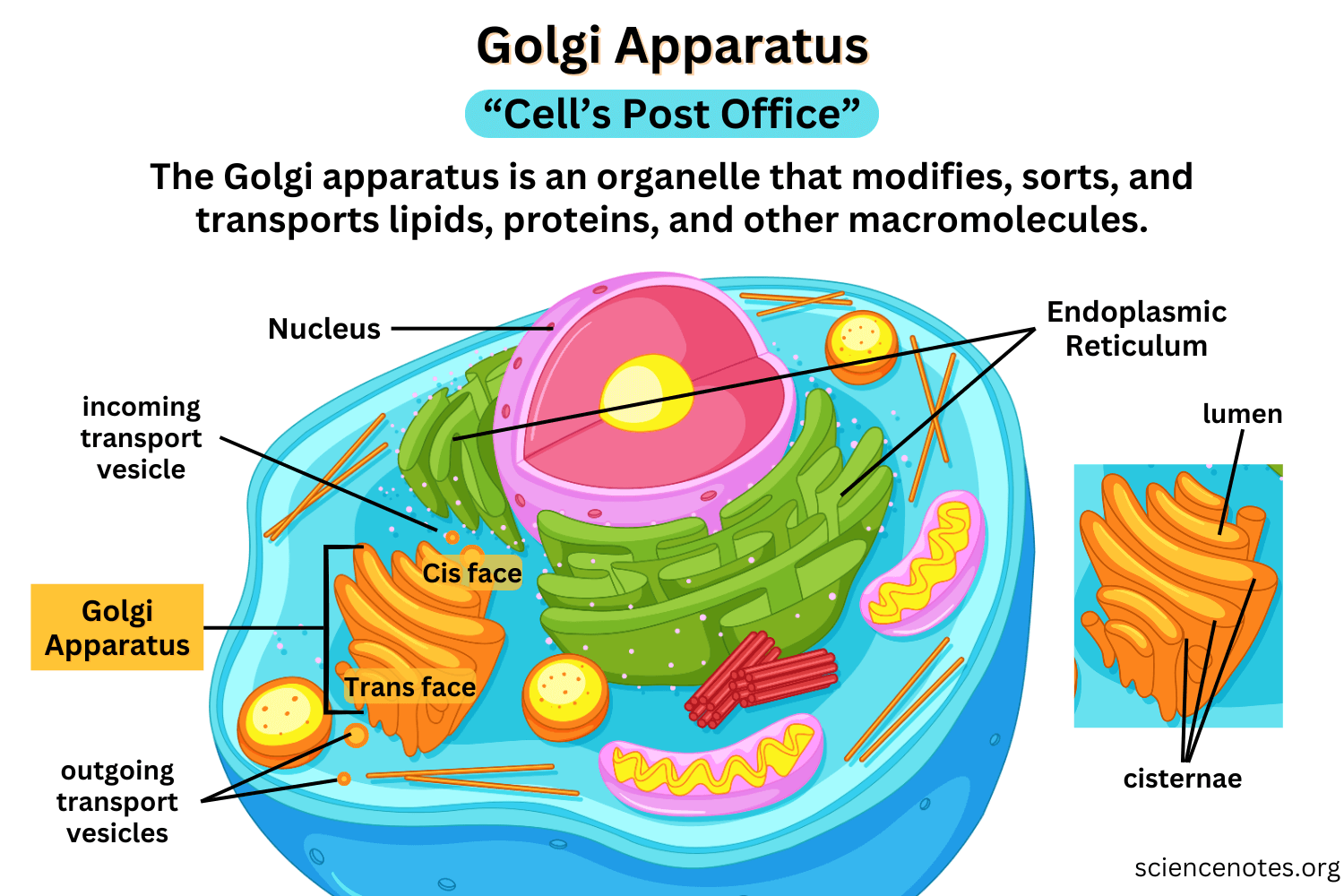
The golgi
a central organelle that processes, modifies, and sorts proteins and lipids received from the ER for delivery to their final destinations.
🔹 Key Functions
Sorting & Dispatching
Directs ER-exported cargo to the plasma membrane, lysosomes, or secretory vesicles
Uses signal sequences and membrane domains for targeting
Carbohydrate Synthesis
Synthesizes complex polysaccharides, including those for the cell wall (in plants) and extracellular matrix (in animals)
Oligosaccharide Attachment
Adds and remodels glycan chains on proteins and lipids
Includes N-linked trimming and O-linked glycosylation
Extracellular Matrix & Cell Wall Components
Packages and modifies structural molecules for secretion and tissue architecture
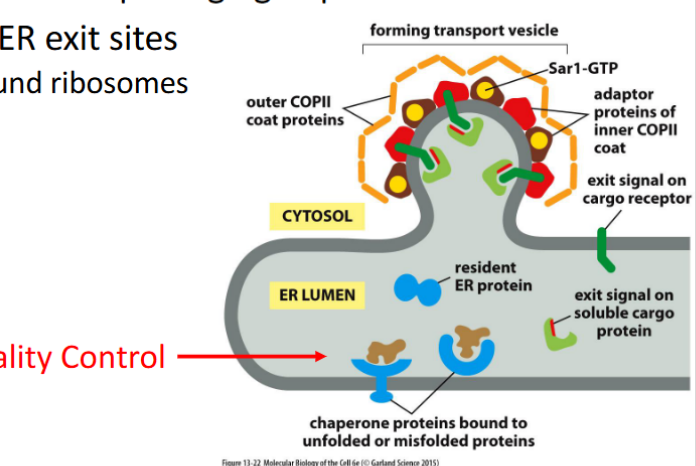
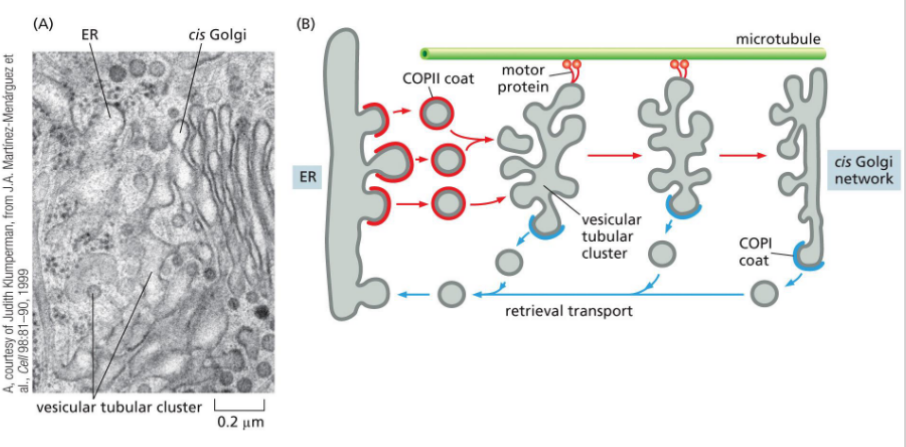
Leaving the ER
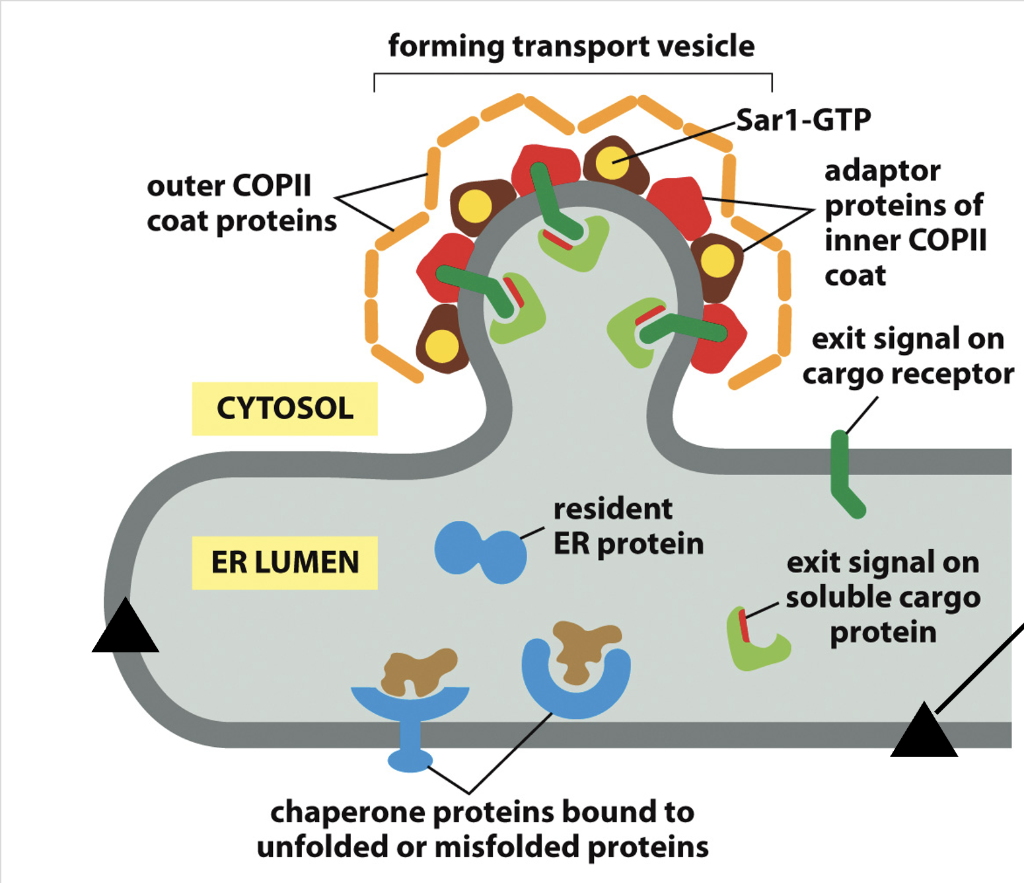
Proteins exit the ER in COPII-coated vesicles, which selectively package cargo and bud from specialized regions of the ER membrane.
🔹 Key Features
COPII-Coated Vesicles
Formed by the action of Sar1 GTPase and inner/outer coat proteins (e.g., Sec23/24, Sec13/31)
Drive anterograde transport from ER to Golgi
Selective Packaging
Proteins with ER exit signals (e.g., di-acidic or di-hydrophobic motifs) are actively selected for export
Recognized by cargo receptors and adaptor proteins
Circumstantial Packaging
Some proteins lack explicit exit signals but are passively included if they associate with selected cargo
ER Exit Sites (ERES)
Vesicles bud from ribosome-free zones of the ER membrane
These are specialized domains enriched in COPII machinery
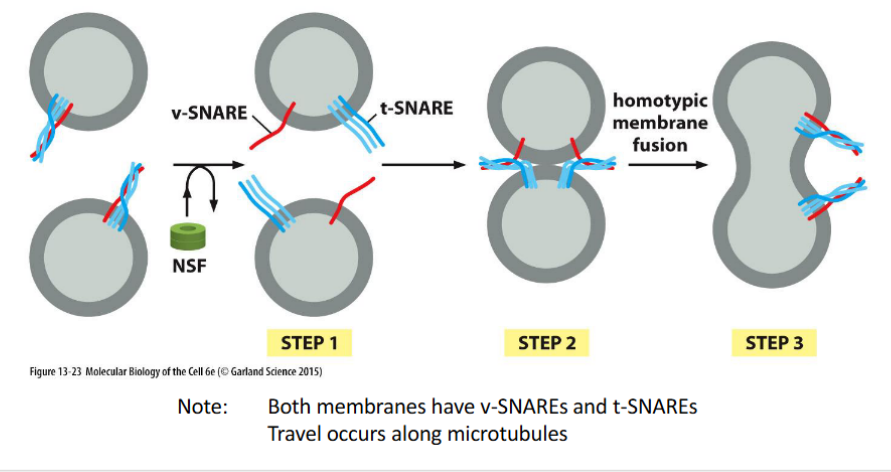
Homotypic fusion of vesicles
the merging of vesicles with identical membrane origin, forming a vesicular tubular cluster known as the ER-Golgi Intermediate Compartment (ERGIC).
🔹 Key Features
ERGIC Formation
Vesicles budding from the ER fuse with each other to form a transient compartment between the ER and Golgi
This cluster acts as a sorting and staging area for cargo en route to the Golgi
SNARE Logic
Both vesicle membranes contain v-SNAREs and t-SNAREs, enabling mutual recognition and fusion
SNARE pairing drives membrane merging even between vesicles of the same origin
Microtubule-Based Transport
ERGIC clusters travel along microtubules toward the cis-Golgi
Movement is powered by motor proteins like dynein or kinesin
COPI-coated vesicles
Mediate retrograde transport (retrieval pathway) from Golgi to ER by recognizing ER retention signals; coat switching mechanism is unclear.
ER protein retention
Definition: Proteins resident in the ER are retained or retrieved using C-terminal signals:
Soluble proteins carry a KDEL sequence, which binds to the KDEL receptor in the Golgi and triggers retrograde transport via COPI-coated vesicles.
Membrane proteins use cytosolic signals that directly interact with the COPI coat.
Binding is pH-dependent — acidic Golgi pH promotes receptor binding; neutral ER pH triggers release.
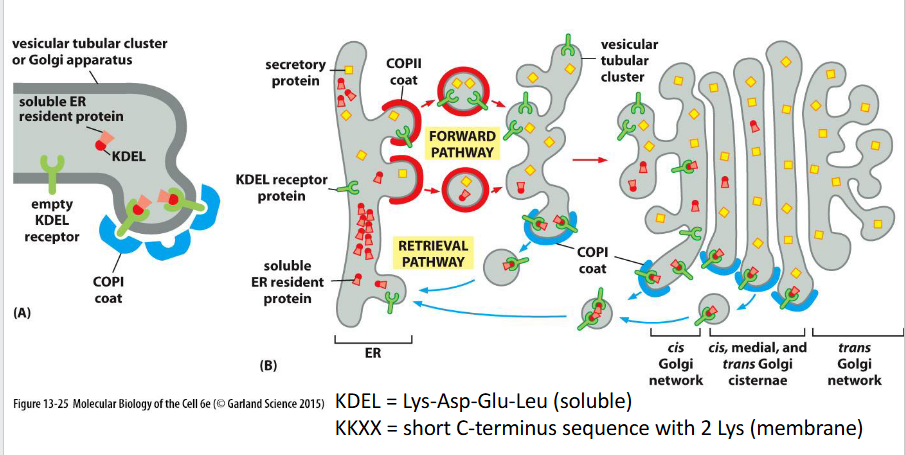
Resident proteins
are maintained in their target organelle by two mechanisms:
Retention — proteins remain due to physical or biochemical properties (e.g., membrane affinity or complex formation).
Retrieval — proteins that escape are recognized and returned via retrograde transport. → Some resident complexes are too large to be packaged into transport vesicles, reinforcing retention.
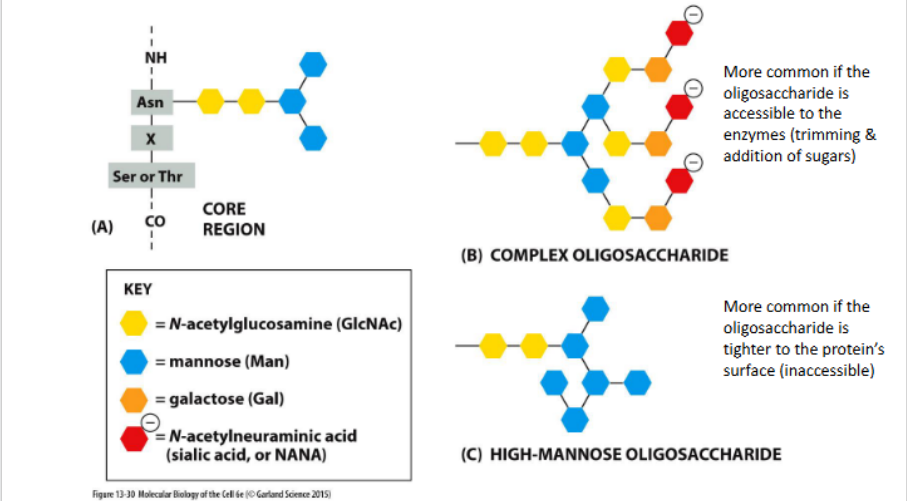
Modifications to N-linked glycosylations
Processing depends on the position of oligosaccharide in the protein (folded shape of the protein)
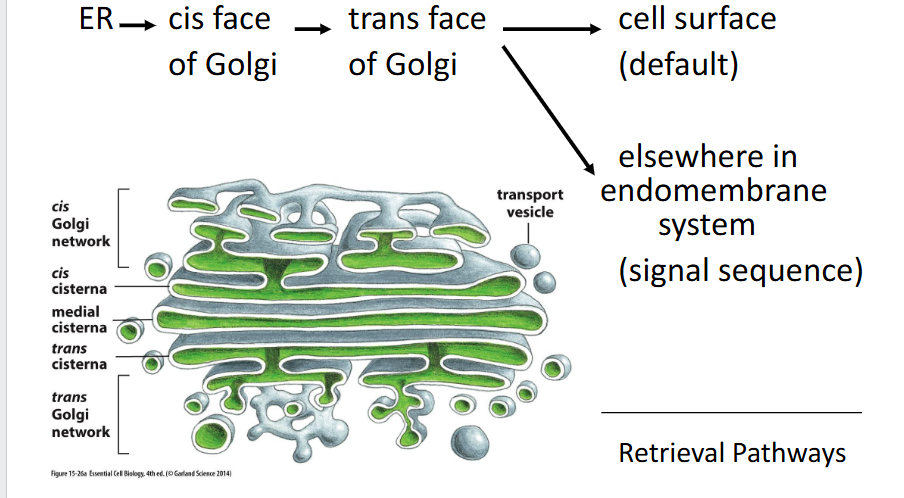
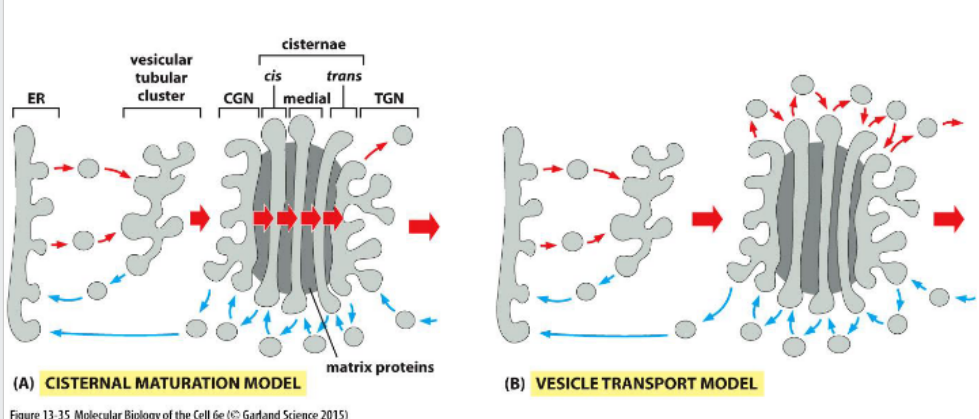
Movement through the Golgi
Protein movement through the Golgi involves cisternal maturation, where compartments progress from cis to trans. Golgi-resident enzymes are recycled backward via vesicles to maintain compartment identity.
Default pathway
travel to the cell surface via
constitutive secretory pathway
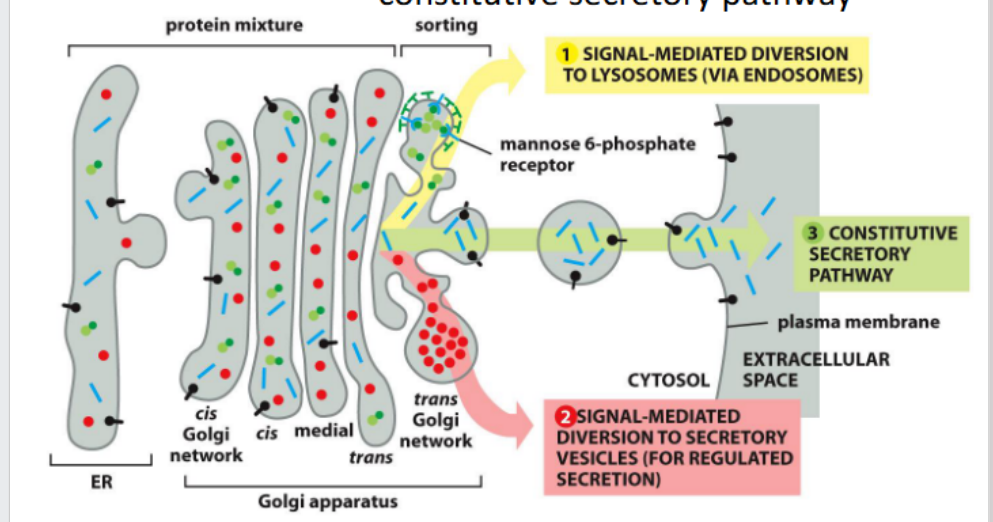
Pathways to the lysosome
From Golgi → Lysosome
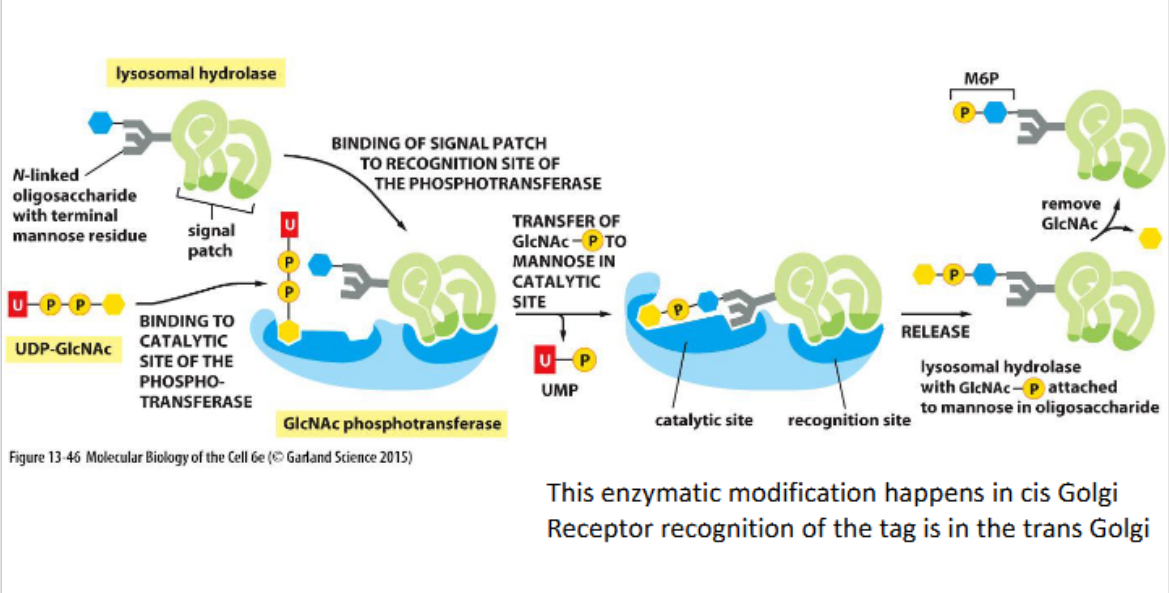
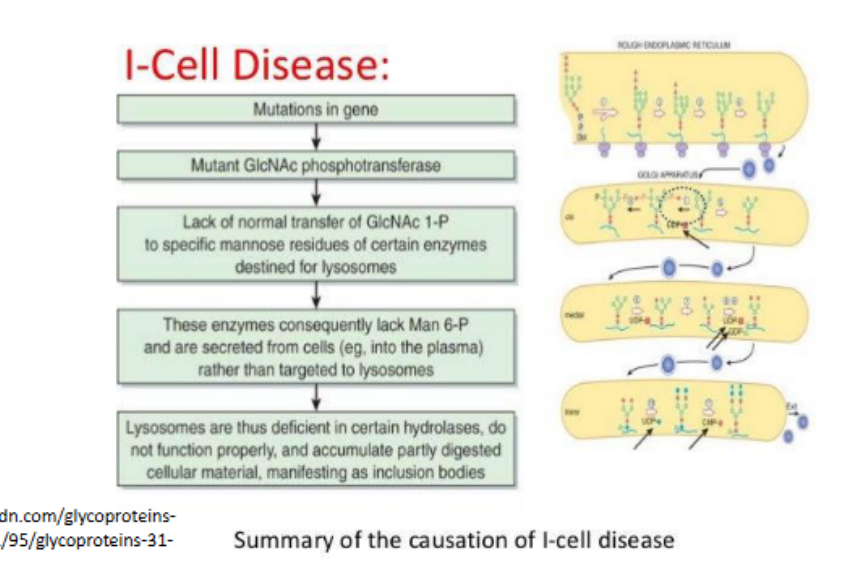
In animal cells, lysosomal hydrolases are tagged with mannose-6-phosphate (M6P) in the Golgi. M6P receptors recognize these tags and direct the proteins into clathrin-coated vesicles for delivery to endosomes en route to lysosomes.
A hydrolase is an enzyme that catalyzes the breakdown of molecules using water, typically splitting large molecules into smaller ones.
Lysosomal storage diseases
Occur when there is a problem with a lysosomal hydrolase (an enzyme that catalyzes the hydrolysis of chemical bonds using water, breaking down complex molecules into simpler ones.)
• Most severe = I cell disease
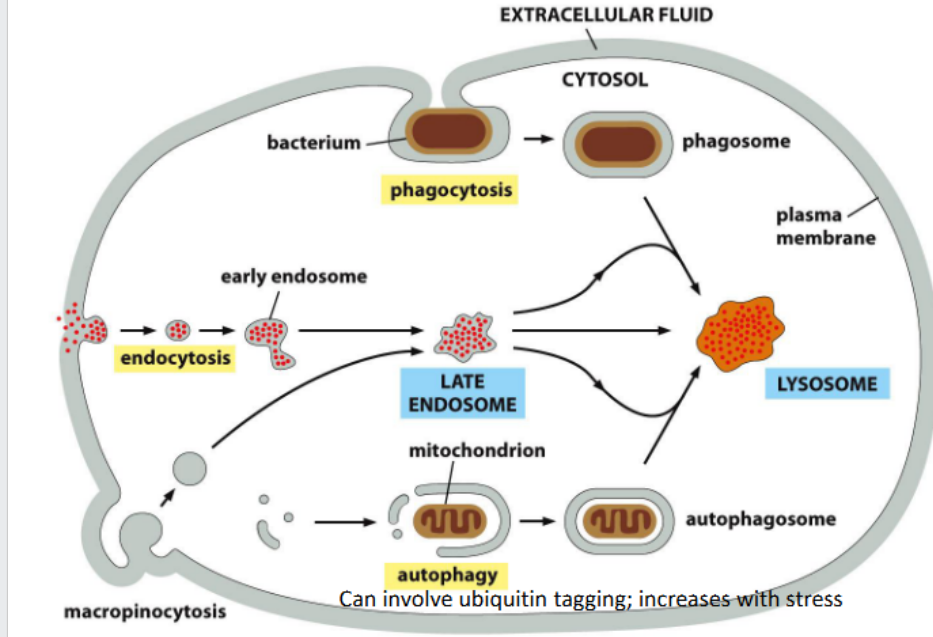
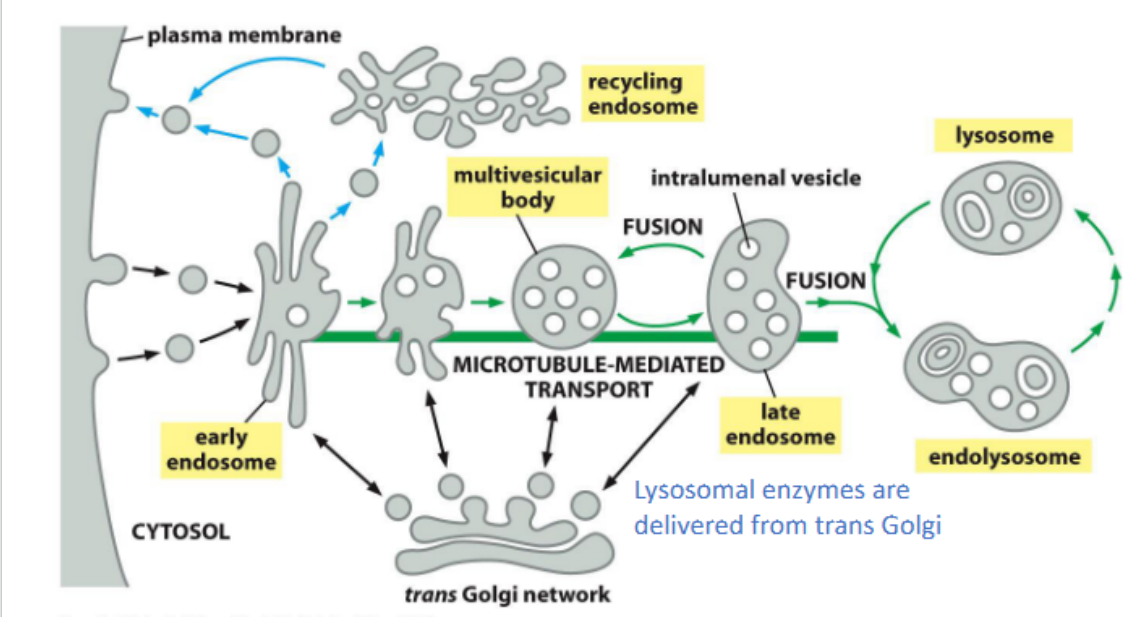
Early endosomes
act as sorting hubs in the endocytic pathway, directing internalized cargo either back to the plasma membrane for recycling or forward to late endosomes and lysosomes for degradation.
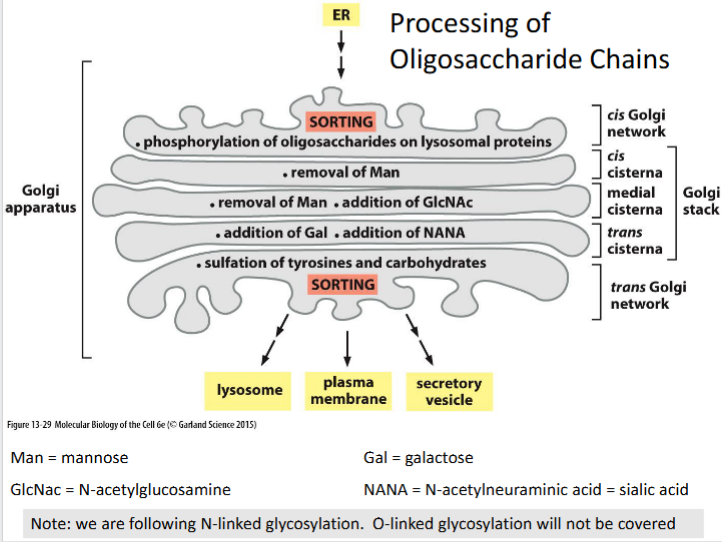
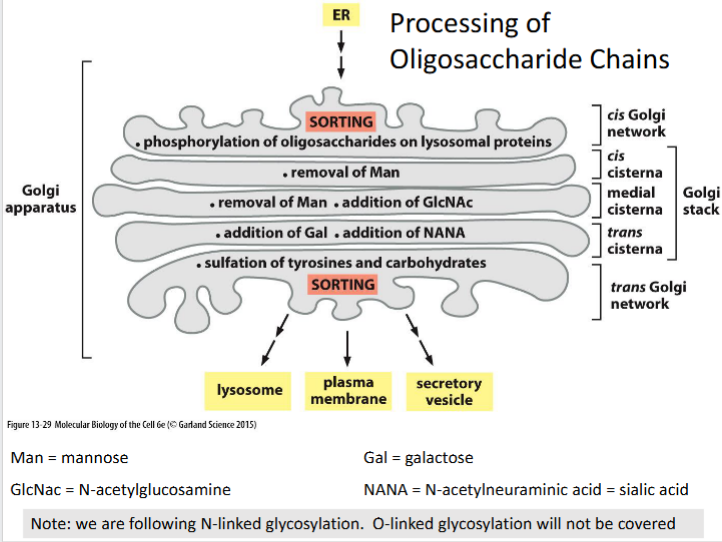
Cis Golgi network
Entry compartment of the Golgi where lysosomal proteins are phosphorylated on their oligosaccharide chains to initiate targeting to lysosomes.
Trans golgi
Final processing compartment where galactose (Gal) and N-acetylneuraminic acid (NANA or sialic acid) are added to the glycan structure.
Trans golgi network (TGN)
Sorting hub at the exit of the Golgi where processed glycoproteins are directed to lysosomes, the plasma membrane, or secretory vesicles.
N-linked glycosylation
A post-translational modification where oligosaccharide chains are attached to asparagine residues and sequentially processed through the Golgi compartments.
Lysosomal targeting sequence
Phosphorylated oligosaccharides on lysosomal proteins serve as recognition signals for sorting to lysosomes via mannose-6-phosphate receptors.
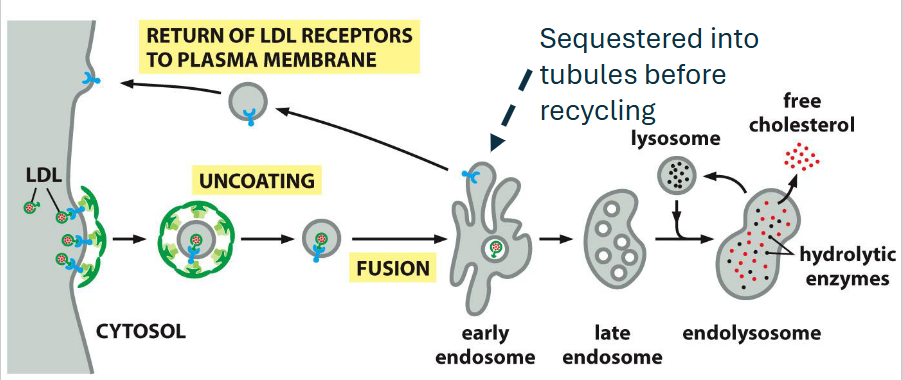
Receptor-mediated endocytosis (recycled receptor)
Example: LDL receptor mediates cholesterol uptake via LDL particles
Adaptor protein: Uses AP2, activated by PI(4,5)P₂ at the plasma membrane
Mechanism: Internalized into clathrin-coated vesicles → delivered to early endosomes
Fate: LDL is released; receptor is recycled back to the surface
Timing: Receptor completes a full cycle approximately every 10 minutes
If not recycled, ubiquitin binding proteins guide
receptors into coated pits → early endosome
Not shown: Rab proteins, phosphoinositide lipids, SNAREs,
tethers, microtubule motor proteins, addition of V-type pumps
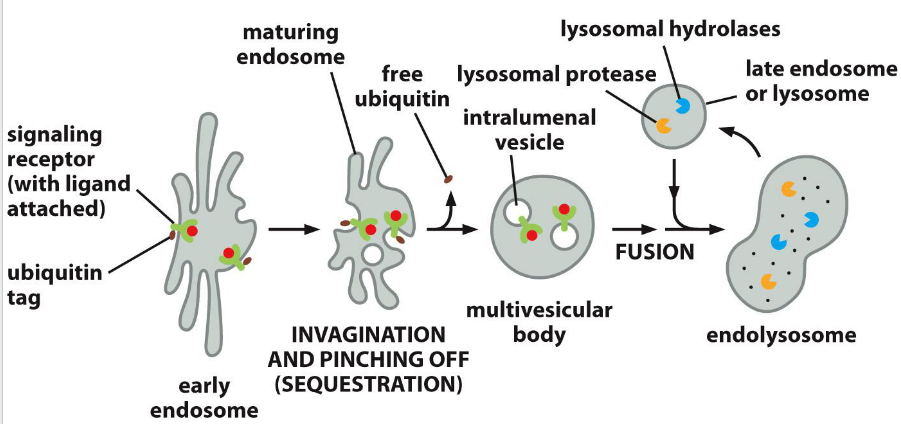
Receptor Down-regulation (to the lysosome)
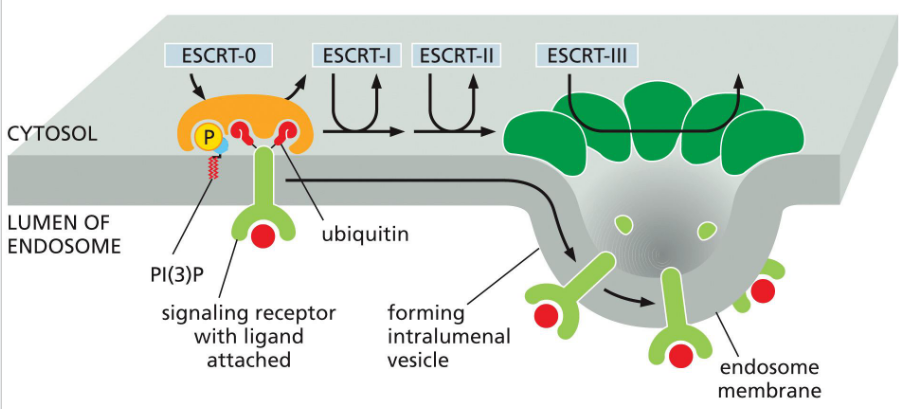
Monoubiquitination or multiubiquitination of lysine residues on the cytosolic tail of membrane receptors marks them for endocytosis and sorting into intraluminal vesicles via the ESCRT pathway. This leads to receptor degradation in the lysosome and termination of signaling.
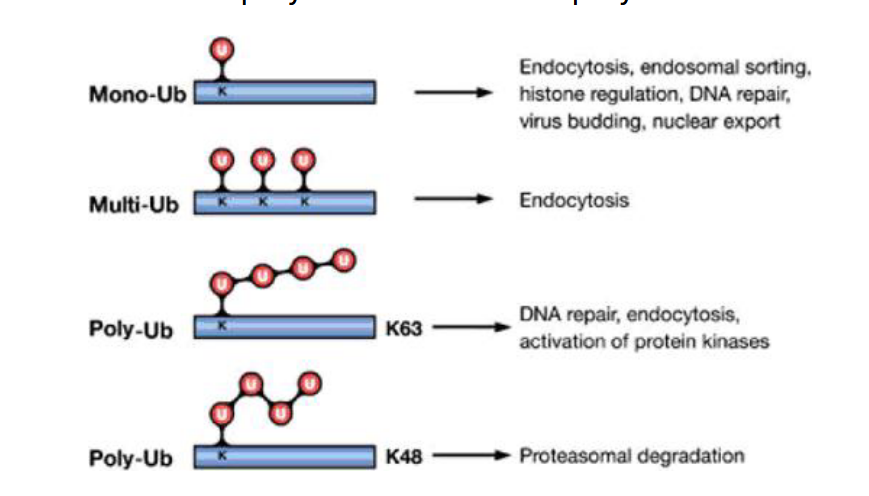
ESCRT (Endosomal Sorting Complex Required for Transport)
complexes mediate membrane remodeling for:
Cargo sorting into multivesicular bodies (MVBs) for lysosomal degradation
Plasma and organelle membrane repair
Nuclear envelope sealing after mitosis
cells that have defective function:
Impaired endosomal trafficking, leading to protein accumulation
Membrane rupture or poor repair, triggering inflammation or cell death
Associated with neurodegeneration, cancer, and viral susceptibility
Early ESCRT proteins (like ESCRT-0 and ESCRT-I) act before Rab5-to-Rab7 conversion, helping sort ubiquitinated cargo into MVBs. This positions ESCRT upstream in the endosomal maturation timeline.
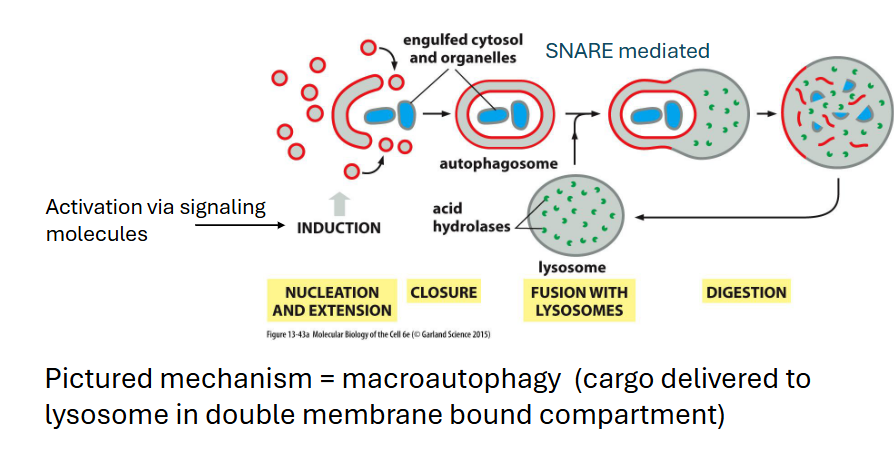
Autophagy
Removal of large objects (macromolecules, large protein aggregates, whole organelles)
maintains cellular homeostasis
• Removes substances that could disrupt cell
function or viability (cargo is selected)
• Mediates turnover of organelles
• Help mediate impacts of short-term ATP or
nutrient depletion (not a long-term solution)
• Plays an important role in development
• Often has roles in diseases like cancer and
neurodegenerative conditions (role is context
dependent)