Chemistry Chapter 1.2: How are gases collected? (Experimental Chemistry)
1/9
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
When is gas syringe used to collect gases?
Any gas
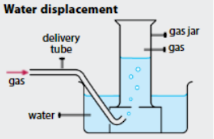
When is Water displacement used to collect gases?
When the gas is INSOLUBLE (CH₄/Methane) or SLIGHTLY INSOLUBLE (CO₂/Carbon Dioxide) in water
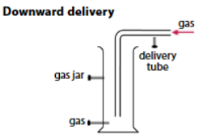
When is downward delivery used to collect gases?
When the gas is more dense than air and soluble in water.
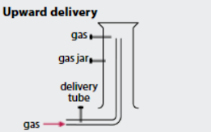
When is upward delivery used to collect gases?
When the gas is less dense than air and soluble in water.
What gases are soluble in water?
Ammonia (NH3), Hydrogen Chloride (HCl), Chlorine (Cl2), Sulfur Dioxide.
What gases are insoluble in water? (Water displacement is used to collect)
Methane (CH₄), Hydrogen.
What’s the Relative Molecular Mass of Air?
29
What type of drying agent can be used for gases which are neutral in nature? What are some neutral gases?
Both acidic and basic drying agents can be used. Some neutral gases are: Carbon Monoxide (CO), Dihydrogen (H2).
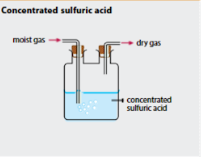
What type of drying agent can be used for gases which are acidic in nature? What are some acidic gases? What’s the most common acidic drying agent?
Acidic drying agent. Hydrogen Chloride (HCl), Sulfur Dioxide (SO2). Concentrated Sulfuric Acid.
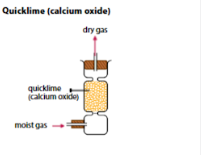
What type of drying agent can be used for gases which are basic in nature? What are some basic gases? What’s the most common basic drying agent?
Basic drying agent. Ammonia (NH3). Powdered Anhydrous Calcium Oxide.