5. TESTS TO IDENTIFY METAL IONS
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
test tube reactions
a qualitative method of working out the identity of unknown metal ions in solution
adding different reagents like sodium hydroxide, ammonia and sodium carbonate
and recording observations help you identify what metal ion is present
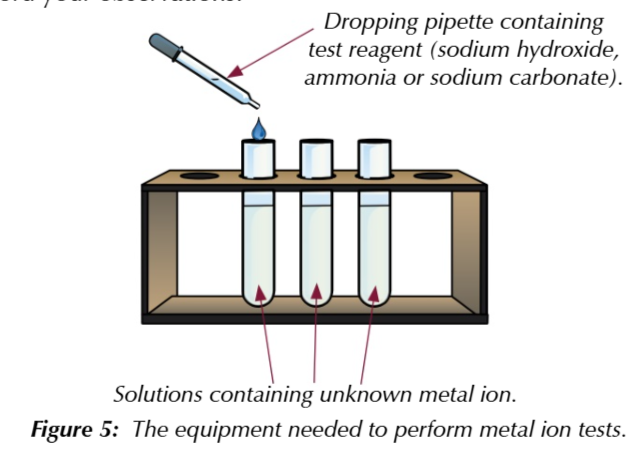
what to do:
measure out samples of unknown metal solutions into 3 seperate test tube
for tt1 add sodium hydroxide dropwise using pipette and record changes, then add more in excess n record changes
to tt2 add ammonia solution dropwise using pipette and record changes then add in excess n record changes
for tt3 add sodium carbonate solution dropwise n record observations
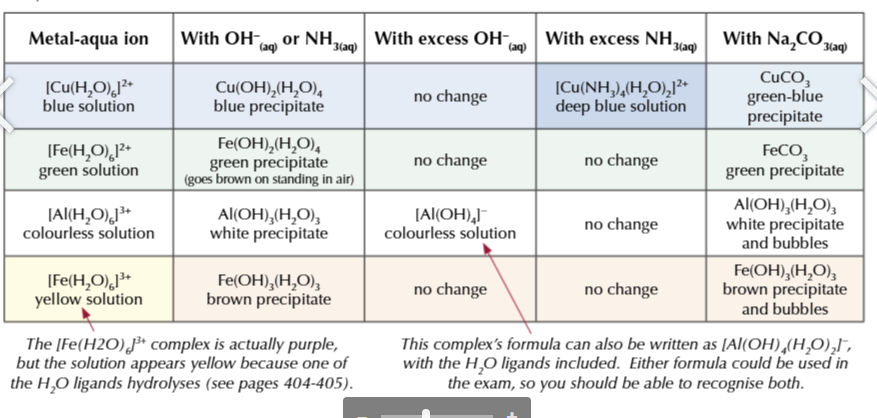
you need to know the reactions for
Cu2+
Fe2+
Fe3+
Al3+
aqua ions

AQUA IONS + NaOH
all form precipitates
only aluminium hydroxide WHITE precipitate will dissolve in excess NaOH
because it is amphoteric
AQUA IONS + AMMONIA
all form precipitates
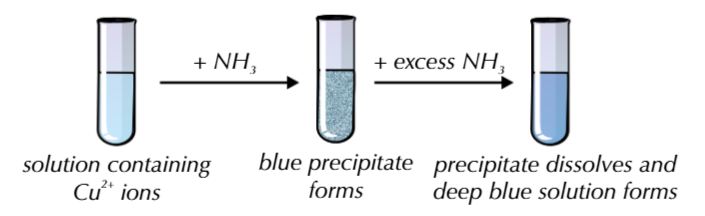
only copper hydroxide precipitate will dissolve in excess ammonia leaving a deep blue solution
because it undergoes ligand substitution
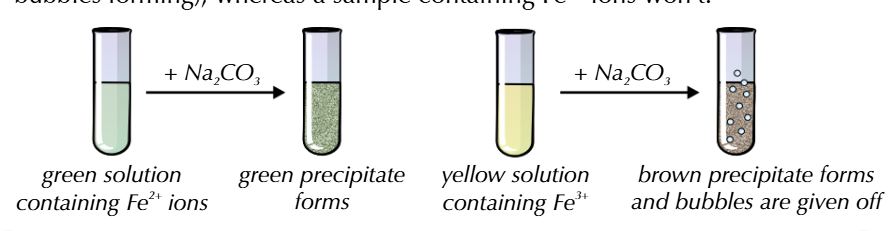
AQUA IONS + Na2CO3
all form precipitates
Al3+ and Fe3+ form CO2 gas, so you will observe bubbling
this test differentiates between green Fe2+→ green ppt and yellow Fe3+→ brown ppt + bubbles
Cu2+ metal aqua ion solution
BLUE
Fe2+ metal aqua ion solution
GREEN
Al3+ metla aqua ion solution
COLOURLESS
Fe3+ metal aqua ion solution
YELLOW