Chemlab Practicals (Experiment 5-8) (FINALS) WITH PICTURES
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms

Schiff’s Test
HCHO
=POSITIVE
Schiff’s solution (fuchsin decolorized by H2SO4) + HCHO —→
=ROSEPINK SOLUTION

Schiff’s Test
C6H5CHO
=POSITIVE (slow reaction)
Schiff’s solution (fuchsin decolorized by H2SO4) + C6H5CHO —→
=ROSEPINK SOLUTION
ACETONE (schiff’s test)
Schiff’s Test
CH3COCH3
=NEGATIVE (does not contain an aldehyde group)
Schiff’s solution (fuchsin decolorized by H2SO4) + CH3COCH3 —→
=COLORLESS SOLUTION


Resorcinol Test
(HCHO only)
HCHO
=POSITIVE
HCHO + .5% Resorcinol + H2SO4 —→
=RED FLOCCULENT PRECIPITATE AT THE JUNCTION OF TWO LAYERS
(sulfuric acid at the bottom, formaldehyde at top)

Tollen’s Test
(Silver Mirror Test)
HCHO (Formalin with 37-40% formaldehyde)
=POSITIVE
HCHO + Ag(NH3)2 —→
=SILVER MIRROR SOLUTION AT THE WALLS OF THE TEST TUBE
BENZALDEHYDE (tollen’s test)
Tollen’s Test
(Silver Mirror Test)
C6H5CHO
=POSITIVE
C6H5CHO + Ag(NH3)2 —→
=SILVER MIRROR SOLUTION AT THE WALLS OF THE TEST TUBE
ACETONE (tollen’s test)
Tollen’s Test
(Silver Mirror Test)
CH3CO3CHO
=NEGATIVE (does not contain an aldehyde group)
CH3CO3CHO + Ag(NH3)2 —→
=COLORLESS SOLUTION

Fehling’s Test
HCHO (Formalin with 37-40% formaldehyde)
=POSITIVE
HCHO + Cu(OH)2 —→
=Cu2O (CUPROUS (I) OXIDE) BRICK RED PRECIPITATE

Fehling’s Test
C6H5CHO
=NEGATIVE
C6H5CHO + Cu(OH)2 —→
=REMAINED BLUE

Fehling’s Test
CH3COCH3
=NEGATIVE
CH3COCH3 + Cu(OH)2 —→
=REMAINED BLUE
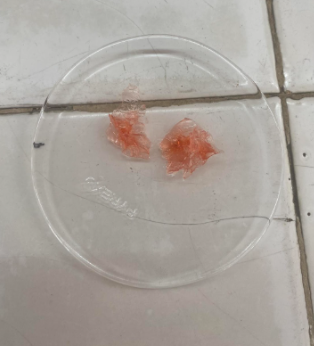
Formaldehyde on Protein Substances
1 ml FORMALIN + 1 ml H2O + GELATIN SHEET
=GELATIN SHEET HARDENED
=insoluble in solution with formalin
=due to crosslinking between molecules
2 ml H2O
=GELATIN SHEET SOFTENED
=soluble in water
C6H5CHO in watch glass
Auto-Oxidation of Benzaldehyde
=WHITE CRYSTALS (BENZOIC ACID)
=C6H5CHO turned to C6H5COOH due to oxidation from the exposure to the atmosphere
C6H5C=O —[O]—>C6H5C-O-OH —[O]—>
|| ||
O O
C6H5C-OH
|| (BENZOIC ACID)
O

Addition of Sodium Bisulfite
=WHITE PRECIPITATE (ACETONE SODIUM BISULFITE)
CH3COCH3 + NaHSO3 ——>
CH3COHCH3
|
SO3Na

Addition of Phenyl hydrazine
=YELLOW-ORANGE PRECIPITATE (ACETONE 2,4-DINITROPHENYLHYDRAZONE)
CH3COCH3 + C6H5NHNH2——>
CH3CCH3
||
NHNHC6H5

Iodoform Test
=Acetone + 10% NaOH + Iodine Solution
CH3COCH3 + 3I₂ + 4NaOH——>
CHI3 +CH3COONa + 3Nai + 3H₂O

Biuret Test
1% Egg Albumin Solution + CuSO4 + 10% NaOH —→
=ROSEPINK SOLUTION

Xanthoproteic Test
1% Egg Albumin Solution + Conc. HNO3 + Conc. NH4OH—→
=YELLOW-ORANGE PRECIPITATE
YELLOW with HNO3
ORANGE with NH4OH after being neutralized

Millon’s Test
-Millon’s solution: dissolving mercury in nitric acid, mercurous or mercuric nitrates
1% Egg Albumin Solution +Millon’s Reagent (HgNO3)
=FLESH TO RED PRECIPITATE
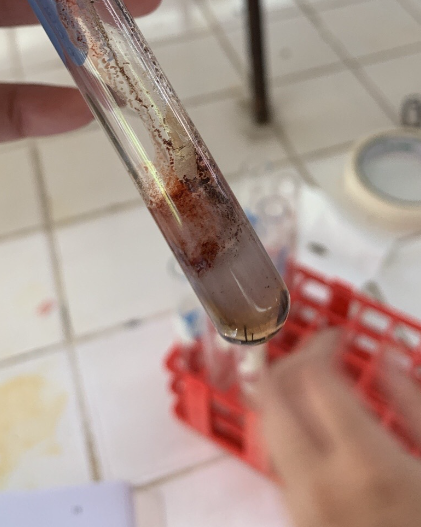
Molisch’s Test
-Molisch Reagent: Alpha naphthol in ethanol
1% Egg Albumin Solution + Molisch Reagent + H2SO4
=VIOLET RING AT THE JUNCTION OF TWO LAYERS

Sulfur Test
1% Egg Albumin Solution + NaOH + Ch3COOH
(Cover with filter paper dipped in Pb(Ch3COO)2——>
=BLACK PRECIPITATE (LEAD (II) SULFIDE PbS

Effect of Heat on Proteins
=PROTEIN COAGULATES
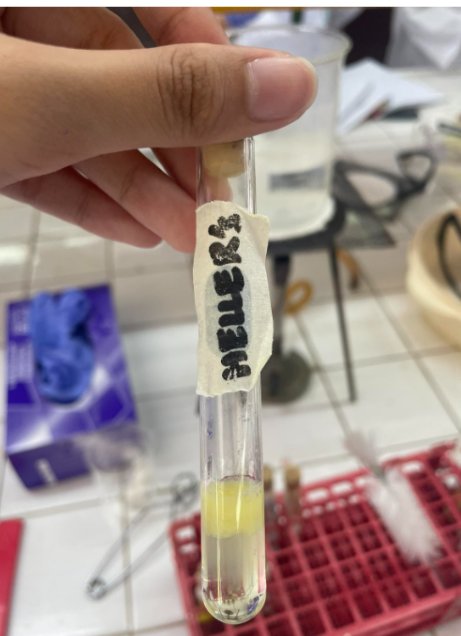
Heller’s Ring Test
1% Egg Albumin Solution + HNO3——→
=WHITE PRECIPITATE AT THE CENTER OF TWO LATERS.
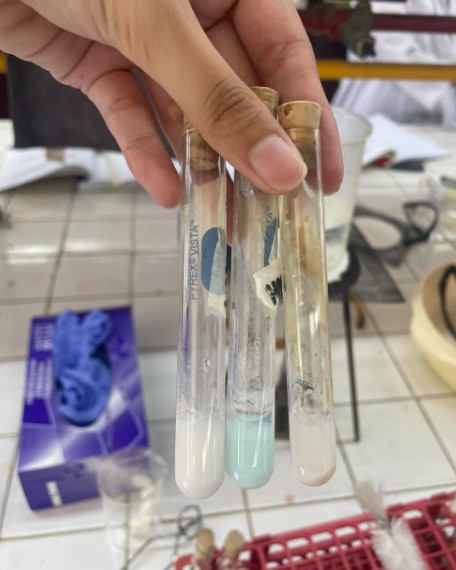
Heavy Metal Salts
Copper (I) Sulfate
= BLUE PRECIPITATE
Lead (II) Acetate
= WHITE RECIPITATE
Silver Nitrate
= WHITE PRECIPITATE
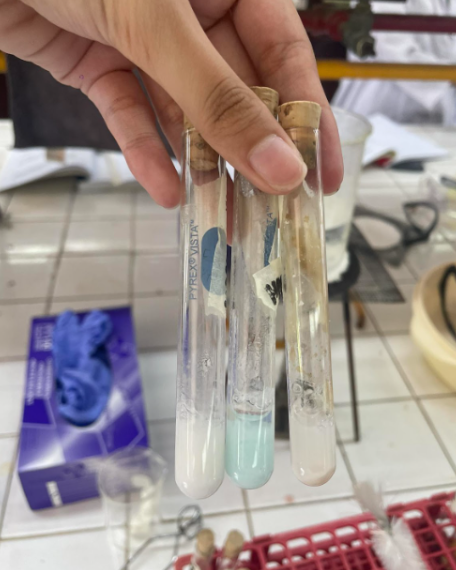
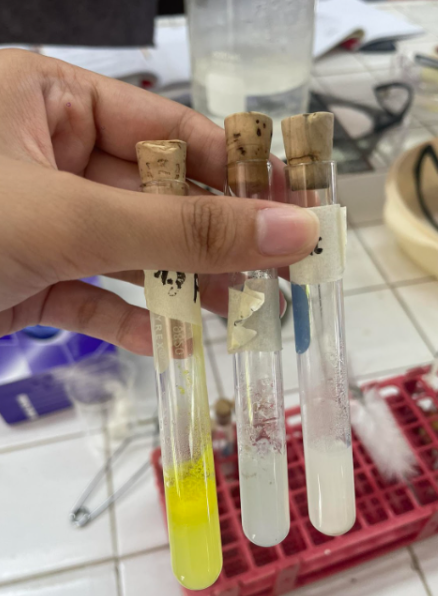
Alkaloidal Reagent
5% Tannic Acid
=BROWN PRECIPIATE
5% Potassium Ferrocyanide
= BLUE PRECIPIATE
conc. Picric Acid
=YELLOW PRECIPIATE
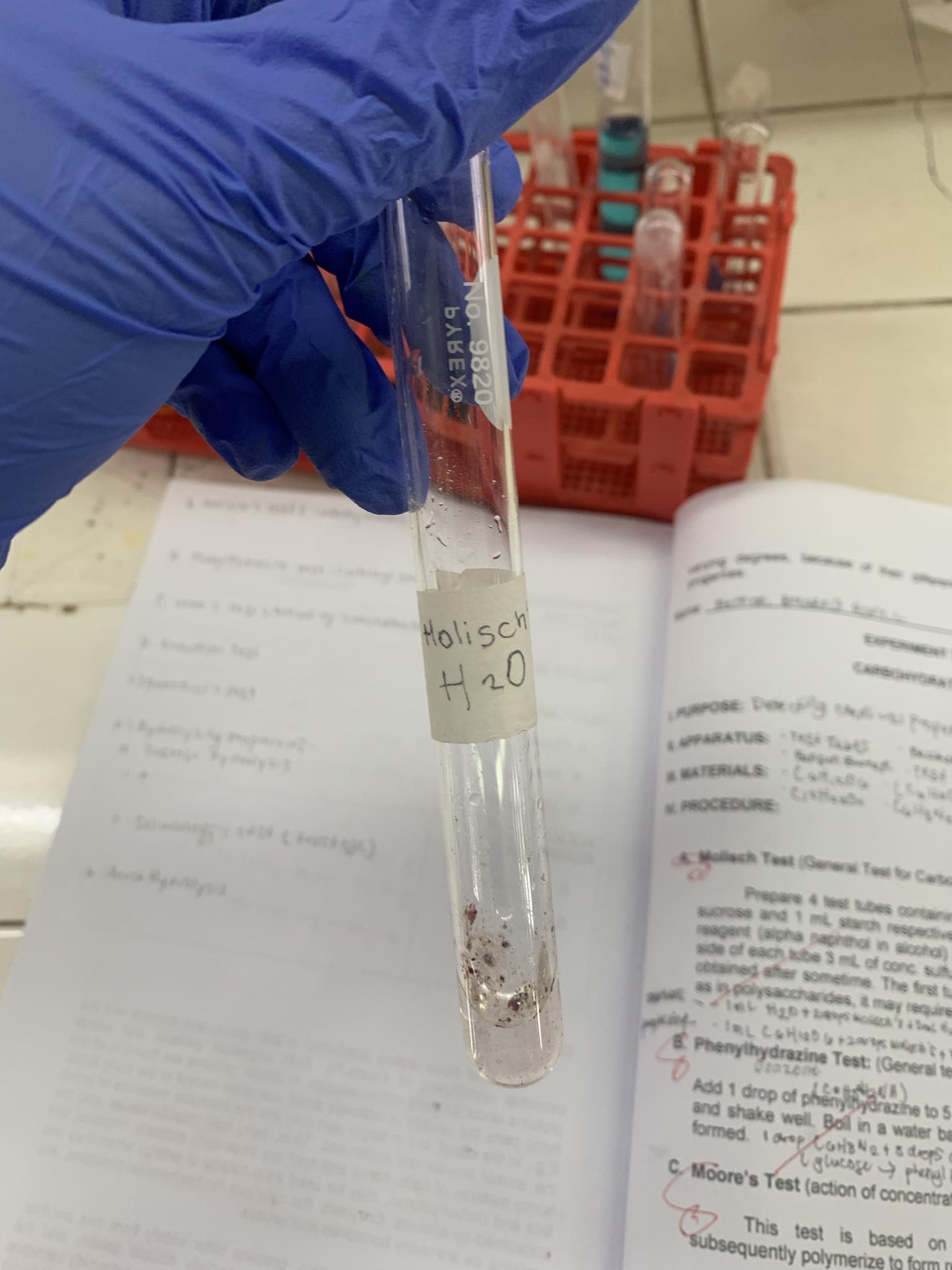
Molisch’s Test
-Molisch Reagent: Alpha naphthol in ethanol
H2O
=NEGATIVE
(water does not contain carbohydrates)
=UNMIXED SOLUTION
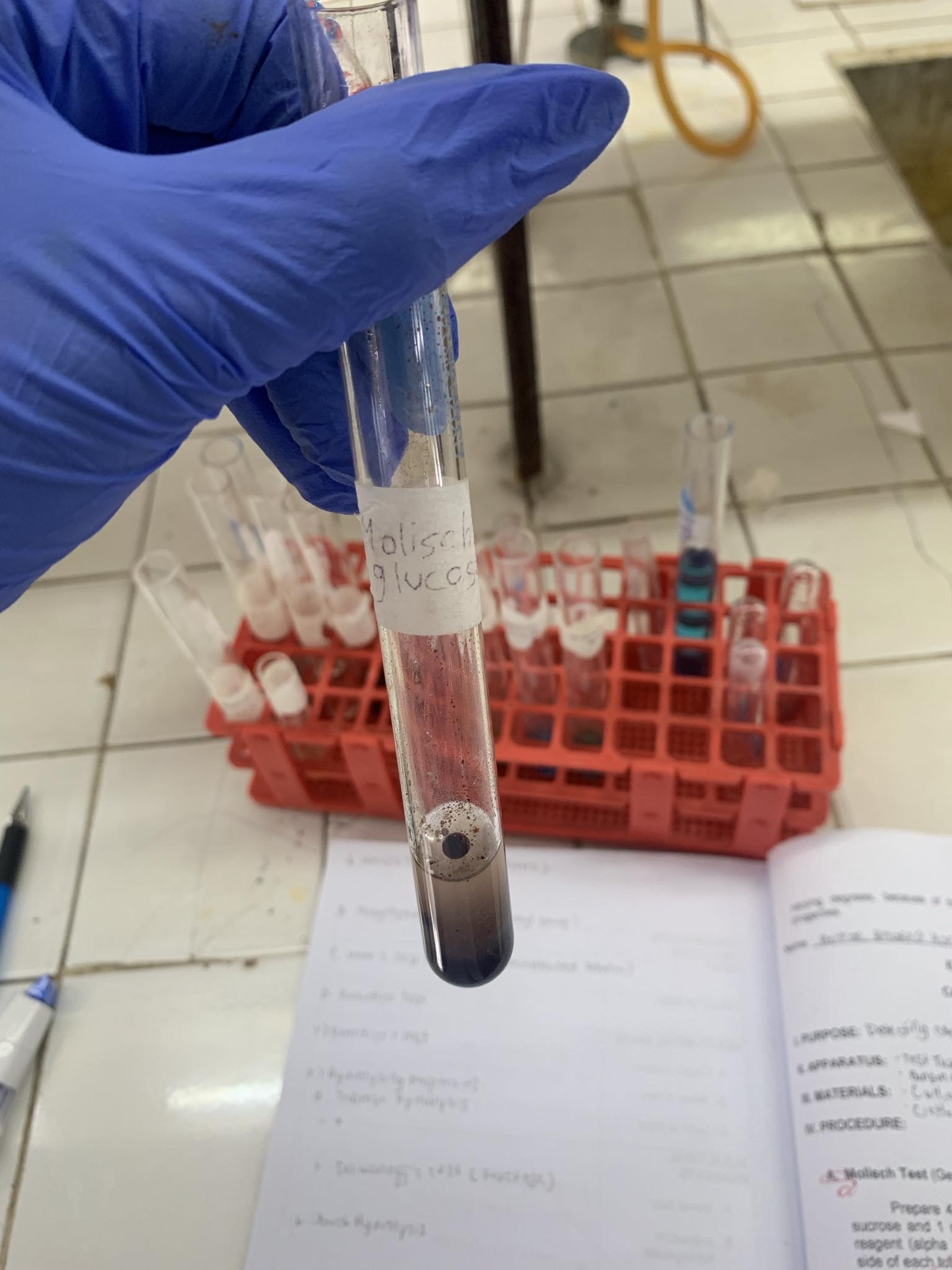
Molisch’s Test
-Molisch Reagent: Alpha naphthol in ethanol
C6H12O5 (glucose)
=POSITIVE
C6H12O5 + Molisch Reagent + H2SO4—→
=VIOLET RING AT THE JUNCTION OF TWO LAYERS
Molisch’s Test
-Molisch Reagent: Alpha naphthol in ethanol
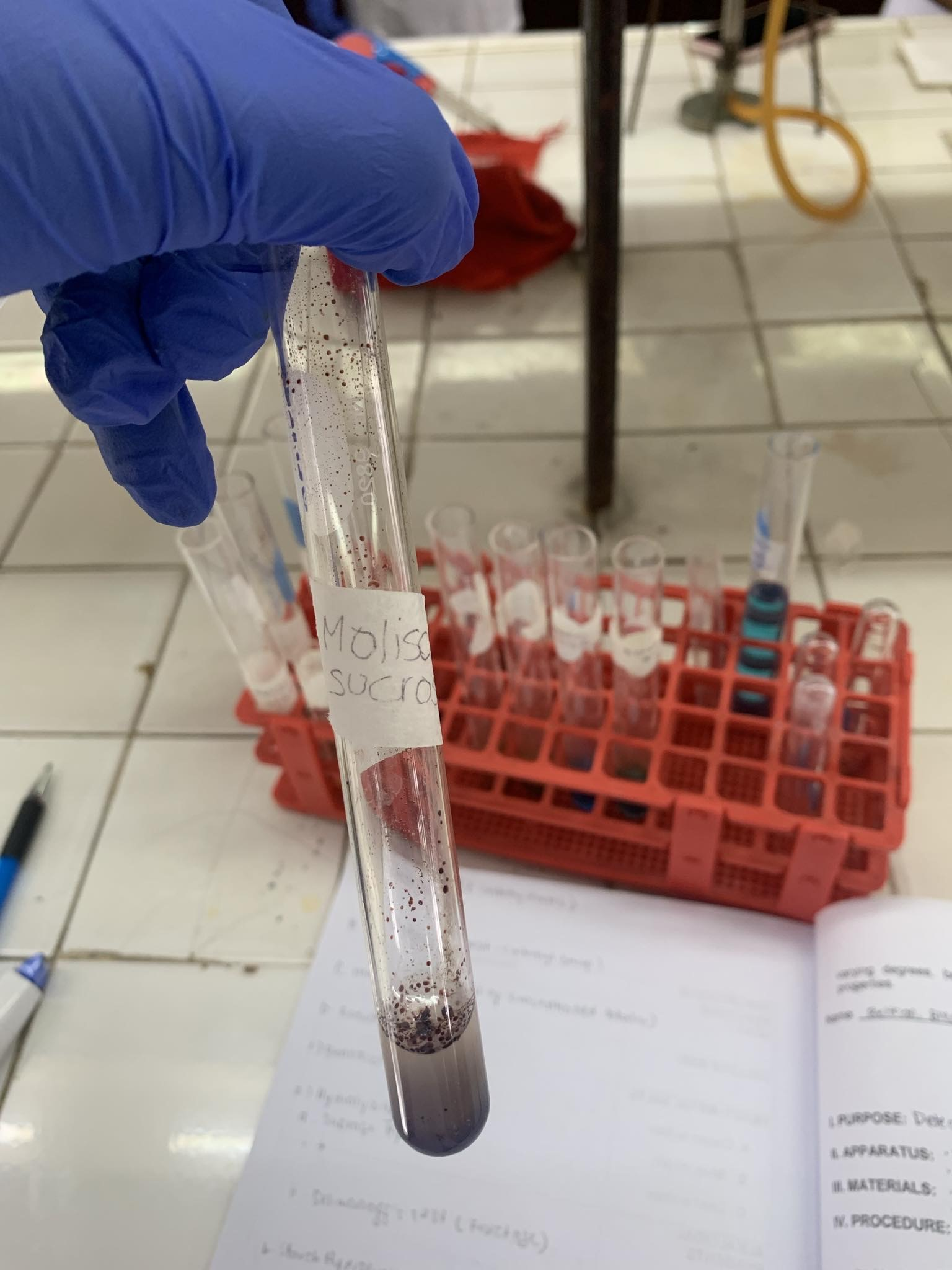
C12H22O11 (sucrose)
=POSITIVE
C12H22O11 + Molisch Reagent + H2SO4—→
=VIOLET RING AT THE JUNCTION OF TWO LAYERS
Molisch’s Test
-Molisch Reagent: Alpha naphthol in ethanol
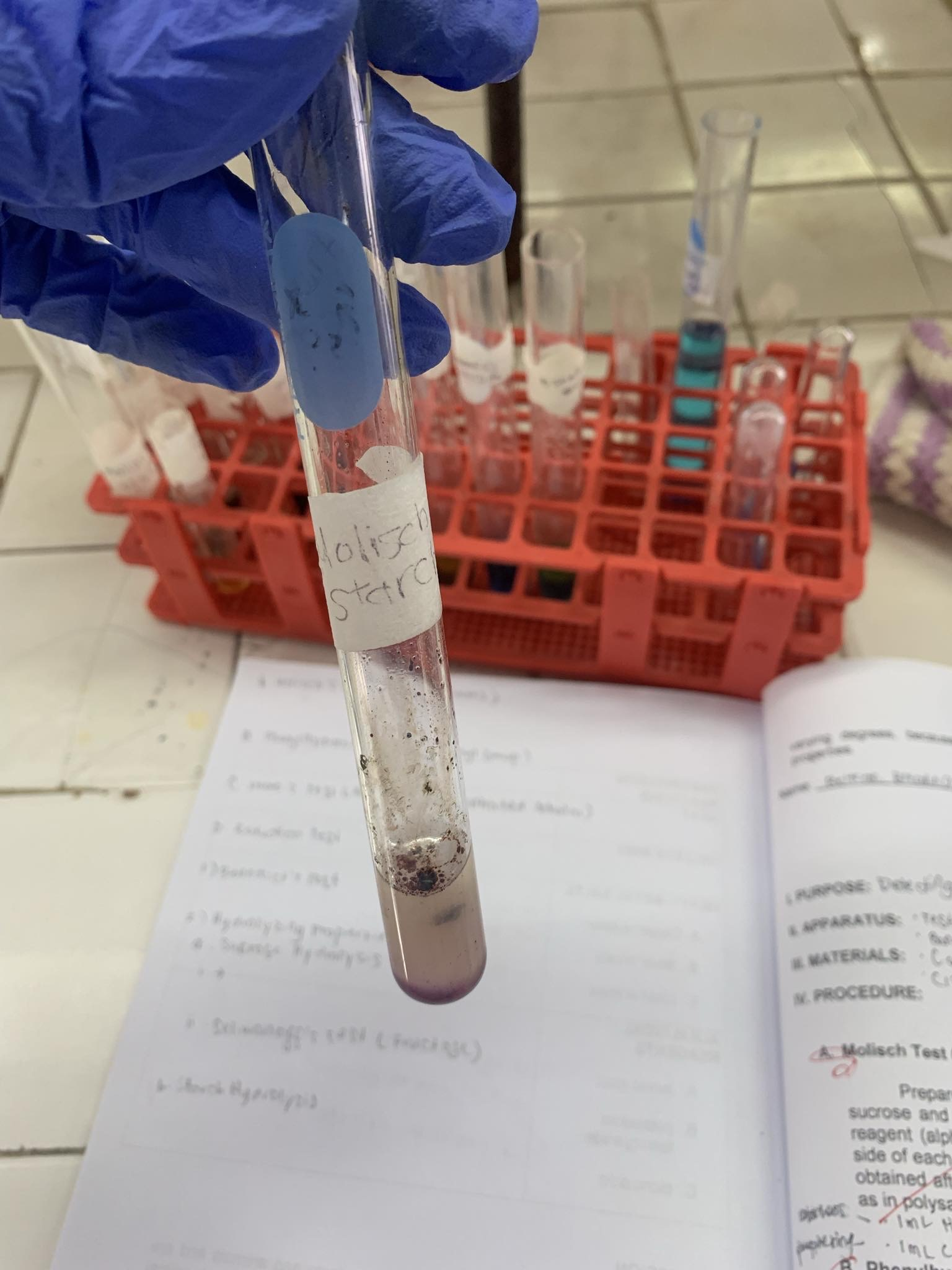
C6H10O5 (starch)
=POSITIVE
C6H10O5 + Molisch Reagent + H2SO4—→
=VIOLET RING AT THE JUNCTION OF TWO LAYERS
Moore’s Test
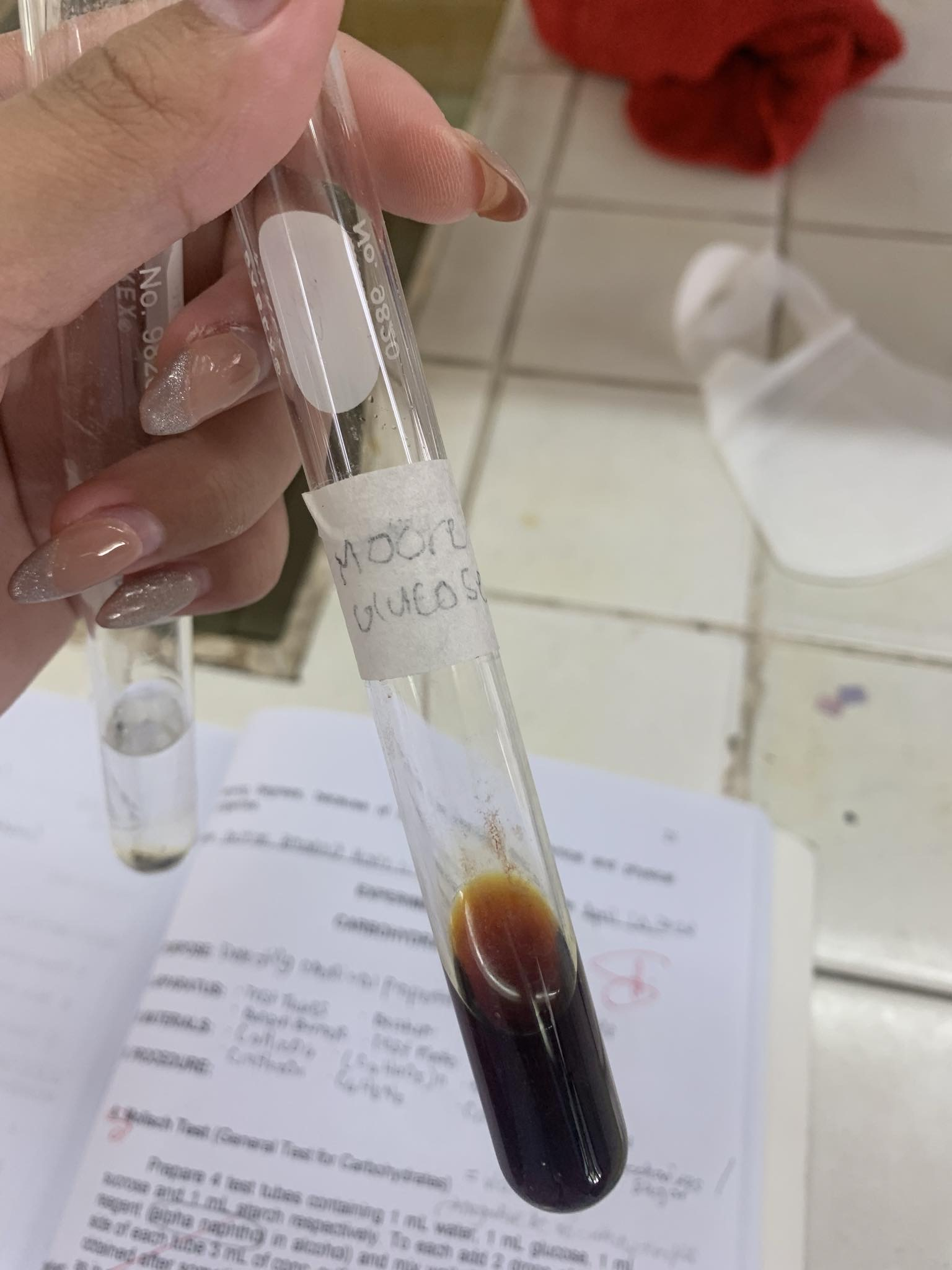
C6H12O5 (glucose)
=POSITIVE
C6H10O5 + 25% NaOH —→
=CARAMEL ODOR (DARK BROWN RESINOUS SUBSTANCE)
Moore’s Test
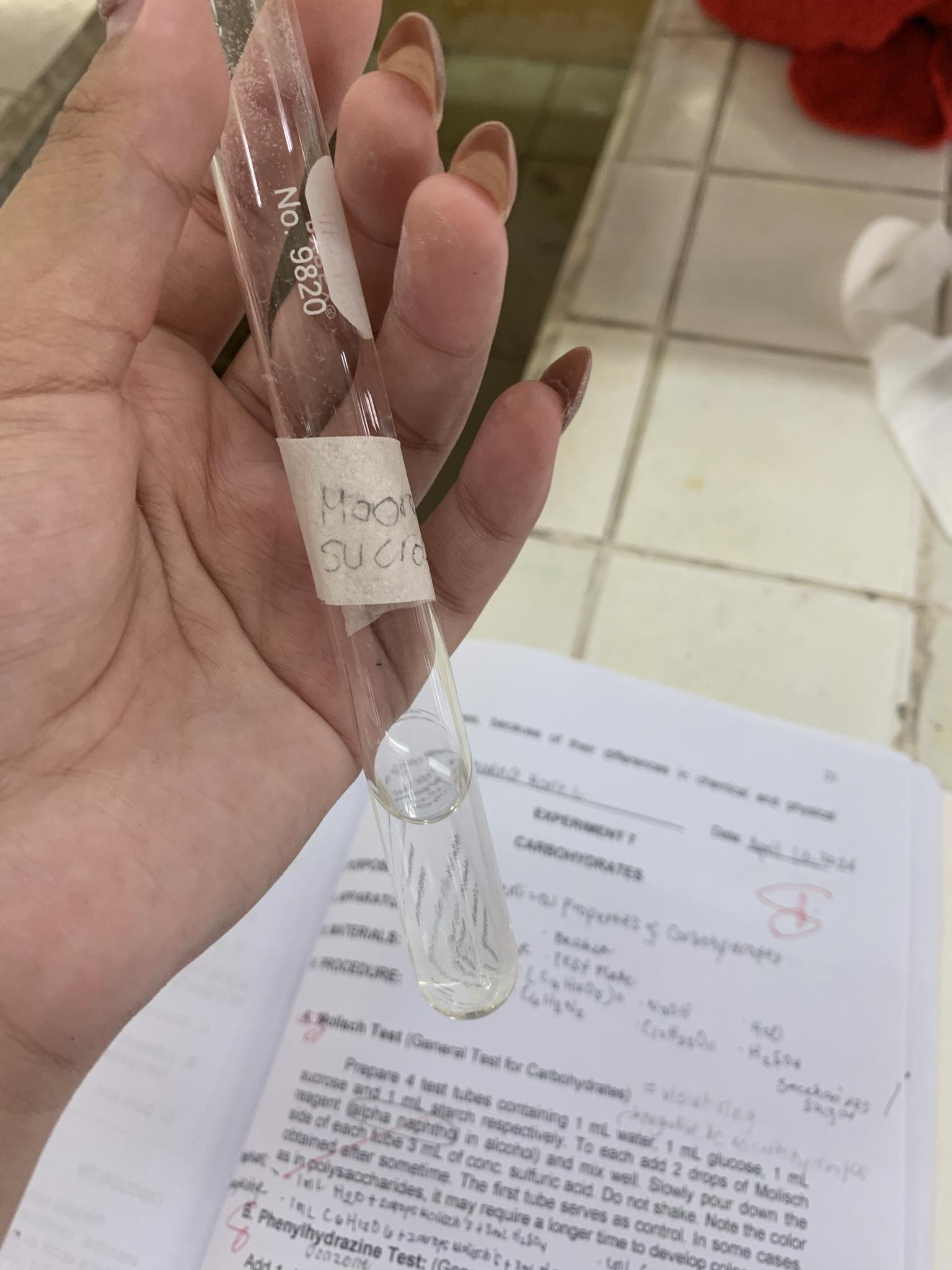
C12H22O11 (Sucrose)
=NEGATIVE
- sucrose is a non-reducing sugar
C6H10O5 + 25% NaOH —→
= COLORLESS SOLUTION
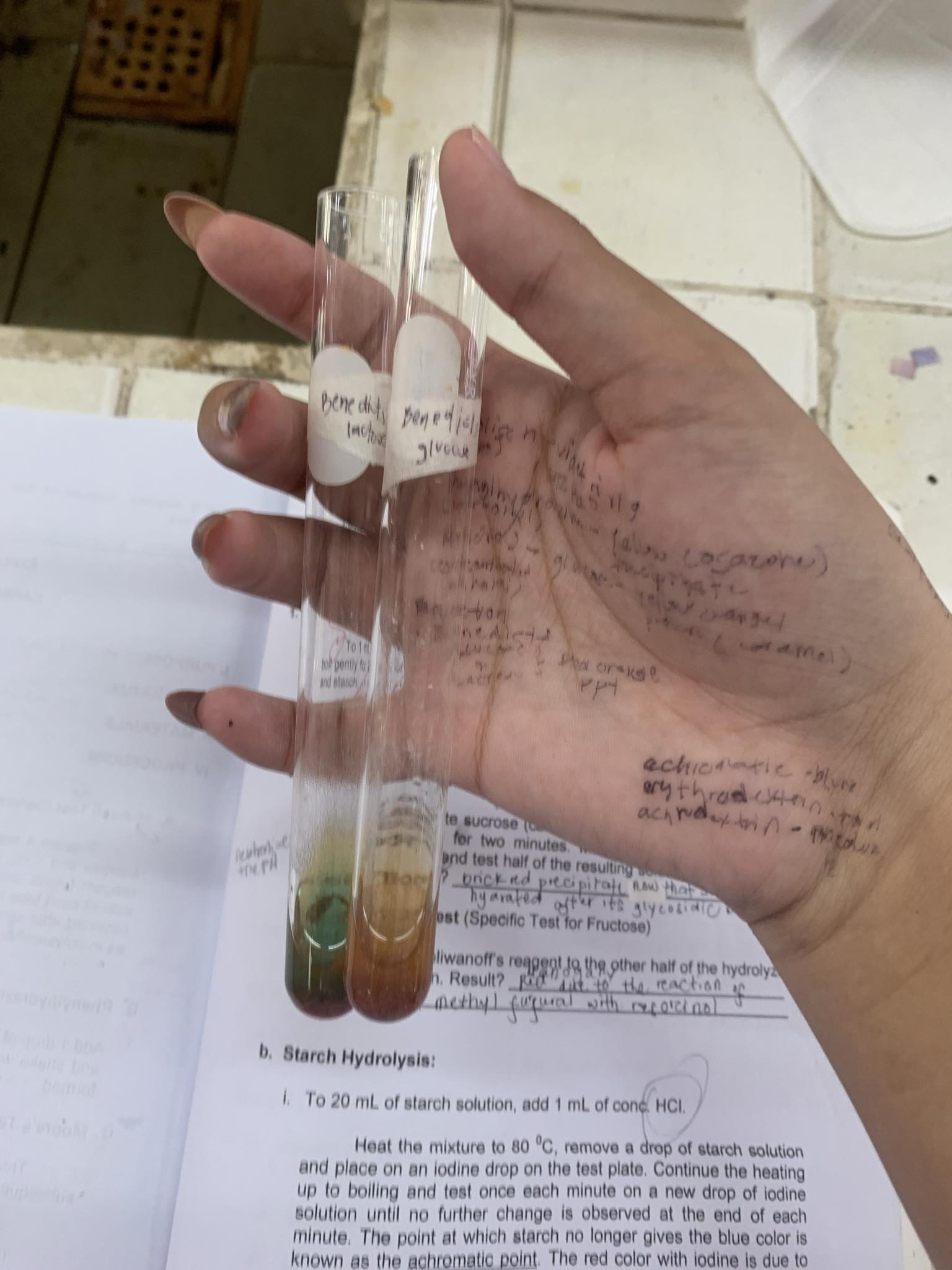
Benedict’s Test
-Benedict’s solution: CuSO4 + Na2CO3 + Sodium Citrate
C6H12O5 (glucose)
=POSITIVE
C6H12O5 (lactose)
=POSITIVE
C6H12O5+ Cu(OH)2 —→
=C6H12O6 + H2O + Cu2O (CUPROUS (I) OXIDE) BRICK RED PRECIPITATE

Benedict’s Test
-Benedict’s solution: CuSO4 + Na2CO3 + Sodium Citrate
C12H22O11 (sucrose)
=NEGATIVE
C6H10O5 (starch)
=NEGATIVE
-both are non-reducing sugars
C6H12O5+ Cu(OH)2 —→
=REMAINED BLUE
HYDROLYSIS OF SUCROSE (C12H22O11)
SUCROSE (C12H22O11) + HCL —→
HYDROLYZED SUCROSE
(broken down into GLUCOSE and FRUCTOSE)

Benedict’s Test
-Benedict’s solution: CuSO4 + Na2CO3 + Sodium Citrate
HYDROLYZED C12H22O11 (sucrose)
=POSITIVE
=now positive, because sucrose has broken into GLUCOSE AND FRUCTOSE
HYDROLYZED C12H22O11+ NaOH —→ Neutralized Sucrose
HYDROLYZED C12H22O11+ Cu(OH)2 —→
=C12H22O12 + H2O + Cu2O (CUPROUS (I) OXIXDE) BRICK RED PRECIPITATE
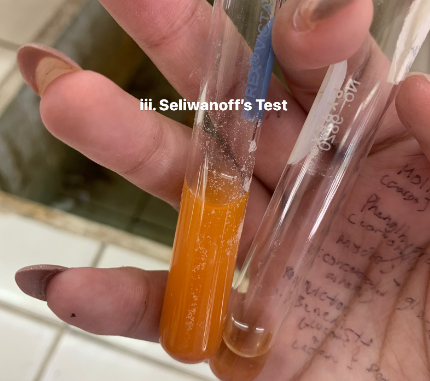
Seliwanoff’s Test
-Seliwanoff’s solution: Resorcinol + HCl
HYDROLYZED C12H22O11 (sucrose)
=POSITIVE
=now positive, because sucrose has broken into GLUCOSE AND FRUCTOSE
=Seliwanoff is test for Fructose
HYDROLYZED C12H22O11+ NaOH —→ Neutralized Sucrose
HYDROLYZED C12H22O11+ Seliwanoff’s reagent (Resorcinol + HCl)—→
=MAHOGANY RED SOLUTION
HYDROLYSIS OF STARCH (C6H10O5)
STARCH (C6H10O5) + HCl ——> HYDROLYZED STARCH C6H10O5
=broken down into GLUCOSE
IODINE TEST (for starch)
SOLUBLE STARCH (blue) ——> AMYLODEXTRIN (purple) ——> ERYTHRODEXTRIN (red) ——> ACHRODEXTRIN (colorless) ACHROMATIC POINT——> MALTOSE (colorless) ——> GLUCOSE (colorless)
=once broken down into glucose, starch has fully hydrolyzed
=IODINE is used to test the presence of STARCH

Benedict’s Test
-Benedict’s solution: CuSO4 + Na2CO3 + Sodium Citrate
HYDROLYZED STARCH C6H10O5
=POSITIVE
=now positive, because sucrose has broken into GLUCOSE
C6H10O5+ Cu(OH)2 —→
=C6H10O6 + H2O + Cu2O (CUPROUS (I) OXIXDE) BRICK RED PRECIPITATE
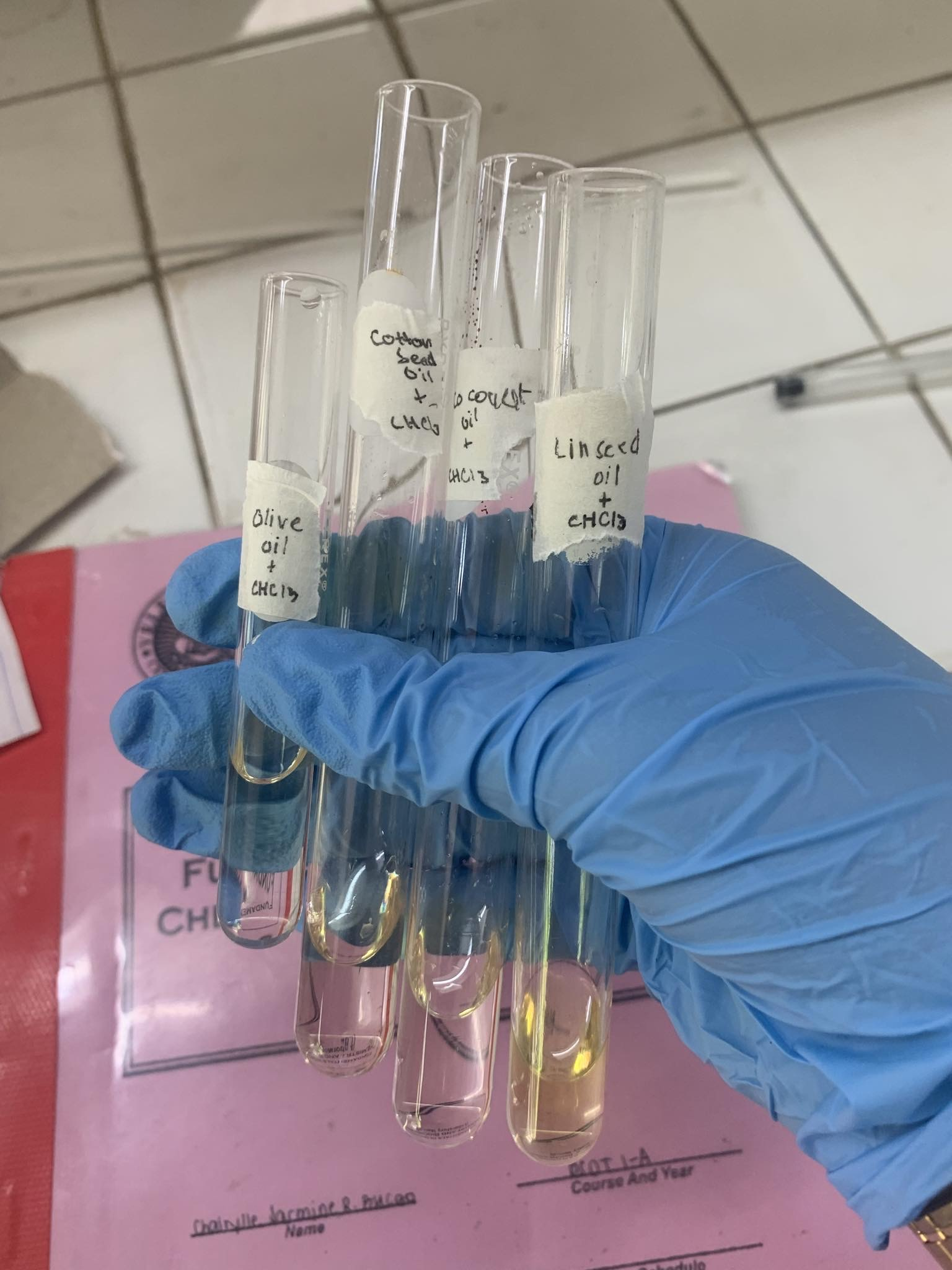
MISCIBILITY TEST
H2O (POLAR)
-all oils was immiscible with H2O
-OIL is NONPOLAR, H2O is POLAR
CH3CH2OH (HAS BOTH POLAR AND NONPOLAR PROPERTIES)
-all oils was immiscible with CH3CH2OH
-OIL is NONPOLAR, CH3CH2OH is BOTH NONPOLAR AND POLAR
CHCl3 (NONPOLAR)
-all oils was miscible with CHCl3
-OIL is NONPOLAR, CHCl3 is NONPOLAR
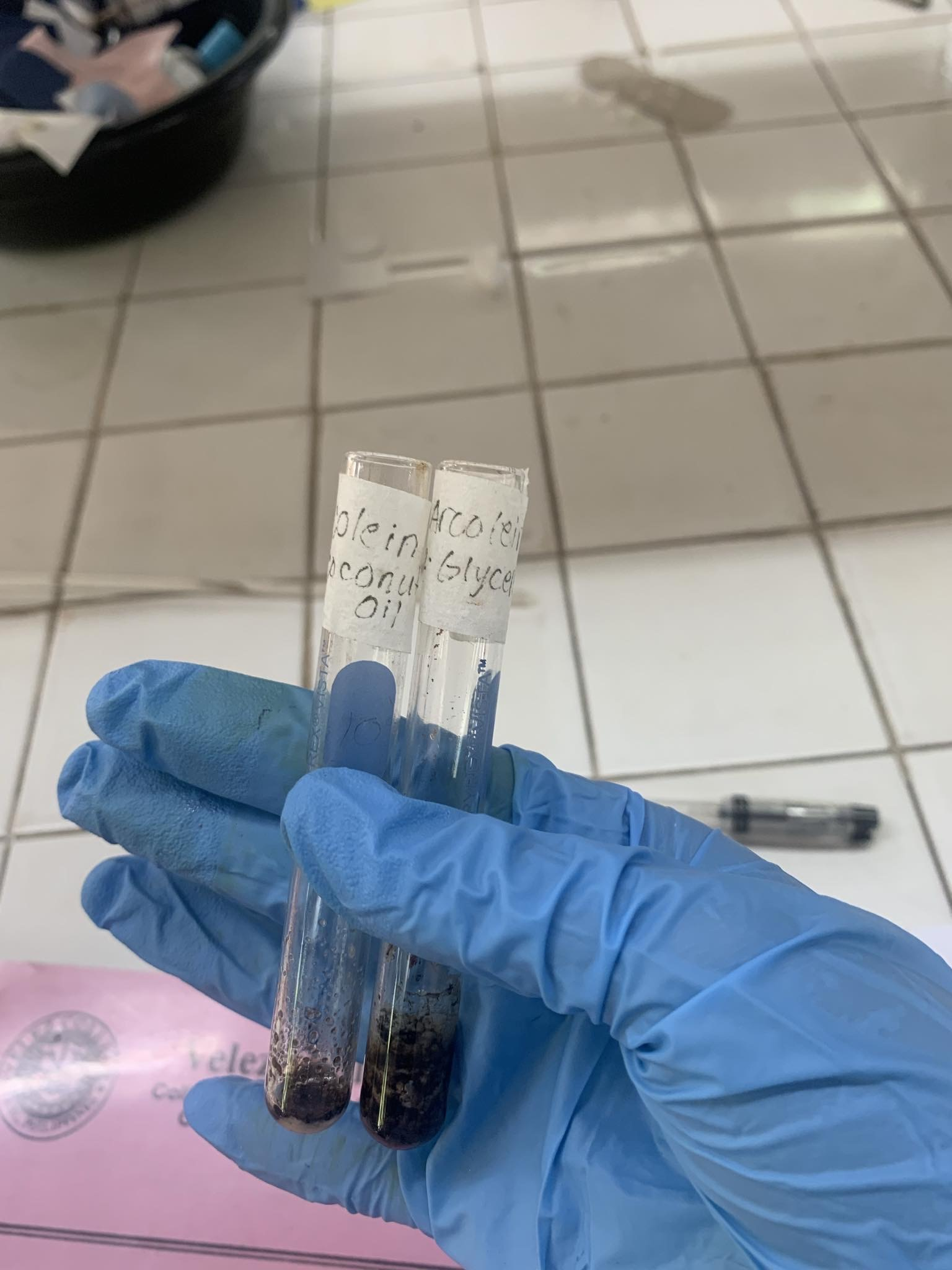
Acrolein Formation
Coconut Oil (has Lauric acid) + KHSO4
=MILD IRRITATING ODOR
Glycerol (CH2OHCHOHCH2OH) + KHSO4
=PUNGENT IRRITATING ODOR/ ACROLEIN (2-PROPENAL) CH2CHCHO
EQUATIONS
Coconut Oil + KHSO4 ——> Lauric Acid + CH2OHCHOHCH2OH
CH2OHCHOHCH2OH + KHSO4 ——>
CH2CHCHO (ACROLEIN)
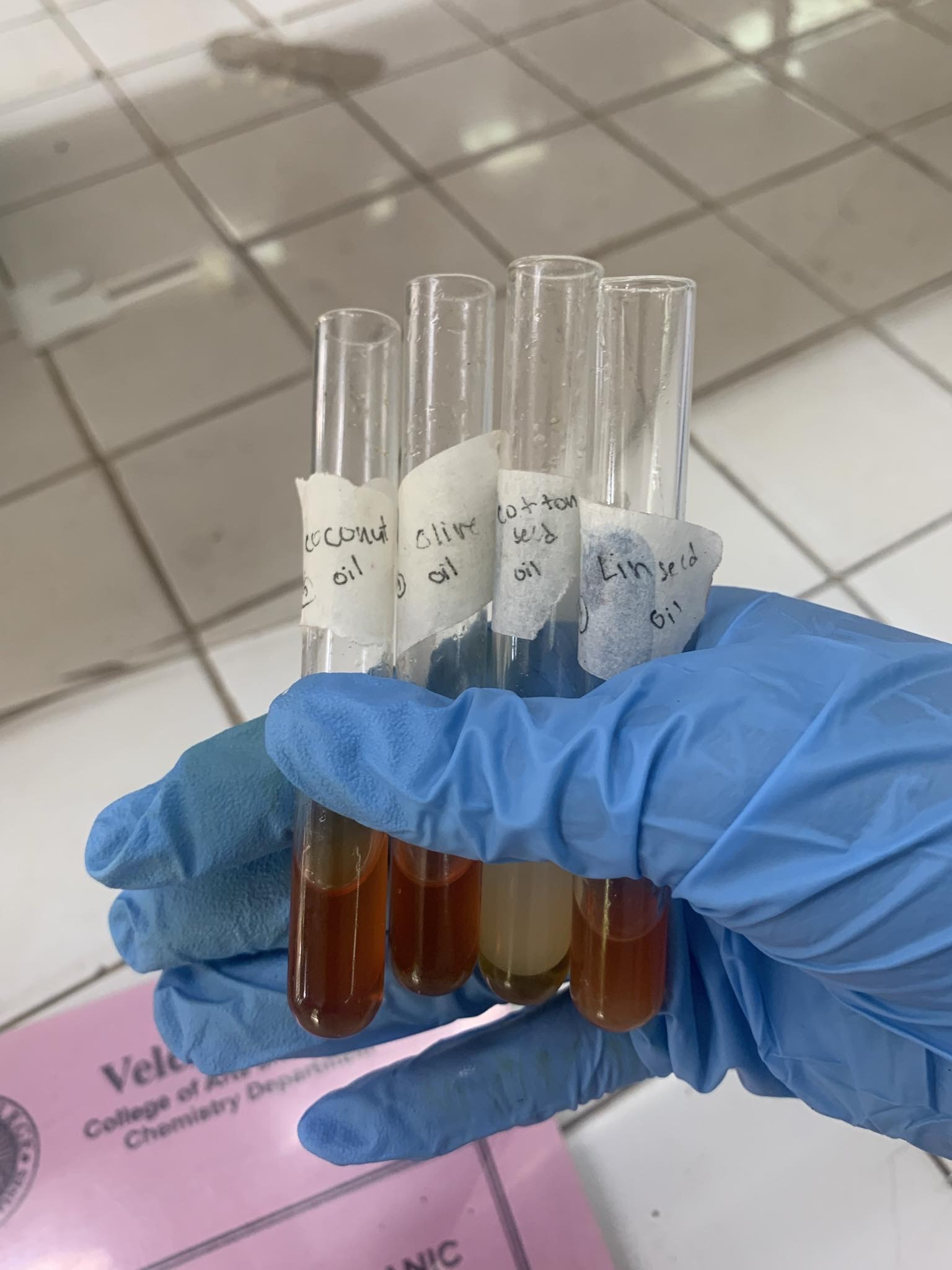
SOLUBILITY TSET (HUBLE’S TEST)
=the more a substance discolorizes IODINE, the more UNSATURATED
=unsaturated substances ABSORB IODINE, fads the color the of the IODINE
LINSEED OIL
=Linolenic Acid
=MOST UNSATURATEDCOTTONSEED OIL
=Linoleic Acid
=UnsaturatedOLIVE OIL
=Oleic Acid
=UnsaturatedCOCONUT OIL
=Lauric Acid
=SATURATED / NOT UNSATURATED
=did not discolorize I2 solution
Coconut Oil + 10% NaOH ——>
Glycerol+ 10% NaOH ——>
Saponification
=hydrolysis in a basic medium
Coconut Oil + 10% NaOH ——> CH2OHCHOHCH2OH + 3C11H23COONa (soap/metallic salt)