Ethics in Research and IRB- Krysiak
1/20
Earn XP
Description and Tags
If it says FYI, I added it for understanding the material, you don't have to memorize.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
In 1979 because a lot of shady human experiments were going on a committee issued the “Ethical Principles and Guidelines for the protection of human subjects of research” AKA the __________________.
FYI
Belmont Report
What are the 3 fundamental ethical principles that must be the basis of all research w/ human subject, that are stated in the Belmont Report?
respect for persons
beneficence
justice

How is the “respect for persons” principle of the Belmont Report demonstrated?
through the INFORMED-CONSENT process
The informed-consent process is a process by which…
a potential study subject is presented w/info about the study and willingly volunteers to participate
YOU HAVE TO
have ongoing dialogue (convos) between study personnel and participants
info must be presented in an understandable manner
understanding of info must be verified
no coercion, influence is allows
if you have diminished autonomy (ex: mental disorder) you may require special safeguards so you aren’t exploited
The “beneficence” principle of the Belmont Report is demonstrated through what 2 general rules?
do not harm and
maximize possible benefits and minimize possible harms
(FYI: this principle is usually applied by a risk-benefit analysis, which is basically weighing the pros and cons)
How is the “justice” principle of the Belmont Report explained?
there must be a sense of fairness in distributing the burdens and benefits of research
What is the Institutional Review Boards (IRB)?
a committee that is formally designated to approve, monitor, and review biomedical and behavioral research
The mission of the IRB is to …
protect HUMAN SUBJECTS from physical or psychological harm caused by experimental research
Before study enrollment can occur, the IRB reviews and approves what?
all study protocols involving human subjects research
What is the definition of a “human subject”?
a living individual about whom an investigator (professional or student) conducting research obtains:
data through interaction or intervention w/ the individual
identifiable private info or biospecimens from the individual
What 3 things are not classified as “human subjects”?
cadaver studies
(a cadaver is a dead human body)
studies where the subjects are institutions or geographic areas do not involve human subjects
reviews or meta-analyses of existing published literature do not involve human subjects
Basically, anything that doesn’t involve anything LIVING
Every study has a research protocol that leads to standardization for implementation across investigators and sites. What is the definition of a research protocol?
a standardized document that covers ALL aspects of a study- very specific details
For Investigational New Drugs (IND), ________ must approve ALL clinical protocols before administering the investigational agent to humans.
FDA
All protocols involving human subjects must be approved by an _______________________.
Institutional Review Board (IRB)
If I had no human subjects involved in my research study, would I need IRB approval?
nope- (no human subjects=no IRB)
What does an IRB exemption mean in terms of human subjects research?
certain categories of human subjects research are “exempt” from all human subjects protection requirements:
no IRB needed
no subject informed consent needed
(FYI: An example of an exemption could be observation of public behavior)
NO MATTER WHAT, even if there are exemptions, ________ still applies to use of protected health info.
HIPAA
Regarding data confidentiality, Common Rule Law requires that every effort should be taken to maintain participant confidentiality.
What are some ways to maintain this confidentiality?
interactions w/ subjects should be conducted in private
data generated should be held confidentially
secure servers and encryption software
HIPAA (Health Insurance Portability and Accountability Act) limits how health info can be used/disclosed.
How can a patients health information be shared/used for research?
patient MUST CONSENT to its use through a research authorization
this authorization must describe PURPOSE OF THE RESARCH and HOW the data will be SECURED or SHARED
Review:
What is the role of the FDA and IRB in research protocol?
IRB- any protocols using HUMANS must be approved by IRB
FDA- must approve protocols before administering investigational agent to humans
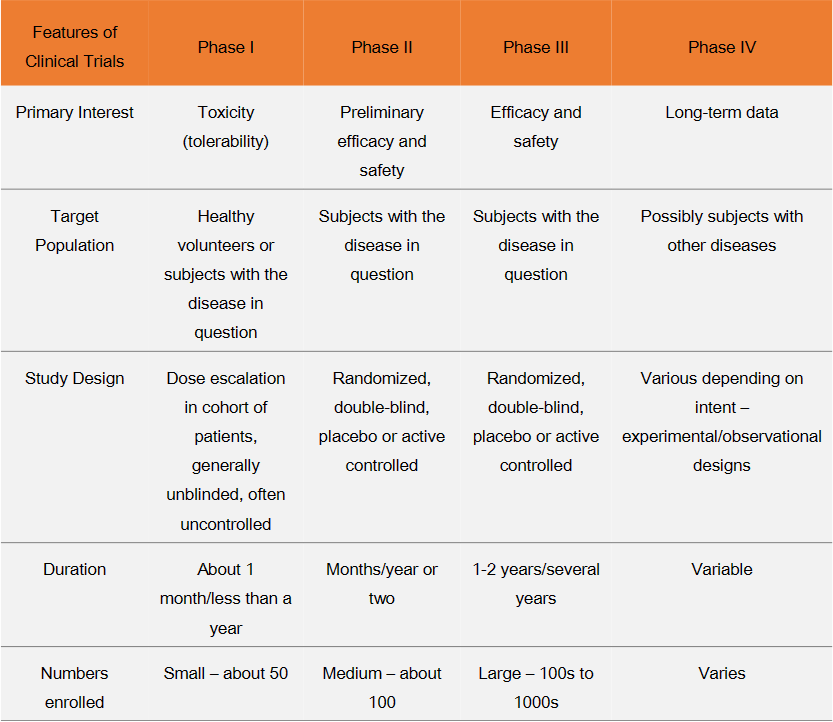
Be familiar with the 4 phases of the drug approval process: