gen chem (orbitals)
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
n
principle quantum number (number of orbital shells)
l
angular momentum quantum number (shape of an orbital)

what is this?
s-orbital (l = 0)
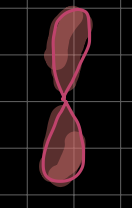
what is this?
p-orbital (l = 1)
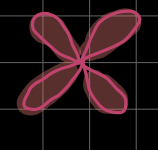
what is this?
d-orbital (l = 2)

what is this!
f-orbital (l = 3)
how do you find l
n - 1
ml
magnetic angular quantum number
how do you find the ml
± L
nodes
where the electrons in an orbital are absent
how do you find the total number of nodes?
n - 1
radial nodes
the boundary between two of the same type of orbital with different n values
how do you find the radial node?
= n - l - 1
angular node
a plane that passes through the nucleus and forms boundaries between orbitals in the same energy level
how do you find the number of angular nodes?
= l
ms
magnetic spin quantum number (describes the direction of the spin of the electron)
what can ms be?
± 1/2