Class 10-Proteins + A.A.
1/36
Earn XP
Description and Tags
Mary Hendrickson , sept 30th
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
amino acids are made of xx, and these elements:
= building blocks of proteins
cooH, amine group, H, and R group
made of carbon, nitrogen, oxygen, sometimes sulphur
what are the conditionally essential AA?
Arginine
Cysteine
Glutamine
Glycine
Proline
Tyrosine
how does a protein become functional?
Several strands cluster together into a functioning unit
OR
A metal ion (mineral) or a Vit. join to the unit and activate it
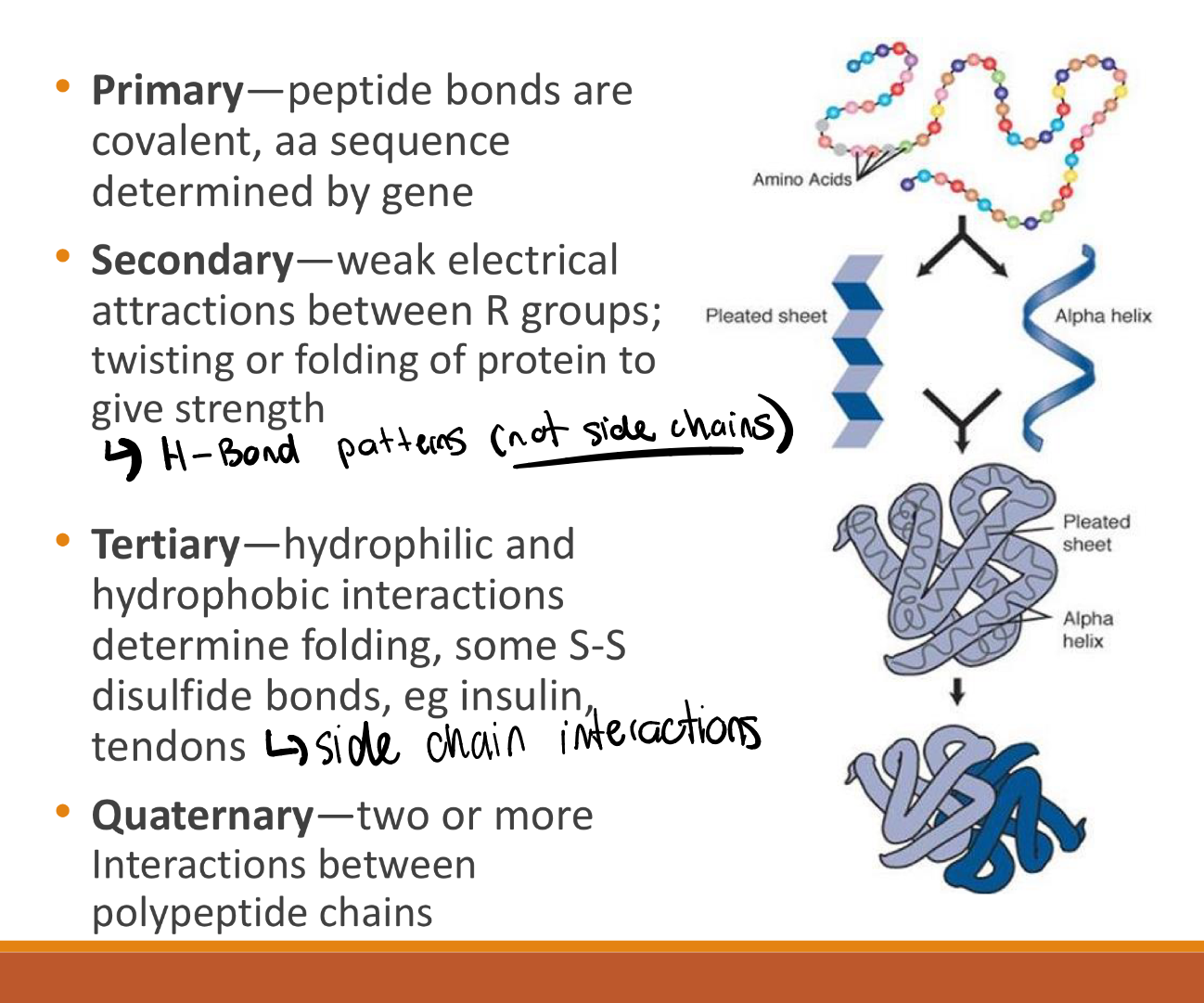
primary, secondary, quaternary, and tertiary structures
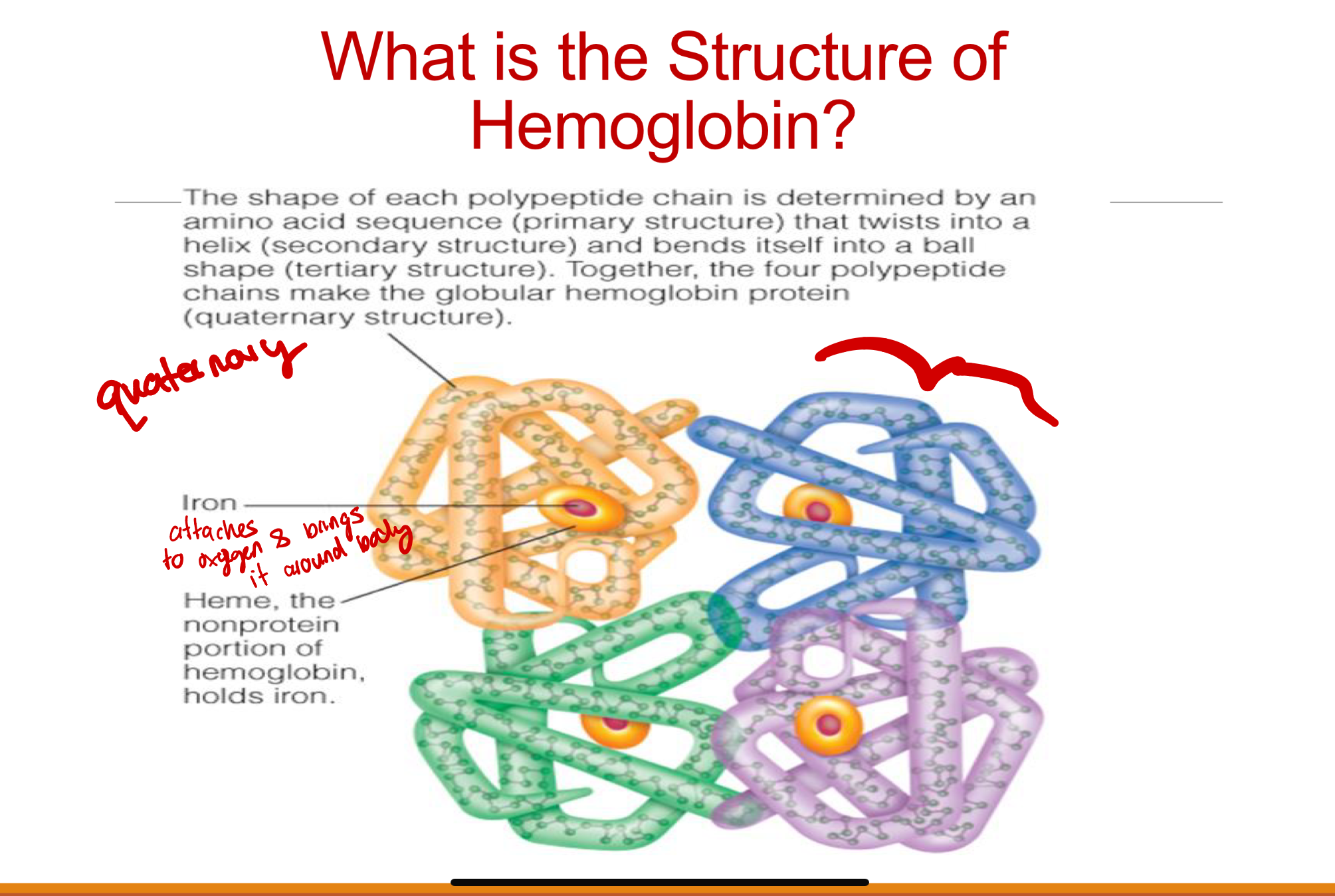
structure of hemoglobin
what is proteome mean
A proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time.
single gene disorders vs multi-gene disorders
Single-Gene Disorders
◦ Mutations inherited at birth (e.g., PKU, sickle cell,CF)
◦ Damage to the individual exerted early in life
Multigene Disorders: Chronic disease– eg CHD
◦ Influence of several genes
◦ Sensitive to interactions with the environment like pullustion, etc (risk factors)
◦ Single nucleotide polymorphisms (SNPs)
what is the key part about how inherited variations of AA sequence can negatively impact XXX of yyy
inherited variations of AA sequence can negatively impact FUNCTION of a PROTEIN
What is PKU
= Phenylketonuria
Non-functioning Phenylalanine Hydroxylase, can’t digest phenyl alanine AA
PHE is not hydroxylated to TYR
PHE builds up and is metabolized by alternate pathway to Phenylketones
Damages the developing brain
Neonatal screening programs
Medical Nutrition Therapy
◦ Keep PHE and phenylketones low
◦ TYR is an essential amino acid
What is cystic fibrosis
‘Every person has 2 copies of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. A person must inherit two copies of the CFTR gene that contain mutations — one copy from each parent — to have cystic fibrosis’
Poorly functioning chloride channel CFTR means:
◦ Thick mucus in lungs…infections – difficult to fix
◦ Pancreatic enzyme replacement…oral coated enzymes to facilitate digestion – easy to fix
*Medical Nutrition Therapy – the cornerstone of treatment for growth and immune
function
what is nutritional genomics
Nutritional genomics examines the interactions of genes and nutrients. These interactions include both nutrigenetics and nutrigenomics.
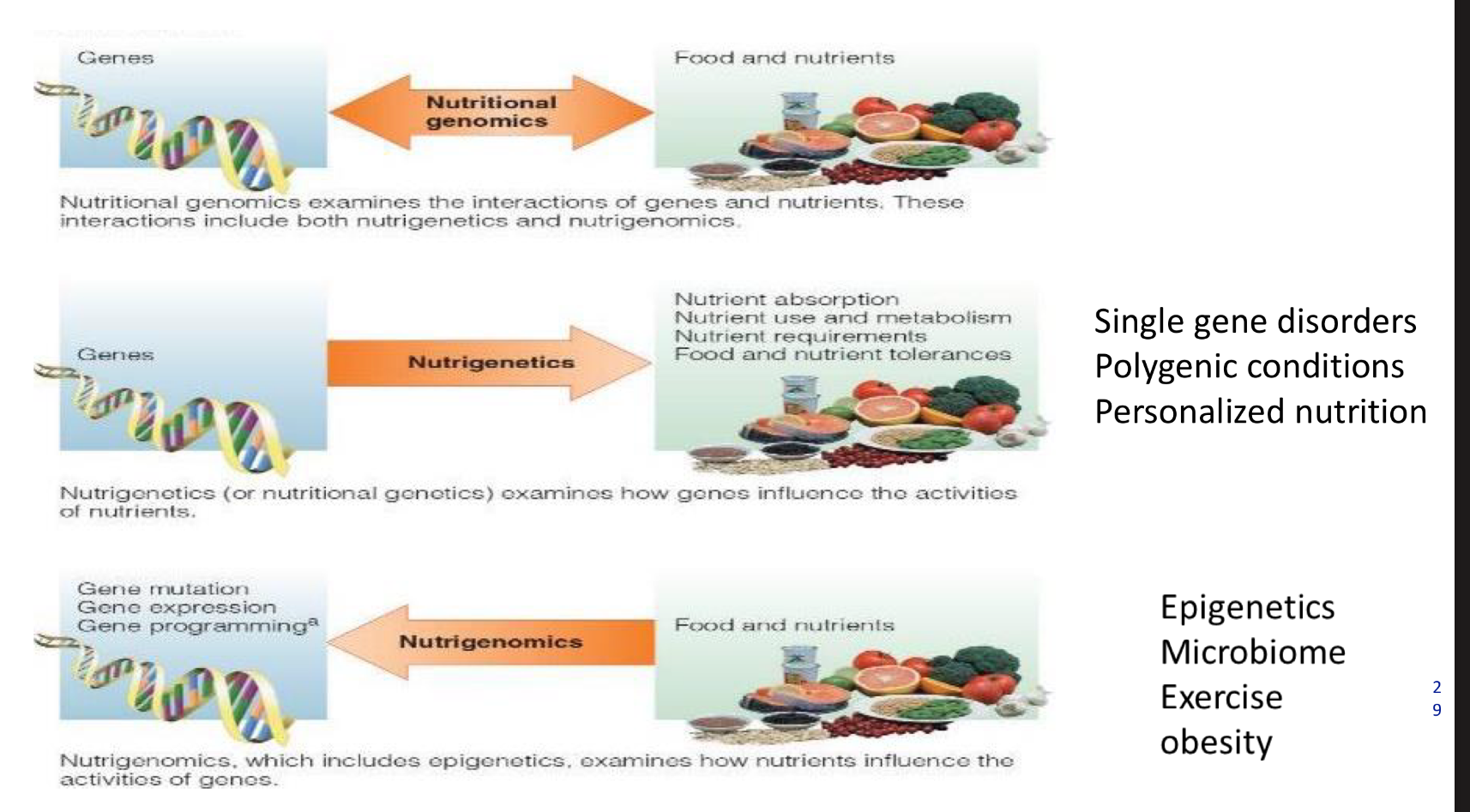
what is nutrigenetics
Nutrigenetics (or nutritional genetics) examines how genes influence the activities of nutrients.
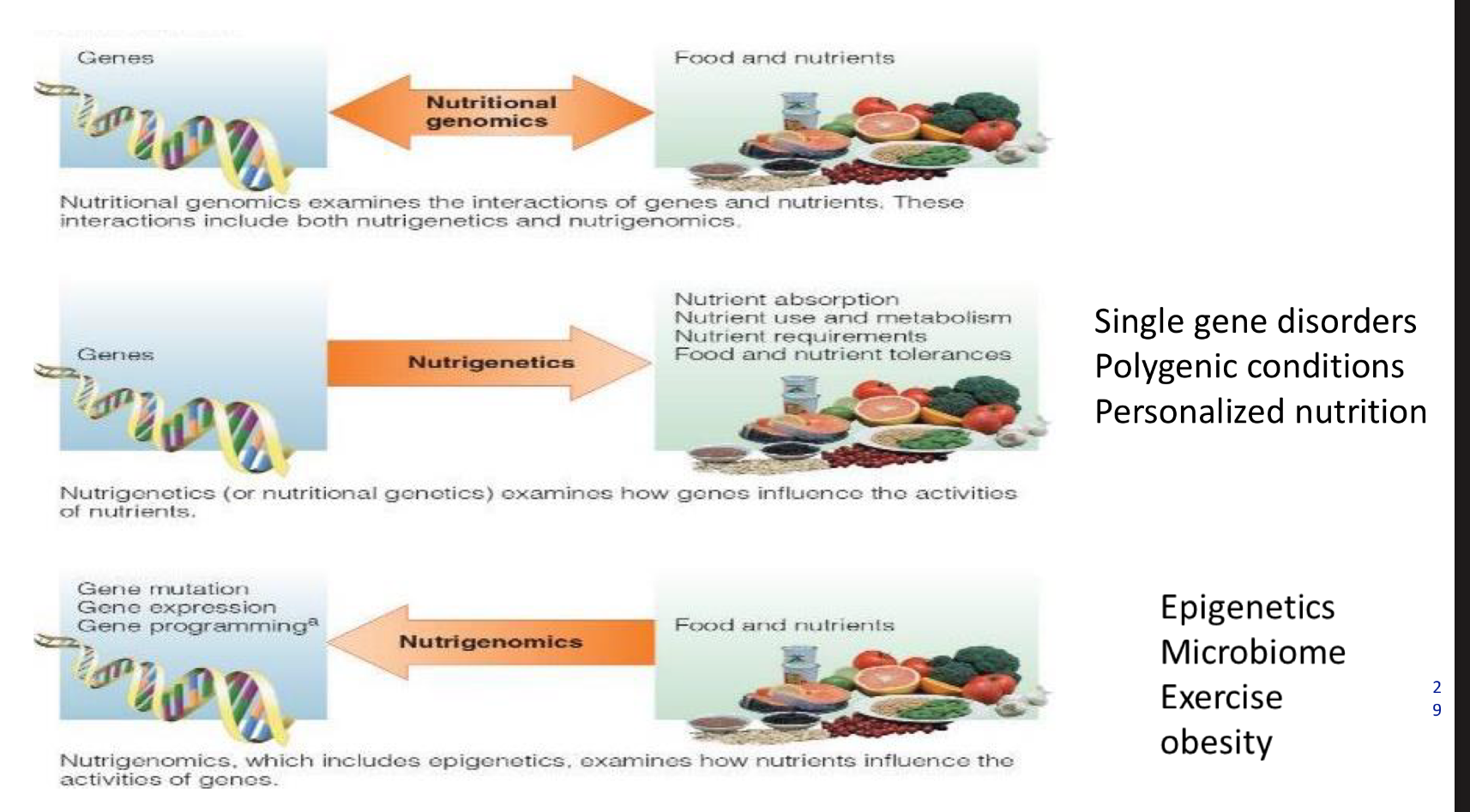
what is nutrigenomics
Nutrigenomics, which includes epigenetics, examines how nutrients influence the activities of genos.
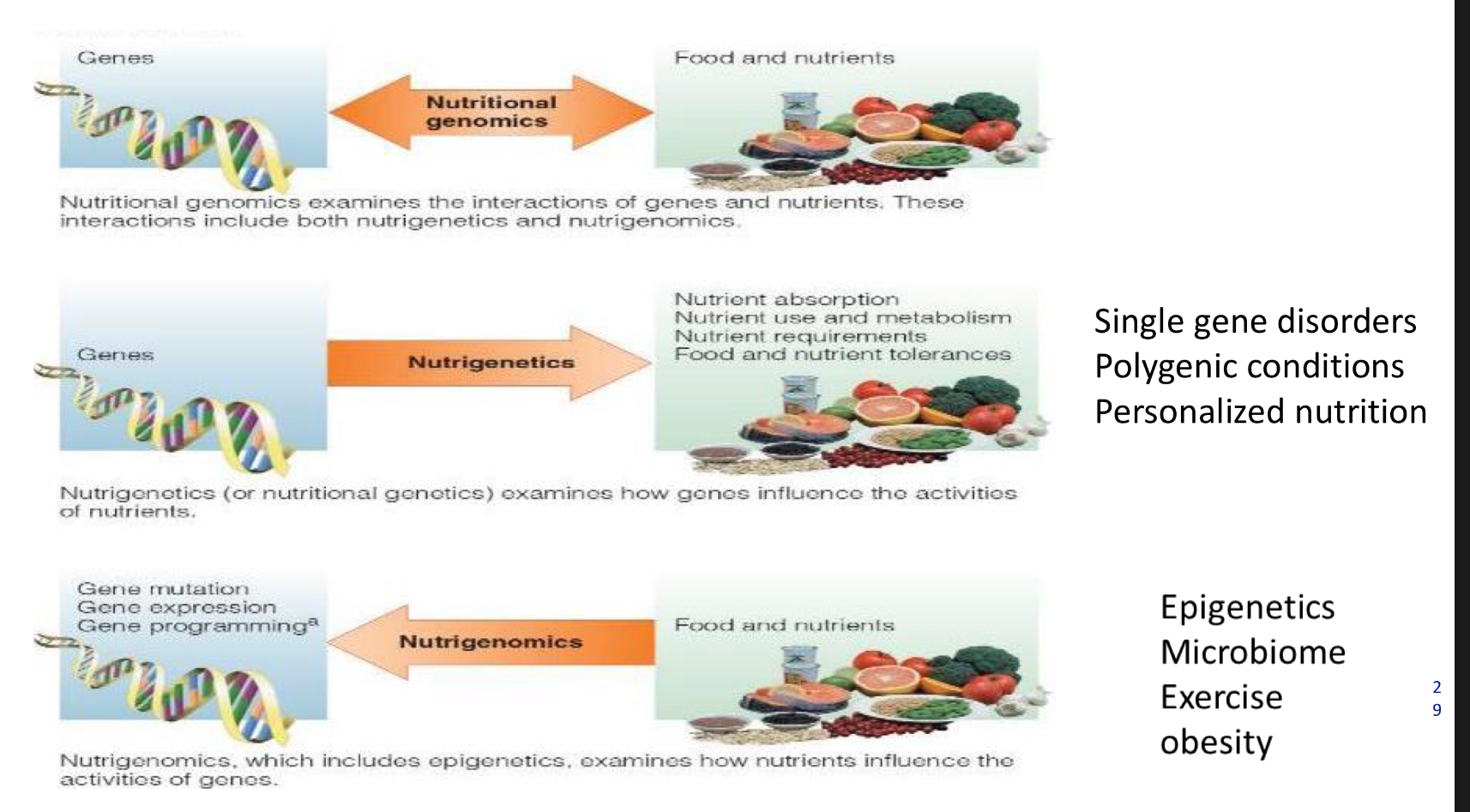
how do nutrients and phtyochemicals play a role in genetics?
what are some causes for protein denaturation?
Caused by:
• Agitation
• Heat
• Acid/Base
• Heavy metal salts
• Alcohol
• Digestion
• Radiation
Denaturatio
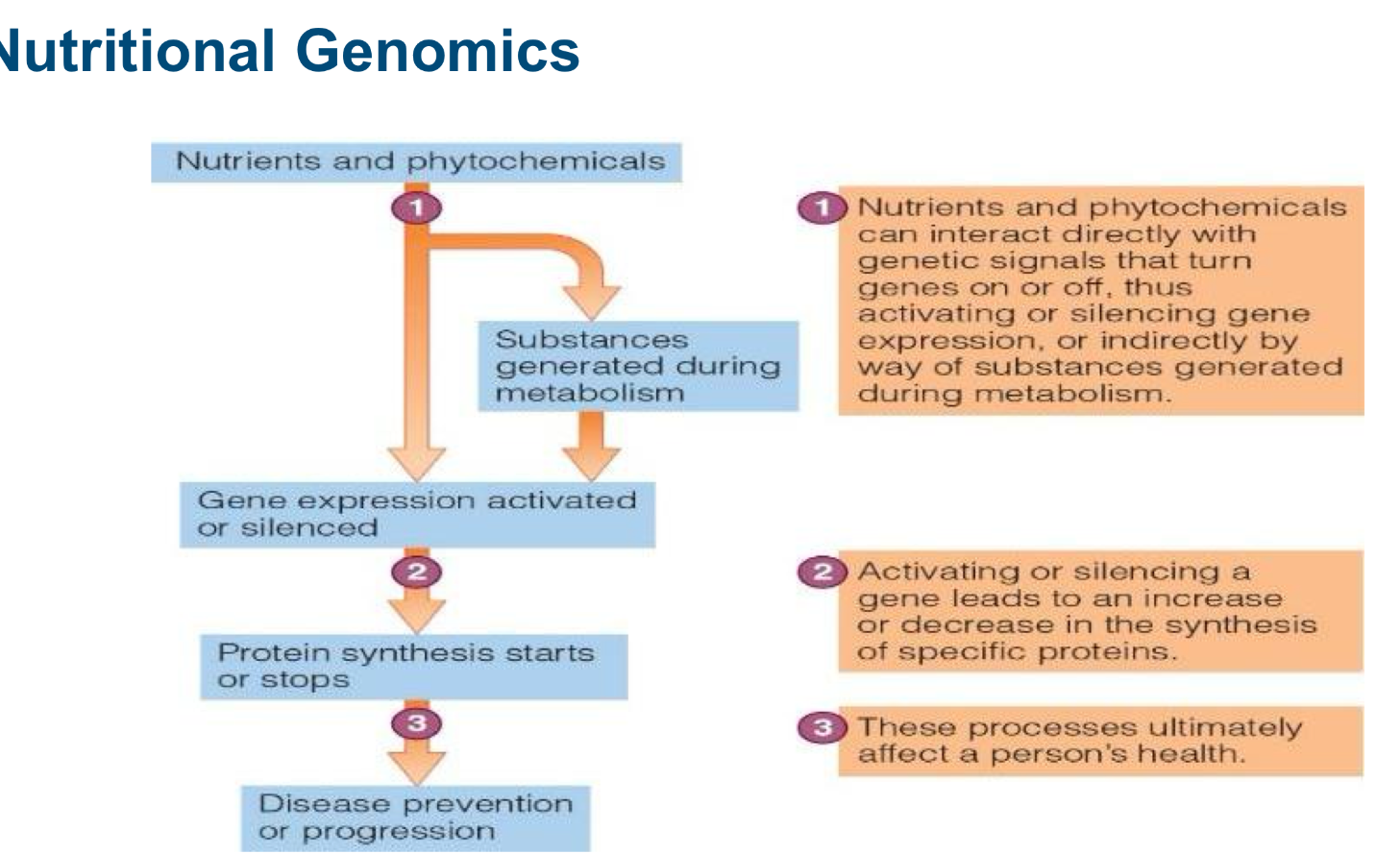
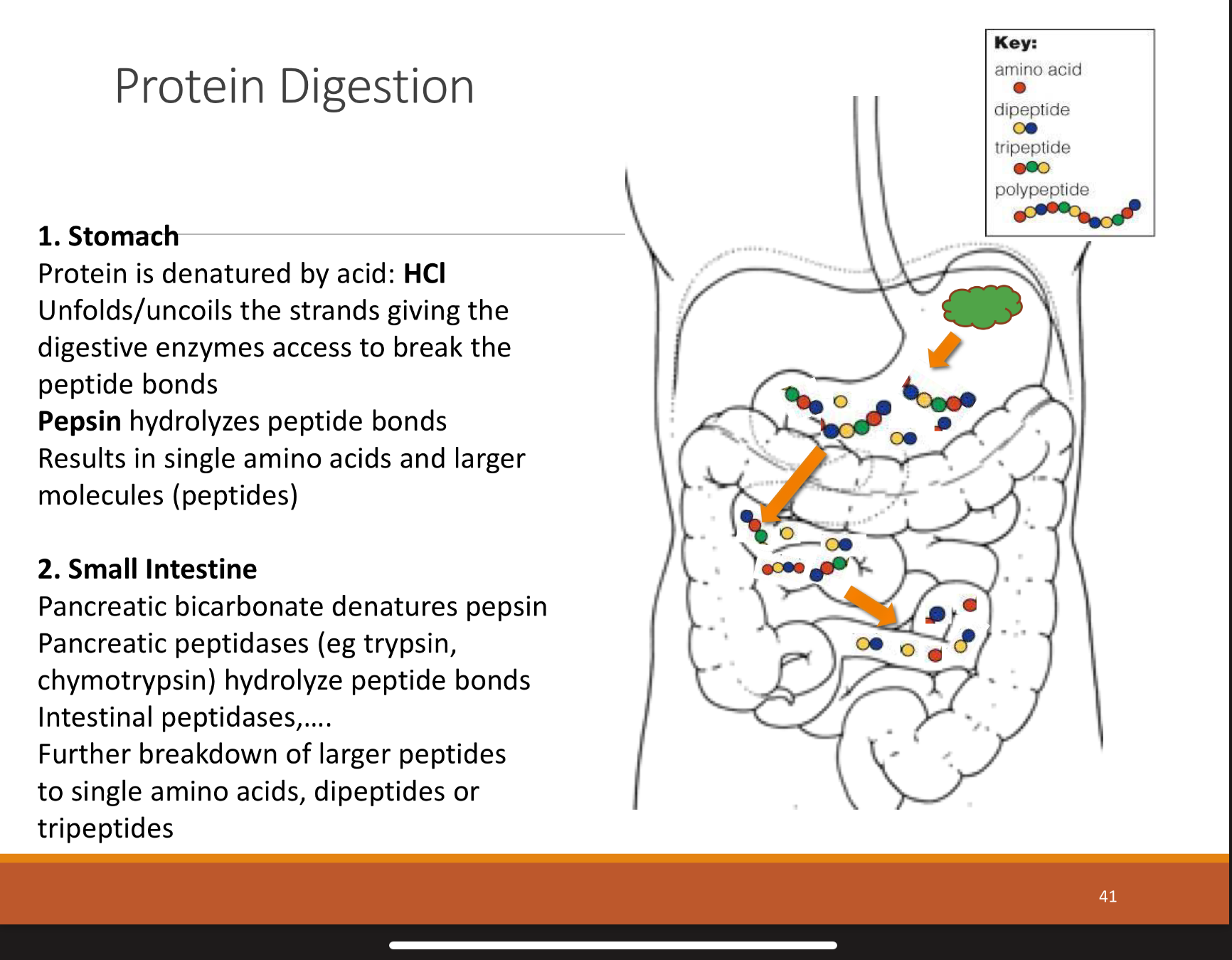
overview of overall protein ingestion
mouth: physical breakdown and moistening from chewing and saliva
stomach: HCL starts to denature and activates stomach enzymes
pancreas + small ints: enzymes from pancreas and small intestine split poly peptides further
small intestine: enzymes on surface of small intestine. hydrolyze peptides and now cells can absorb them
2 main molecules regarding protein digestion in the STOMACH
HCL, hydrochloric acid= denatures protein strands, activates pepsinogen to pepsin
Pepsin= cleaves proteins to smaller polypeptides+some free AA , inhibits pepsinogen synthesis

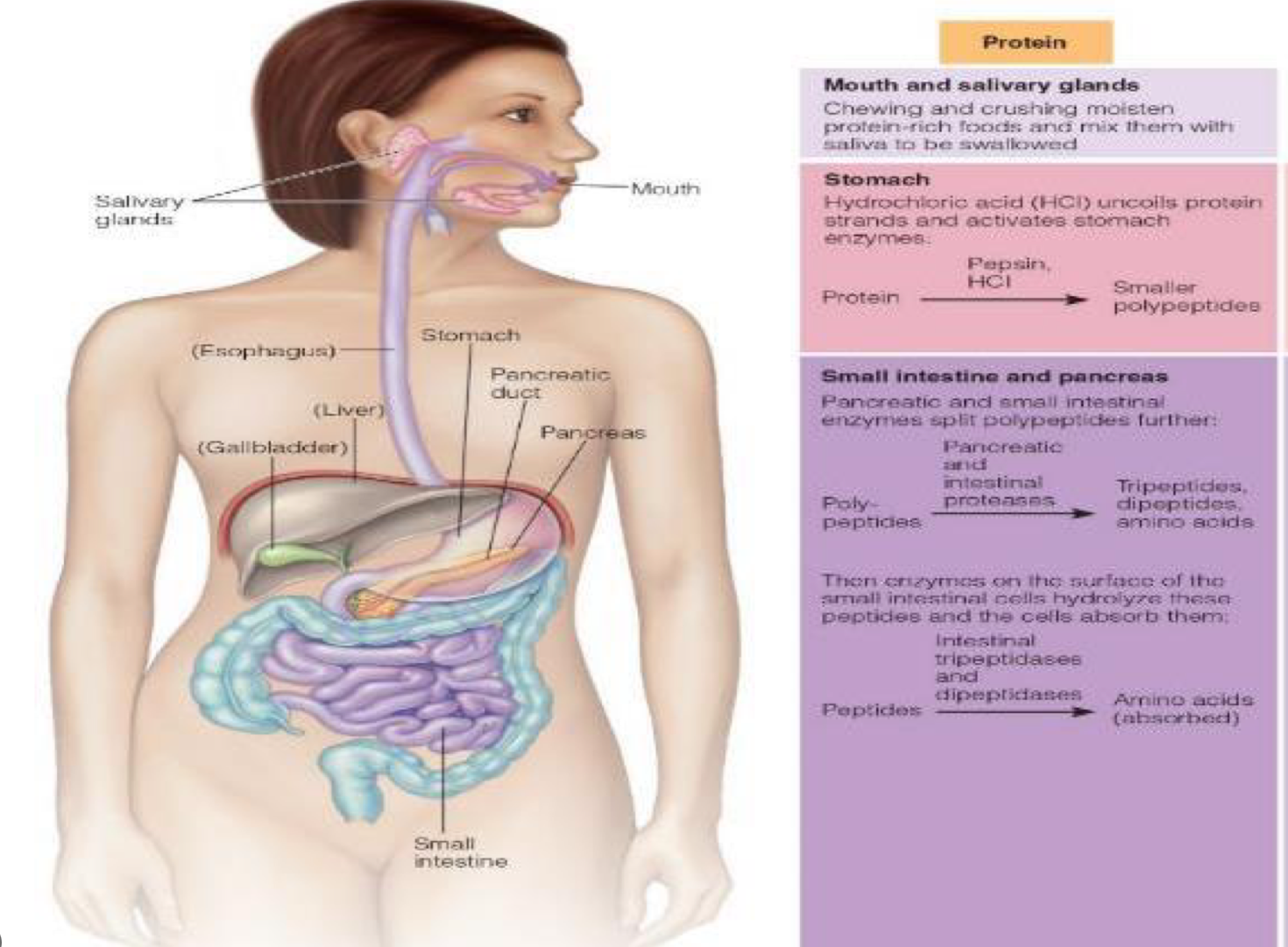
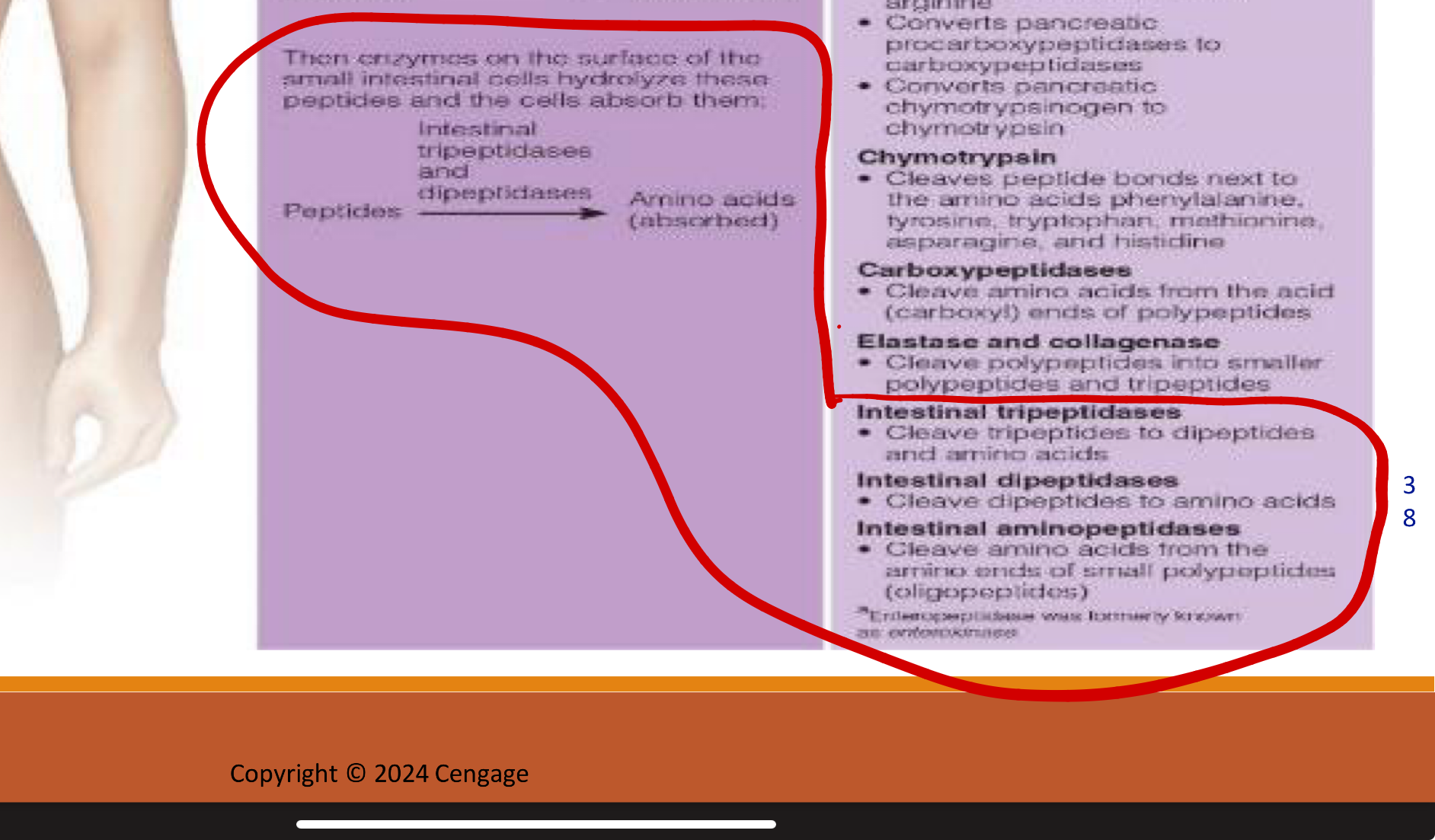
first couple steps of protein digestion in stomach in pancreas and small intestine (polypeptides to peptides)
1.Enteropeptidase (from intestinal brush border)
Converts trypsinogen → trypsin (key master activator).
Trypsin then activates procarboxypeptidases → carboxypeptidases and chymotrypsinogen → chymotrypsin.
Also cleaves peptide bonds between lysine and arginine.
PANCREATIC PROTEASES (MADE IN PANCREAS BUT WORK IN SMALL INST.)
2.Chymotrypsin: cleaves bonds between Cuts after aromatic and bulky residues → Phe, Tyr, Trp, Met, Asn, His.
Carboxypeptidases: Cut amino acids from the C-terminal end
Elastase & Collagenase: Cleave polypeptides into smaller peptides and tripeptides
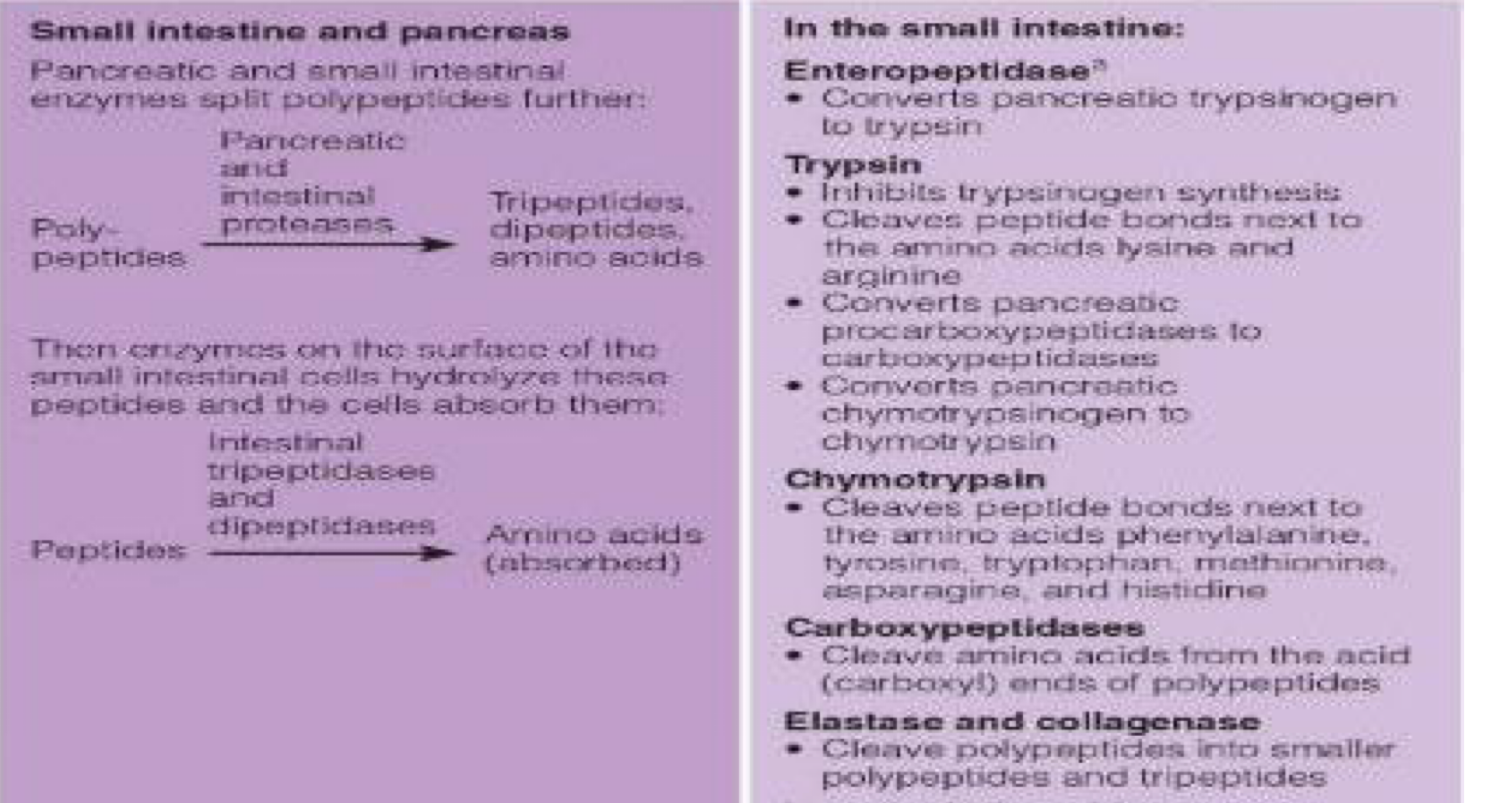
second steps of protein digestion in small intestine (peptides to absorbed amino acids)
3. Enzymes on border of small inst. cells hydrolyze peptides so cells can absorb them
Intestinal Tripeptidases: Break tripeptides → dipeptides + amino acids.
Intestinal Dipeptidases: Break dipeptides → amino acids.
Intestinal Aminopeptidases: Chop amino acids off from the N-terminal end of oligopeptides
summary of proteins going through stomach then small intestine
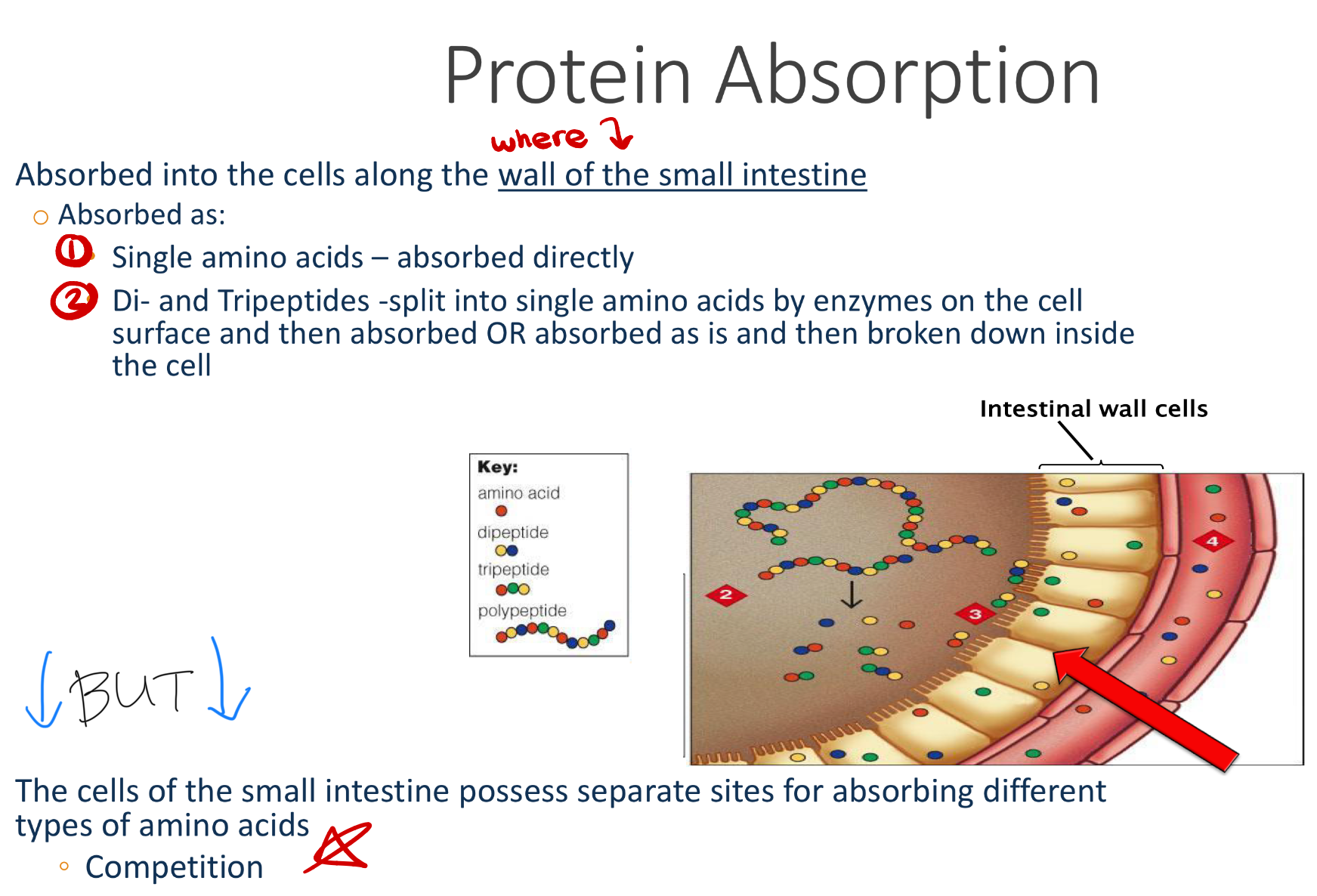
how are single AA versus di/tri-peptides absorbed, where are they absorbed?
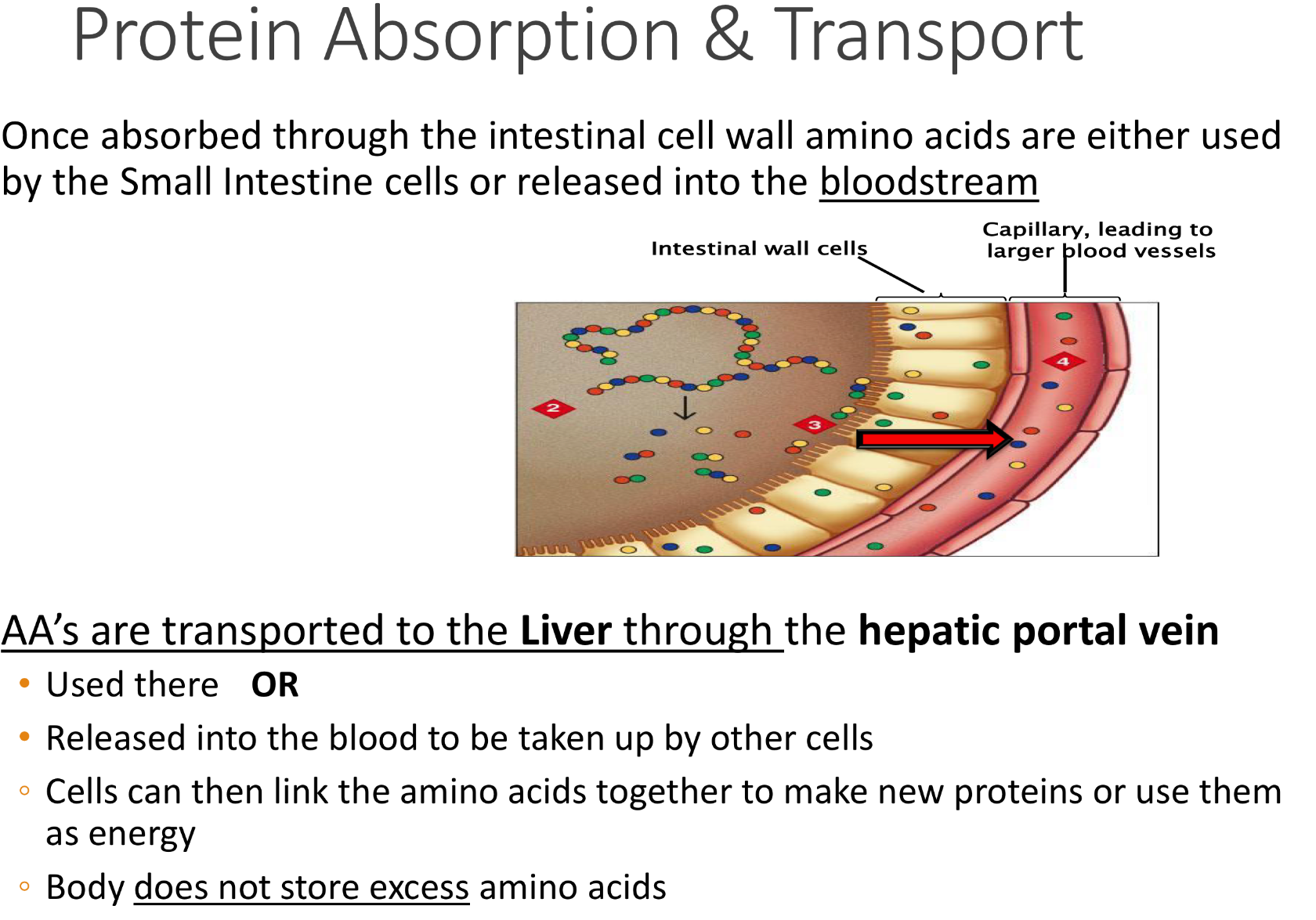
once AA are absorbed through xxx, they are used by yy and zzz. once in zzz, they are transported to liver by the ——
Once absorbed through the intestinal cell walls, AA are either used by small intestines. or released into the bloodstream
- through blood, AA’s are transported to the Liver through the hepatic portal vein and used there
OR
- Released into the blood to be taken up by other cells
◦ Cells can then link the amino acids together to make new proteins or use them as energy
!Body does not store excess amino acids!
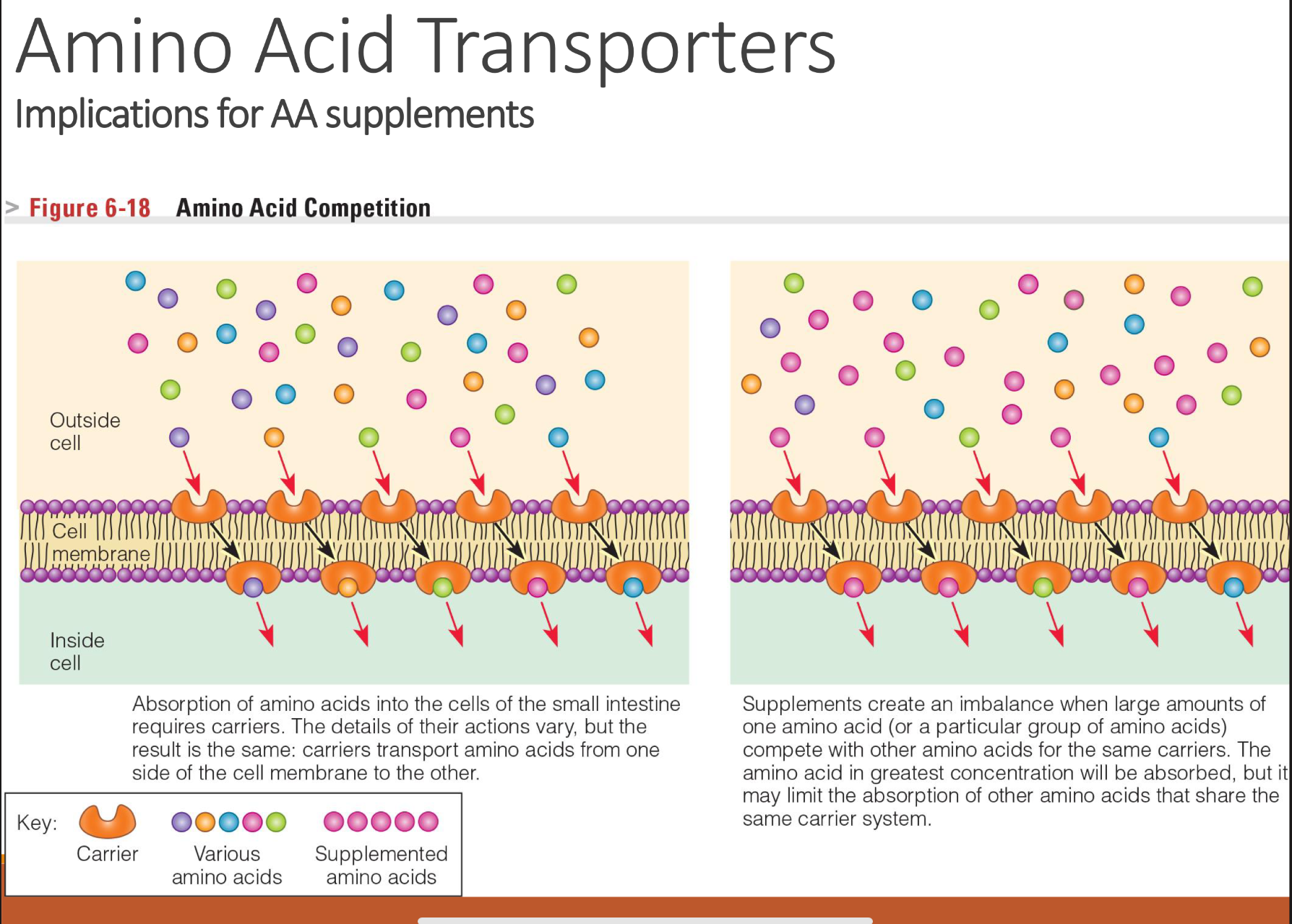
explain AA competition in cell membranes
Unbalanced supplementation (like huge doses of one amino acid) can reduce absorption of others.
Amino acids cannot diffuse freely across membranes.
They need specific transporters (carrier proteins).
Each transporter can carry multiple amino acids with similar size, charge, or structure.
→ This leads to competition: if two amino acids use the same transporter, they can interfere with each other’s absorption or uptake.
just a few out of the many roles of proteins in the body
1 Supporting Growth and Maintenance
2 Building Enzymes and Hormones
3 Building Antibodies
4 Maintaining Fluid and Electrolyte Balance
5 Maintaining Acid-Base Balance
6 Source of Energy
7 Blood clotting, etc
how do proteins support growth and maintenance?
Amino acids must be continuously available for:
• Red blood cells
• Internal cell structures
• Muscles
• Intestinal cells
• Skin, hair, naills
all these AA are constantly being replaced!!
how are proteins involved in building enzymes and hormones
Enzymes= build substances, break them down,
or transform one substance into another (amino
acid into glucose) —> act as catalyst!!!
Hormones= Messenger molecules released by various glands in response to changes
ex:
• Insulin
• Glucagon• Serotonin(via tryptophan)
• Tyrosine used synthesize the chemical messengers
• epinephrine and norepinephrine
proteins are important for building antibodies because…
protein’s role in maintaining fluid and electrolyte balance
o Proteins attract water found in cells and in plasma
o Regulates the quantity of fluids and
dissolved particles in the compartments
of the body—> Water, sodium, potassium
BUT they Do not normally cross cell membranes,
when they do this causes problems for
the body like Fluid imbalance, e.g., edema
how are proteins important for maintaining acid-base balance
o Blood proteins act as buffers to maintain a normal pH lvl (Blood pH is one of the most rigidly controlled conditions in body)
o Protect against acidosis (excess acid) and alkalosis (excessbase)
how can protein be a source of energy and glucose? what is gluconeogenesis?
RDA=10-35% of total daily energy

protein is xx% nitrogen
protein is 16% nitrogen
6.25g protein contains x grams of nitrogen
6.25g protein contains 1 gram of nitrogen
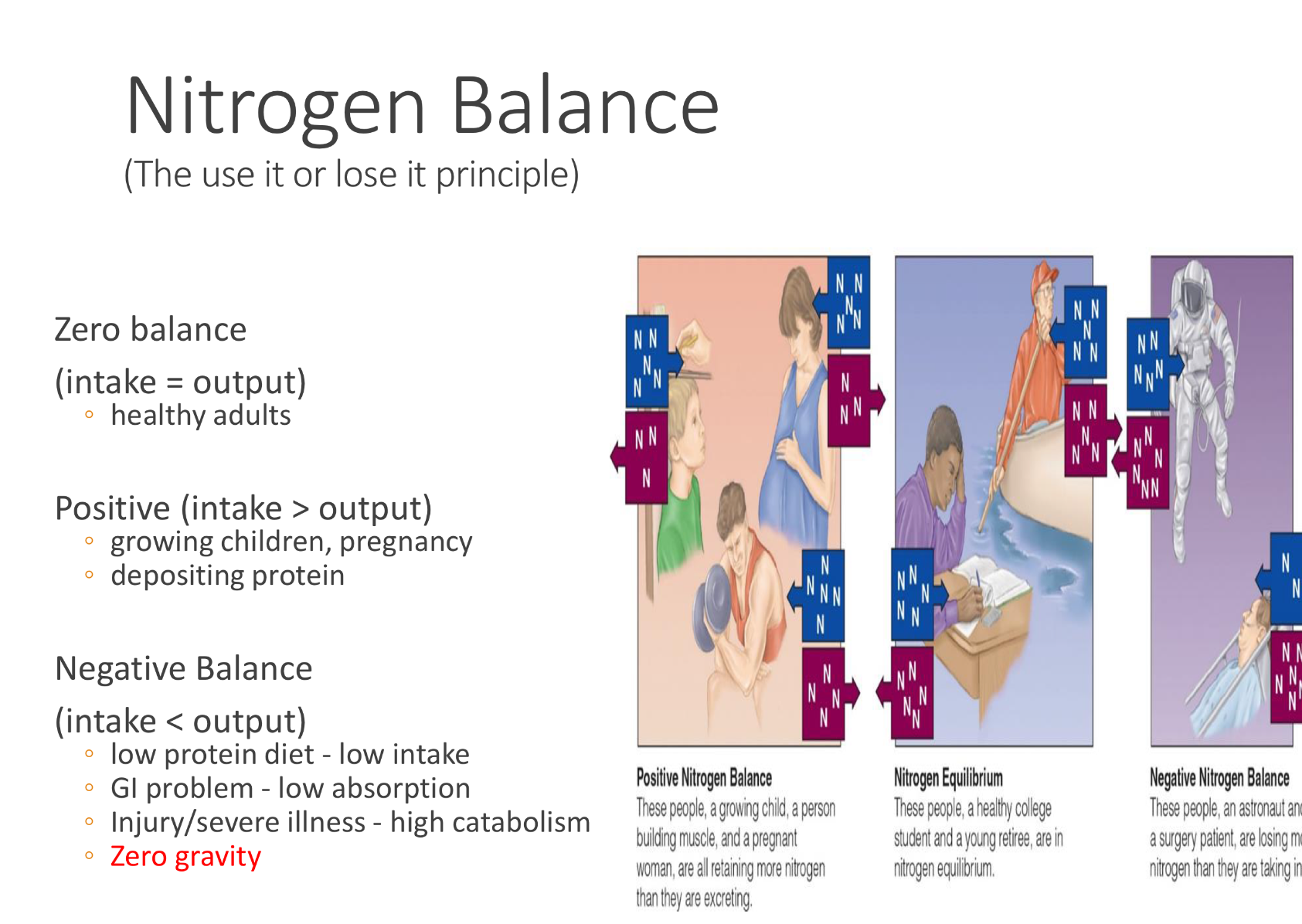
how to calculate nitrogen balance?
nitrogen balance= (N intake)-(Fecal N)- (Urinary N)
the three differnt types of nitrogen balance
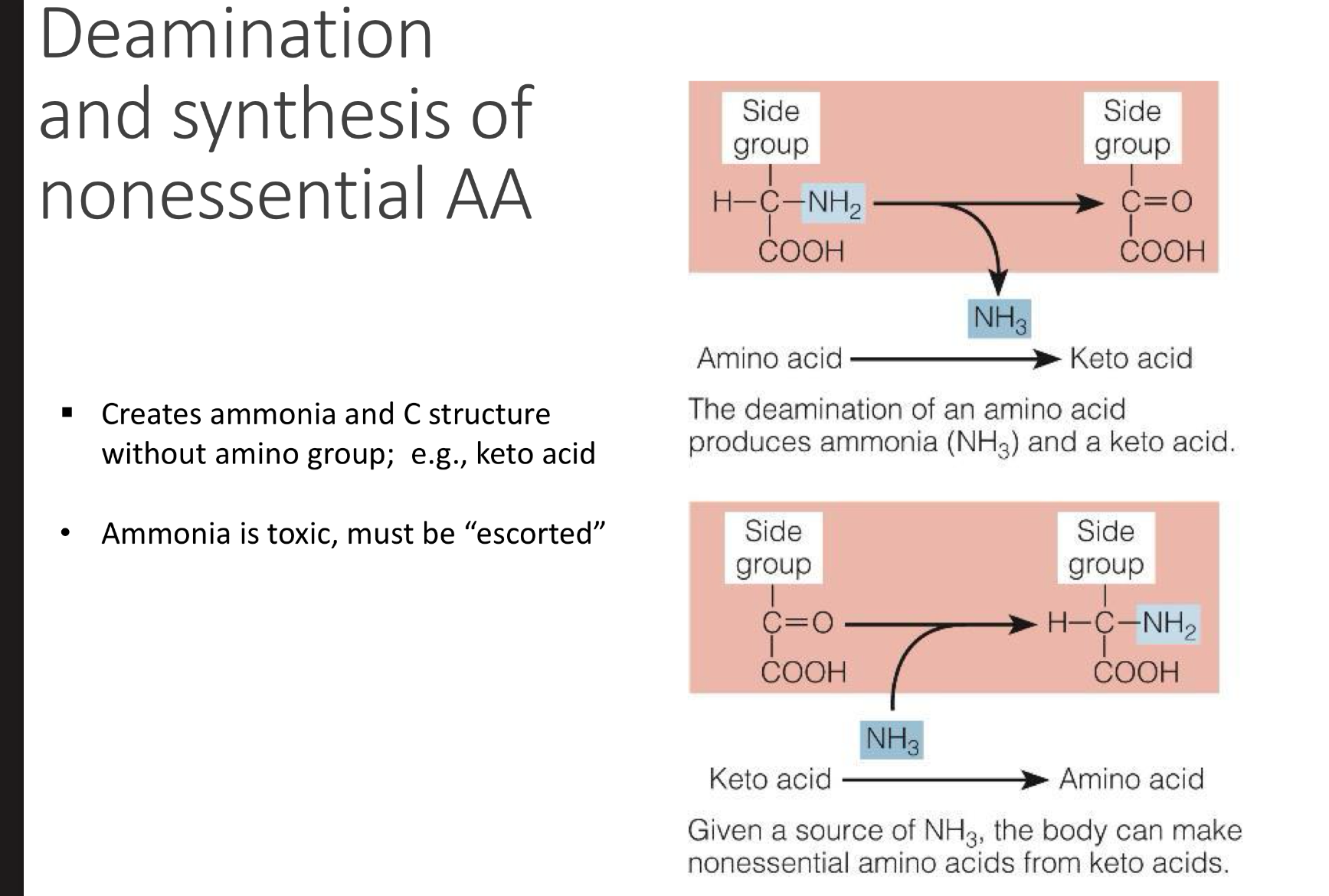
what is deamination and synthesis of nonessential AA
Definition: Removal of the amino group (–NH₂) from an amino acid.
Where/why it happens:
Mainly in the liver.
Happens when amino acids are used for energy or when there’s excess protein.
Process:
The amino group is removed → converted to ammonia (NH₃).
Ammonia is toxic, so it’s quickly converted into urea (via the urea cycle) and excreted in urine.
The remaining carbon skeleton (keto acid) can enter metabolism → used for ATP, glucose, or fat synthesis.
what coenzyme is required for transamination when the body transfers a NH2 from amino to a keto acid to form xxx
VITAMIN B6 is required for transamination when the body transfers a NH2 from amino to a keto acid to form NON-ESSENTIAL AA and new KETO ACID