pcp4- dilutions and pharmaceutical concentrations
1/12
Earn XP
Description and Tags
calculation s- solid, solution preperationcallculate the ratios of two products that are mixed to give a concentration
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Why do we dilute a solution ?
to give a desired strength needed for a patient use
What is the key dilution equation?
c1x v1 = c2 x v2
the number of moles stays the same
1- original
2-new
general method
step1 - use equation , change any units
step2- find out what is missing
step3- solve
example calculation - how many millilitres of a 10% w/v solution of drug A is needed to make 500ml of 4% w/v solution ?
c1= 10 , v1=? , c2= 4, v2=500
10v1=2000
v1= 200
example calculation using - in ratios
How many ml of a 1 in 2000 solution of drug c can be made from 500ml of a 0.25% w/v solution of a compound
1.change units - 1g in 2000ml ( what is in 1ml )
1/2000 × 100 = 0.05%
2.use equation - 0.25× 500 = 0.05 v2
solve- v2= 2500ml
Example - how many of water must be added to 300ml of 20% w/v stock solution of drug d to prepare a 0.8% w/v solution
note- added ( must takeaway 300 from answer)
300 × 20 = 0.8v2
v2 = 75000
7500-3000= 7200ml
what to we need to do when mixing two solutions together our product ?
if we know the concentration or amount of the being mixed , we can use the following approach to calculate the concentration of the final product:
1. calculate the weight of active ingredient in each of the solutions being mixed
2. calculate the total volume of mixture produced
Mixing solutions - example calculation
what is the conc of active ingredient in a solution prepared by mixing 100 ml of 20% w/v , 50 ml of 10% w/v and 200ml of 5% w/v ?
1.find the total weight of active ingredients
100ml of 20% w/v : 20/100 × 100 = 20g
50ml of 10%:w/v 10/100 × 50= 5g
200ml of 5% w/v : = 5/100 × 200= 10g
total weight = 20+10+5= 35g
2.find the total volume
total = 50+100+200= 350 ml
3.combine them and find out what is in 100ml
35g in 350 ml
in 100 35/350× 100= 10g
4.answer
10% w/v
How do we obtain a desired concentration?
mix two preparations of differing concentrations to obtain - DESIRED CONCENTRATION
usually when there is no ready access to the stock solution
but there is a supply of differing concentrations of the products
=ALLIGATION
What are the key principles when doing allegations?
one is greater than the desired concentration and one is less than
use alligation grids to help work out proportions then amounts
Alligation grid
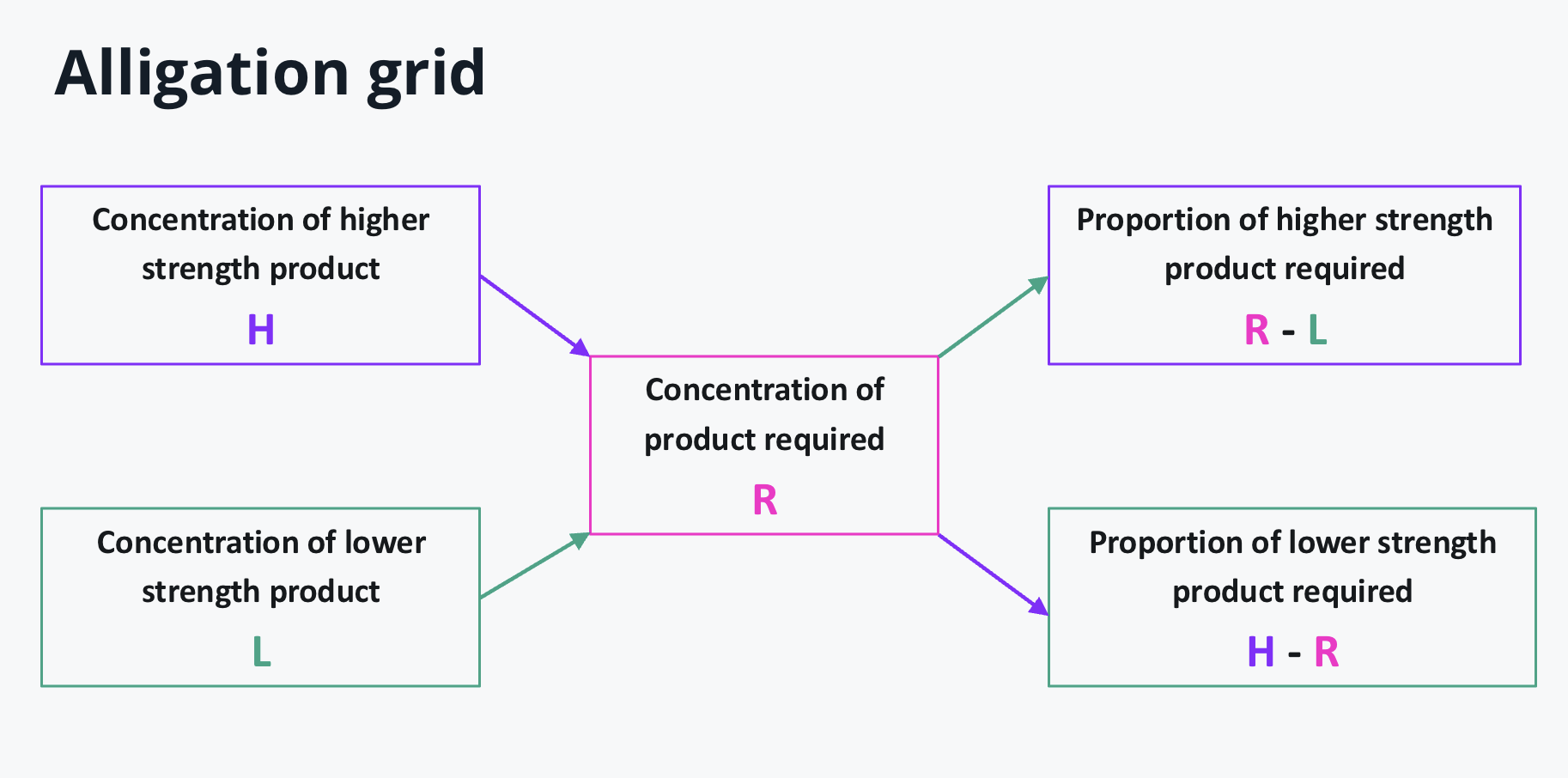
calculation using alligation grid- general. method
1- work out proportions of higher
2- work out proportions of lower
3- apply how much does each part weigh
4- work out each weight using their proportion/parts
Example calculation - alligation grid
You re asked to produce 150g of a 2% cream using stock creams of 0.5% and 4% .How much of each stock cream will you require?
2-0.5= 1.5 parts
4-2 = 2 parts
ratio of higher to lower 1.5 to 2
150 for 3.5 parts , 1 part = 42.857 g
42.8× 1.5= 64.3 g , 42.8 × 2= 85.7 g