Acid-base Reactions
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
What is a Brønsted-Lowry acid?
A substance that donates a proton (H⁺) in a reaction.
What is a Brønsted-Lowry base?
A substance that accepts a proton (H⁺) in a reaction.
Give an example of a Brønsted-Lowry acid.
HCl → H⁺ + Cl⁻ (HCl donates a proton)
Give an example of a Brønsted-Lowry base.
NH₃ + H⁺ → NH₄⁺ (NH₃ accepts a proton)
What is chemical equilibrium?
The state in which the forward and reverse reaction rates are equal, so reactant and product concentrations remain constant over time.
What is the Law of Mass Action?
It states that the rate of a reaction is proportional to the concentration of the reactants, each raised to the power of their coefficients in the balanced equation.
What is the equilibrium constant (K)?
ratio that expresses the relationship between reactant and product concentrations at equilibrium:
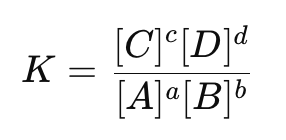
What happens in acid-base reactions in water?
Acids donate H⁺ ions, and bases accept H⁺ ions. The reaction often forms H₃O⁺ (hydronium) and OH⁻ (hydroxide)ions.
Example:
HCl+H2O⇌H3O++Cl−
Why do acid-base reactions form an equilibrium?
Weak acids and weak bases do not fully ionize, leading to a reversible reaction where both reactants and products coexist in solution.
Example:
CH3COOH+H2O⇌CH3COO−+H3O+
What does it mean that equilibria are dynamic?
At equilibrium, the reactions continue to occur, but at the same rate in both directions, so no net change is observed.
What is the velocity equation for a forward reaction?
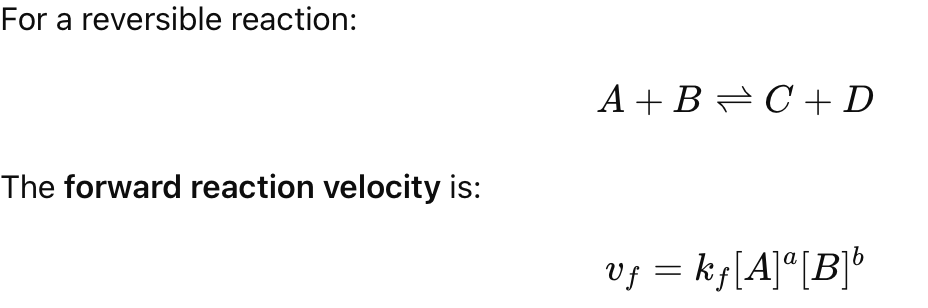
What is the velocity equation for a reverse reaction?
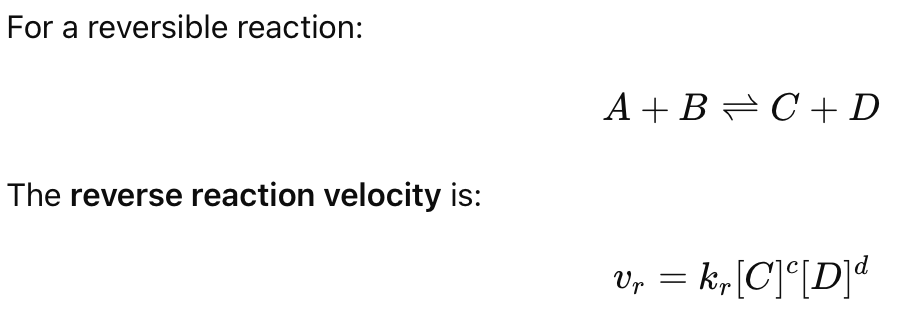
What happens at equilibrium in terms of velocity?
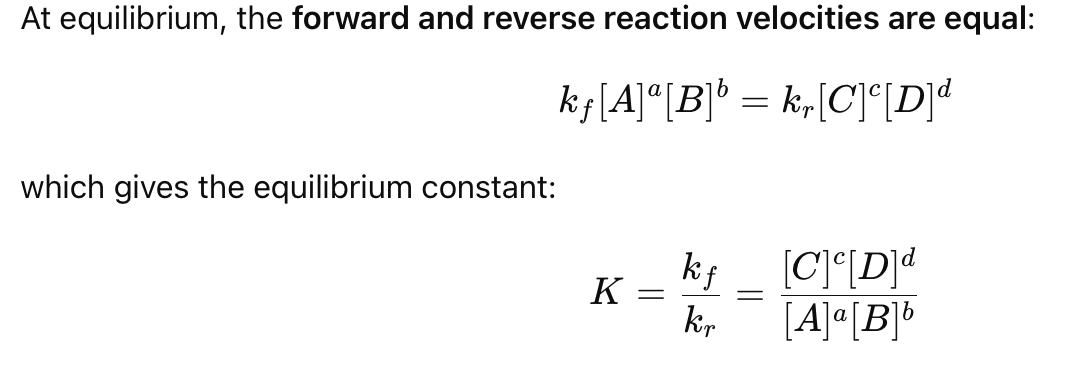
What does reaction velocity depend on?
Velocity depends on concentrations—higher reactant concentration increases reaction speed.
Do both forward and reverse reactions have velocity?
yes! Both the forward and backward reactions occur simultaneously, each with its own rate.
What happens to reaction speeds at equilibrium?
At equilibrium, the forward and reverse reaction speeds are equal, so no net change occurs in reactant and product concentrations.
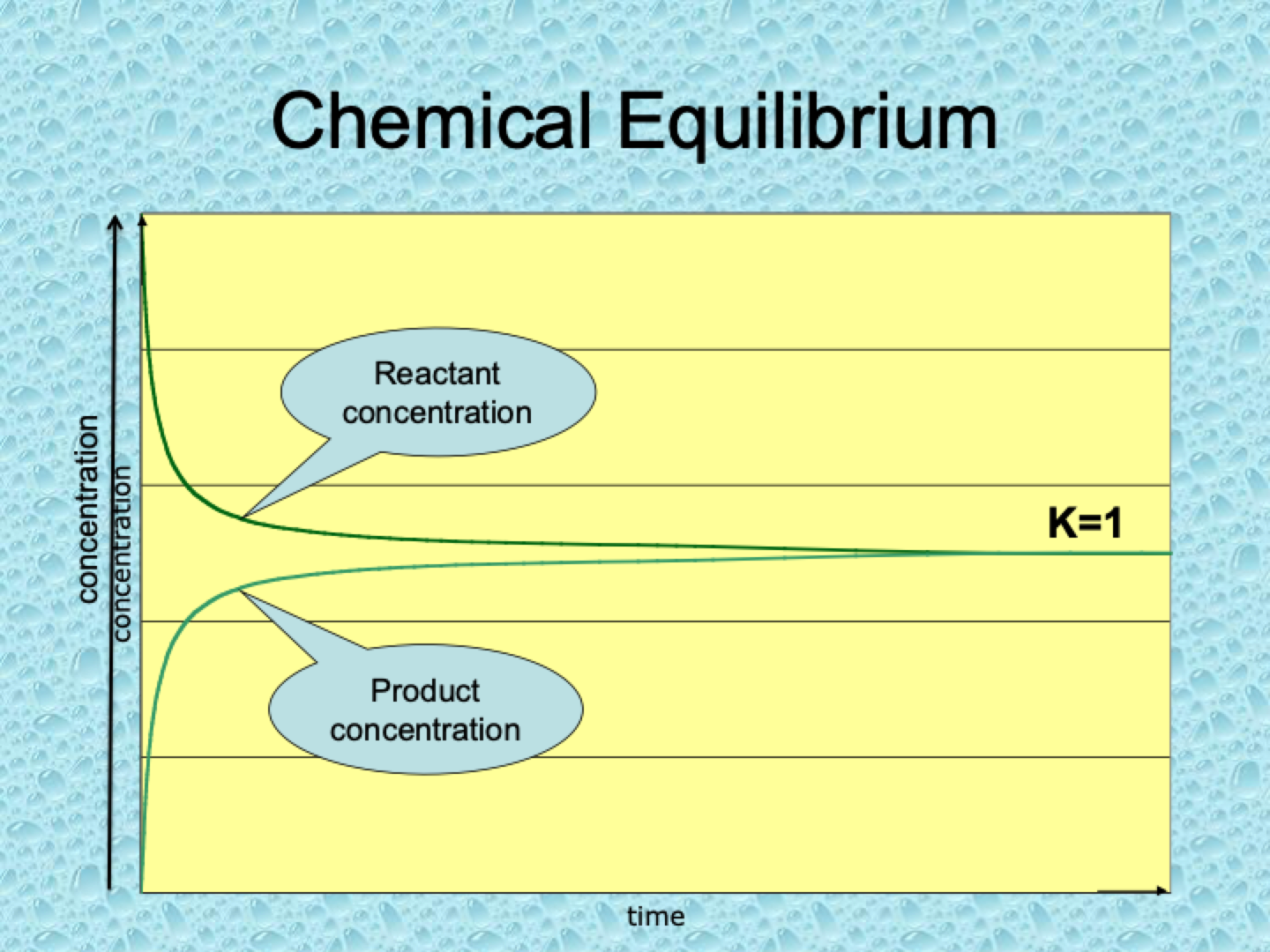
What does it mean if K=1?
When K=1, the concentrations of reactants and products are roughly equal at equilibrium. Neither side is strongly favored.
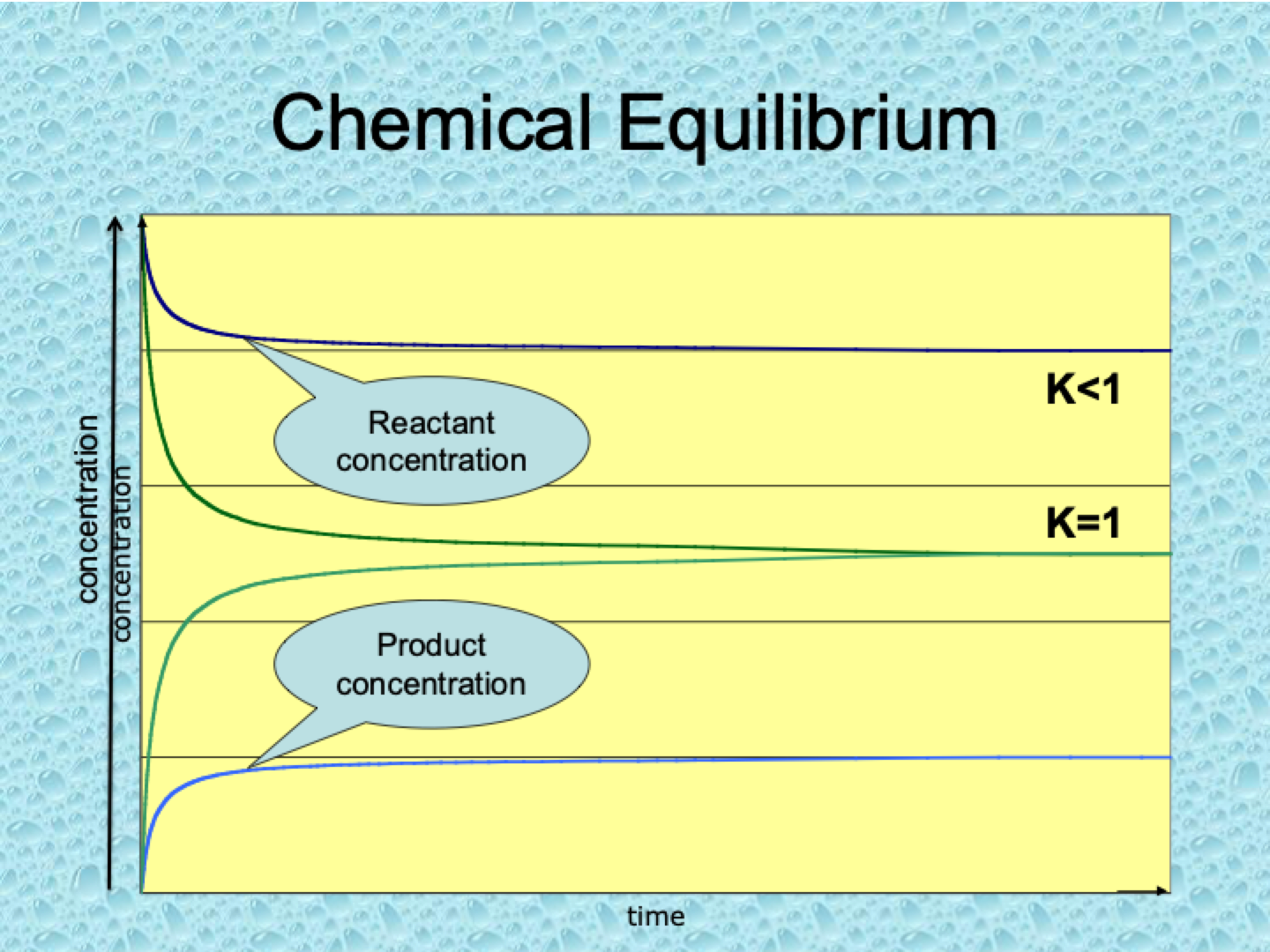
What does it mean if K<1?
When K<1, the reaction favors the reactants at equilibrium, meaning there are more reactants than products in the system. The forward reaction is not very extensive.
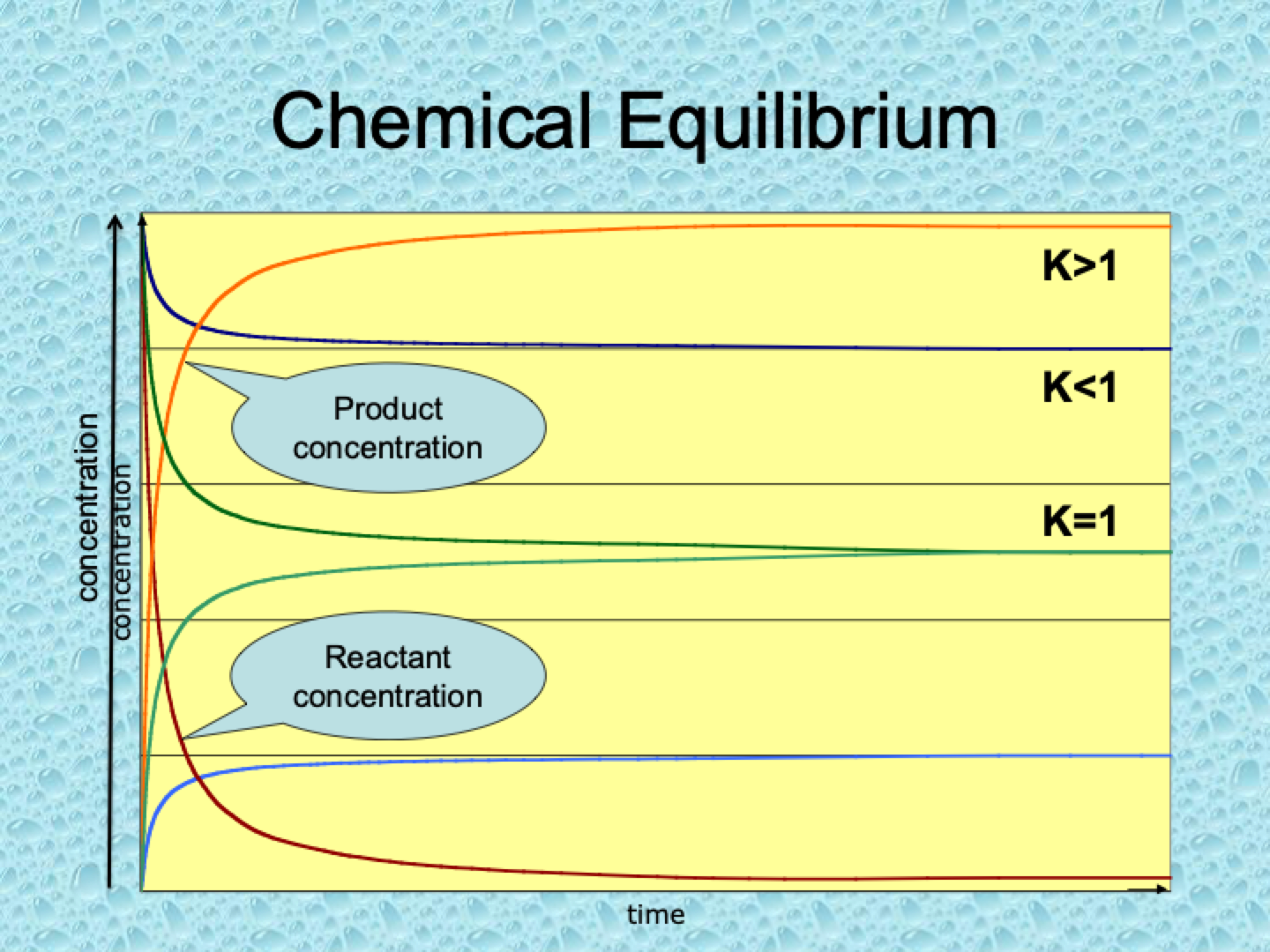
What does it mean if K>1?
When K>1, the reaction favors the products at equilibrium, meaning there are more products than reactants in the system. The forward reaction is more extensive.
What does Le Chatelier’s Principle state?
It states that if a system at equilibrium is disturbed by changing the concentration, temperature, or pressure, the system will shift to counteract the disturbance and restore equilibrium.
What happens when the concentration of a reactant is increased in a system at equilibrium?
What happens when the concentration of a product is increased in a system at equilibrium?
The system will shift toward the reactants to consume the excess product and restore equilibrium.
How does an increase in temperature affect an exothermic reaction at equilibrium?
The system will shift to the left (toward reactants) to absorb the excess heat and restore equilibrium.
How does an increase in temperature affect an endothermic reaction at equilibrium?
The system will shift to the right (toward products) to absorb the added heat and restore equilibrium.
What happens when pressure is increased in a system with gases?
The system will shift toward the side with fewer moles of gas to decrease the pressure.
What happens when pressure is decreased in a system with gases?
The system will shift toward the side with more moles of gas to increase the pressure.
Why is a proton acceptor necessary in an acid-base reaction?
An acid must donate a proton (H⁺), but for the reaction to occur, a base must be present to accept it.
What happens when hydrochloric acid (HCl) dissolves in water?
HCl donates a proton (H⁺) to water, forming Cl⁻ (chloride ion) and H₃O⁺ (hydronium ion).
What happens when ethanoic acid (CH₃COOH) dissolves in water?
Ethanoic acid donates a proton (H⁺) to water, forming CH₃COO⁻ (acetate ion) and H₃O⁺ (hydronium ion).
What is a conjugate acid-base pair?
A conjugate acid-base pair consists of two species that differ by one proton (H⁺)—an acid and its conjugate base or a base and its conjugate acid.
What happens when an acid donates a proton?
It forms its conjugate base (the species left after losing H⁺).
What happens when a base accepts a proton?
It forms its conjugate acid (the species formed after gaining H⁺).
What is the conjugate base of HCl?
Cl⁻ (chloride ion)
What is the conjugate acid of NH₃ (ammonia)?
NH₄⁺ (ammonium ion)
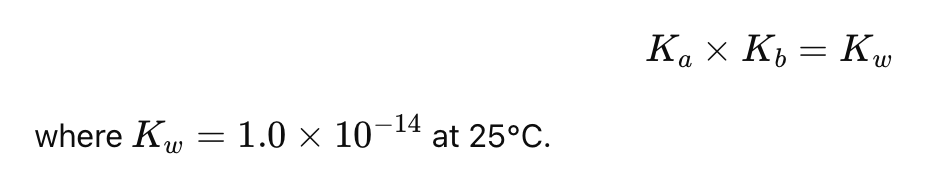
What is the relationship between Ka and Kb for a conjugate acid-base pair?
What is the acid ionization constant (Ka) formula?
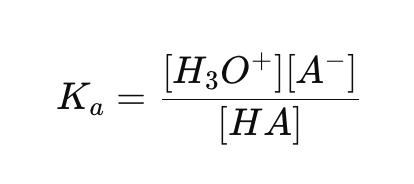
What is the base ionization constant (Kb) formula?
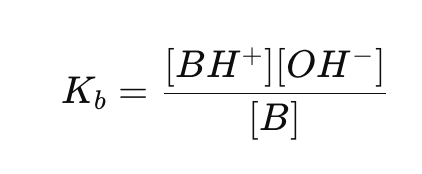
Why is [H₂O] not included in Ka and Kb expressions?
Because water’s concentration is very large (55.56 M) and remains nearly constant.
What is the self-ionization reaction of water?
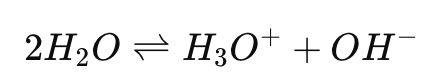
What is the ion product constant for water (Kw)?
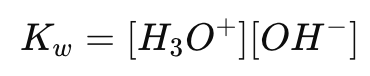
What is the numerical value of Kw at 298K (25°C)?
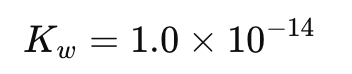
Why does the equilibrium favor the left side in water ionization?
Because water only ionizes slightly, meaning very few ions are present in pure water.
What is the definition of pKw?
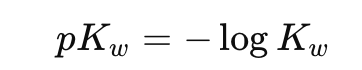
What is the value of pKw at 25°C?
pKw=14.00
How are Kw, pH, and pOH related?
pH+pOH=pKw=14.00
What is the key characteristic of a strong acid/base?
It completely ionizes/dissociates in water, meaning the reaction proceeds almost entirely to the right.
What is the general rule for Ka and Kb for strong acids and bases?
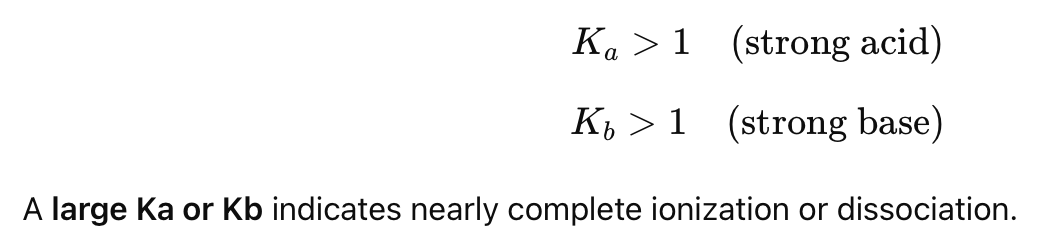
What is the key characteristic of a weak acid/base?
It partially ionizes/dissociates in water, meaning the reaction is incomplete and does not go fully to the right.
What is the general rule for Ka and Kb for weak acids and bases?
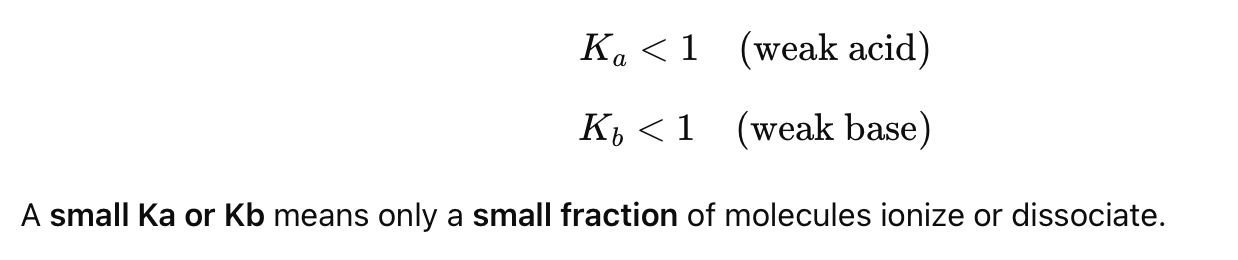
What is the relationship between the strength of a conjugated acid and its conjugate base?
The stronger the acid, the weaker its conjugate base. Conversely, the weaker the acid, the stronger its conjugate base.
What does a low pKa value indicate about an acid?
A low pKa value indicates a strong acid. The lower the pKa, the stronger the acid, and thus the weaker its conjugate base.
What determines the strength of a conjugate base?
The strength of a conjugate base depends on how easily it can accept a proton. A weaker acid has a stronger conjugate base because the conjugate base is more likely to accept a proton.
Table of strength of acid and bases
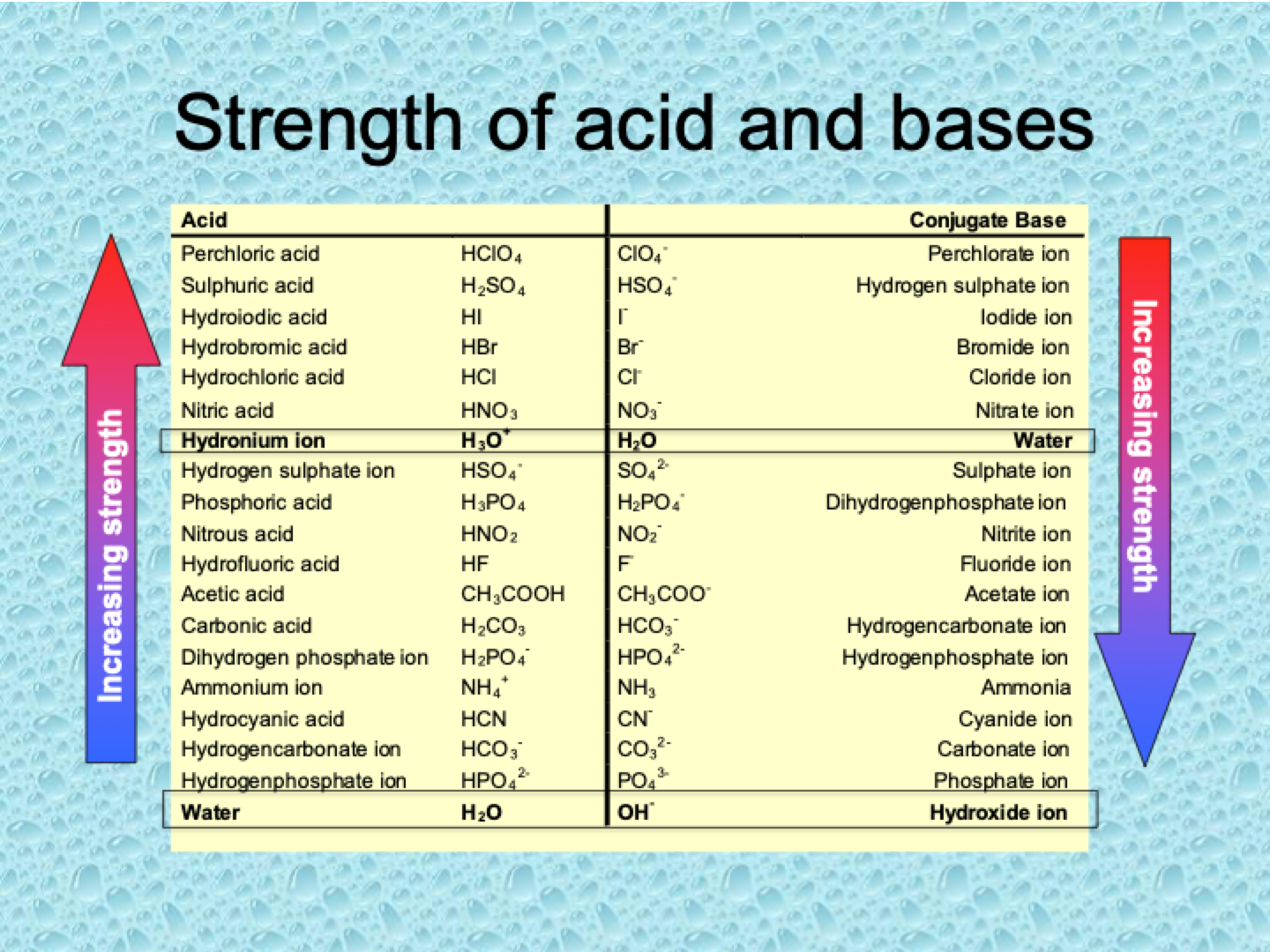
What is the relationship between Ka and Kb for a conjugate acid-base pair?
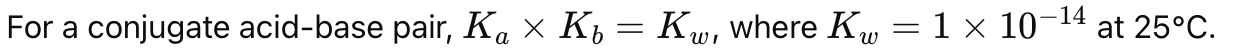
How are pKa and pKb related for a conjugate acid-base pair?
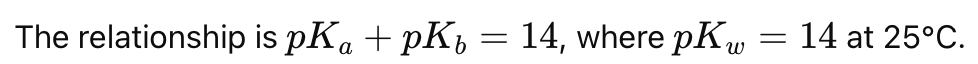
What happens if Ka or Kb is very small (close to KwKw)?
The species has no significant effect on pH.
Examples: Na⁺, Cl⁻ (spectator ions).
What does c0 represent in acid-base calculations?
c0 is the nominal or bulk concentration of the acid or base.
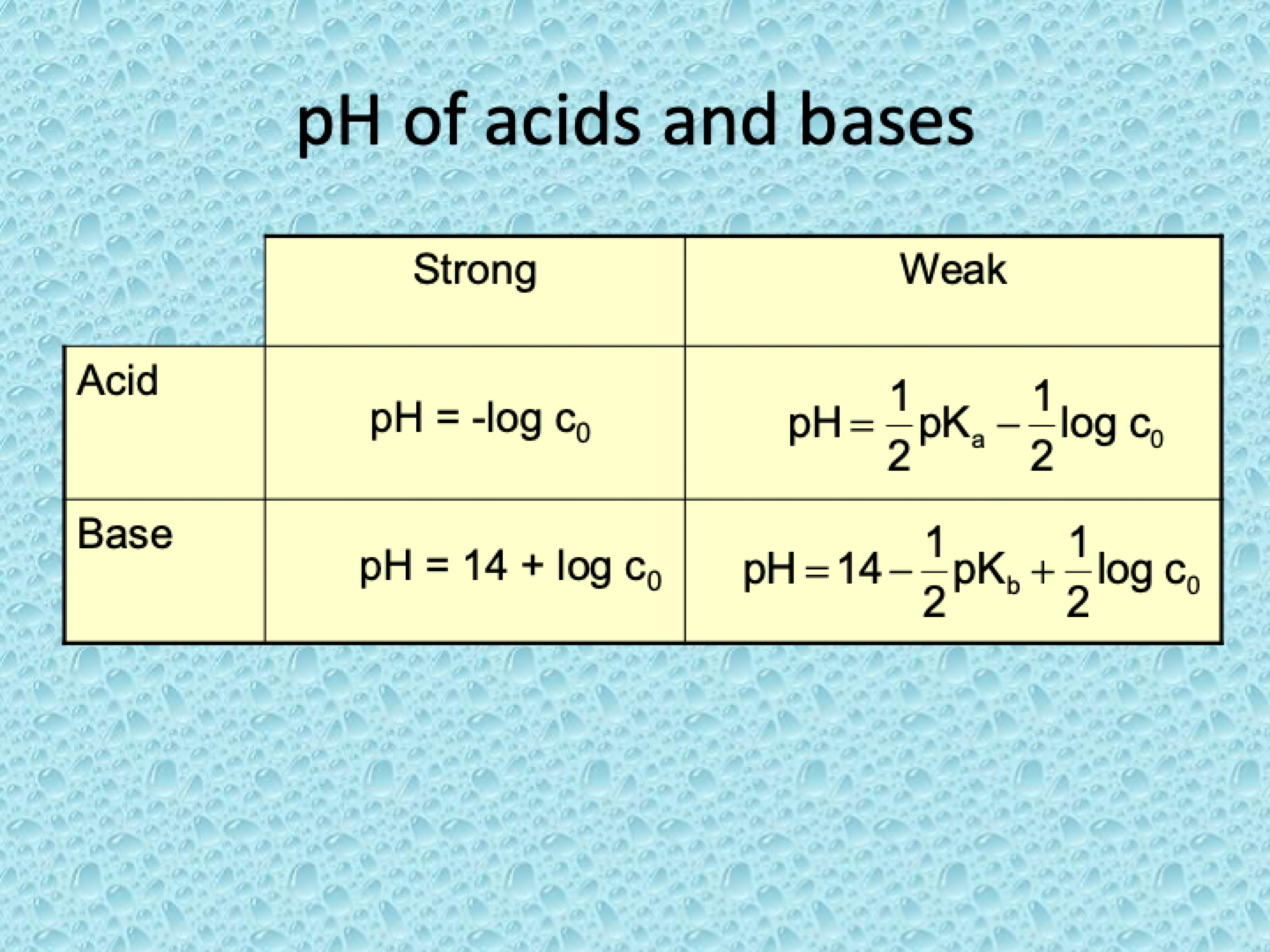
What is the relationship between the concentration of a strong acid and hydronium ion concentration?

How do you calculate the pH of a strong acid?
pH=−log10[H3O+]=−log10c0.
What is the relationship between the concentration of a strong base and hydroxide ion concentration?
Since strong bases fully dissociate, [OH−]=[OH−]0=c0
How do you calculate the pOH of a strong base?
pOH=−log10[OH−]=−log10c0.
How do you find the pH of a strong base?
pH=14−pOH=14+logc0.
What is the mass action law expression for a weak acid (HA)?
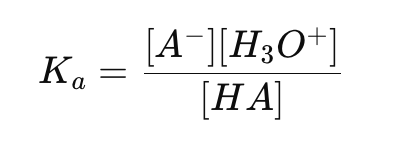
How do you calculate [H3O+] using the mass action law for a weak acid?
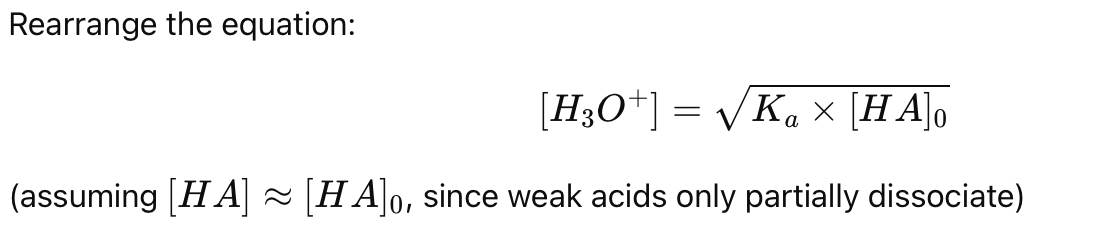
How do you calculate the pH of a weak acid using the mass action law?
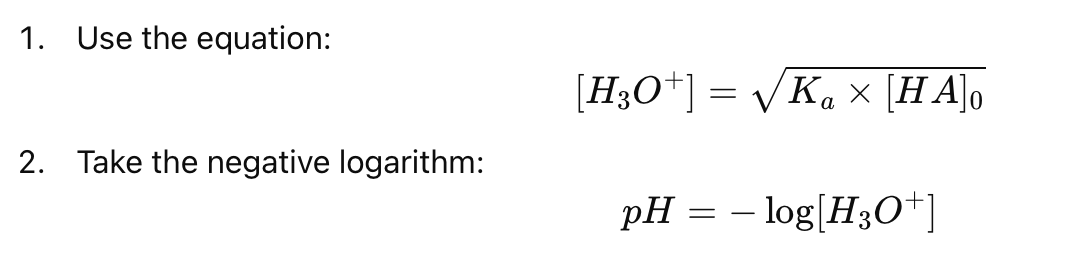
Why can we approximate the equilibrium concentration of a weak acid as nearly equal to its initial concentration?
Weak acids only partially dissociate in water.
Since the dissociation is small, the change in the initial concentration of the acid is negligible.
This allows us to approximate the equilibrium concentration of the acid as nearly equal to its initial concentration
What is the equation for the acid dissociation constant (Ka) in terms of [H3O+] and c0?
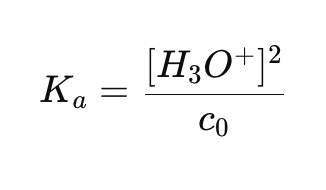
How do you calculate [H3O+] for a weak acid?
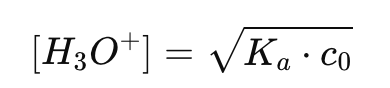
What logarithmic property is used in weak acid calculations?
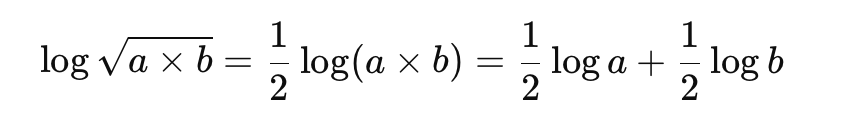
What is the logarithmic form of the [H3O+] equation?
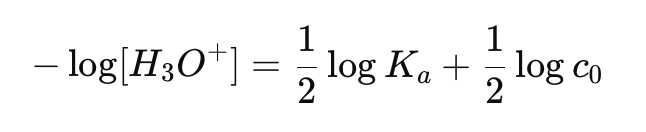
What is the equation for pH of a weak acid?
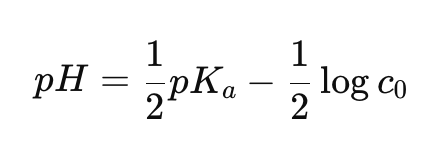
How do you calculate the pH of a weak conjugate acid using pKb?
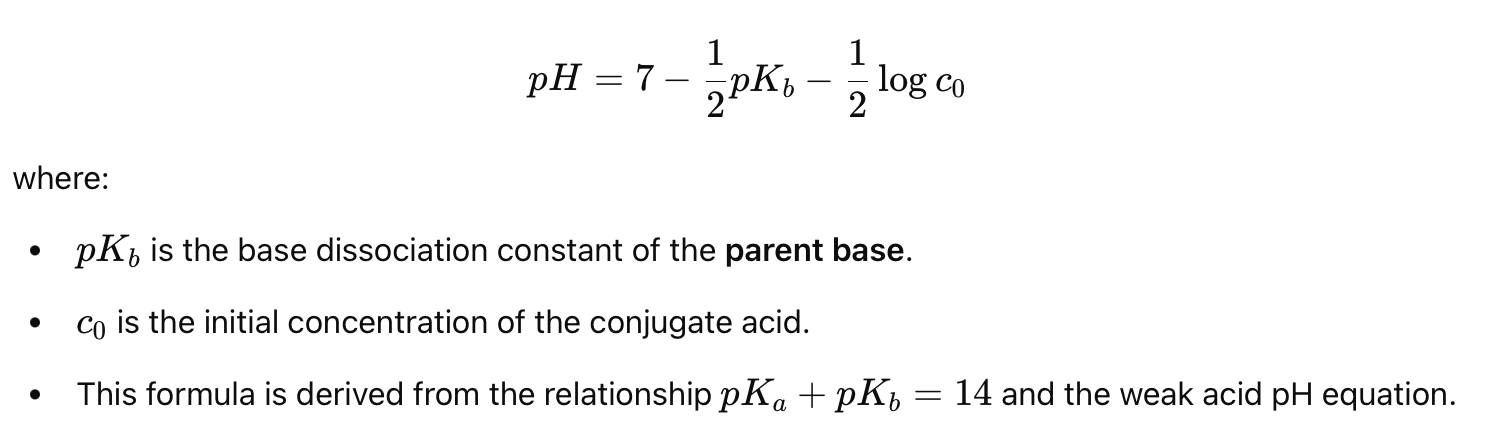
What is the general reaction for a weak base in water?
Weak Base (B) + H₂O ⇌ Conjugate Acid (BH⁺) + OH⁻
What is the formula for the base dissociation constant (Kb)?
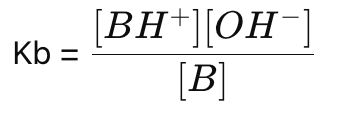
How do you simplify Kb for weak base solutions?
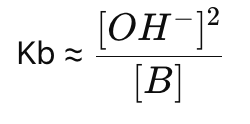
How do you calculate the concentration of OH⁻ in a weak base solution?

How do you calculate pOH for a weak base solution?
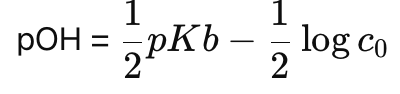
How do you calculate pH for a weak base solution?
pH = ½ pKa - ½ log c0
How do you calculate pH from pOH?
pH = 14 - pOH
How do you calculate pH from pKa and concentration?
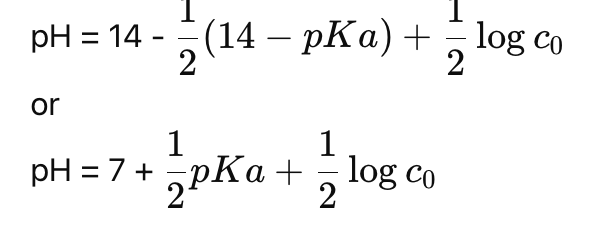
How do you calculate the pH of a weak conjugate base?

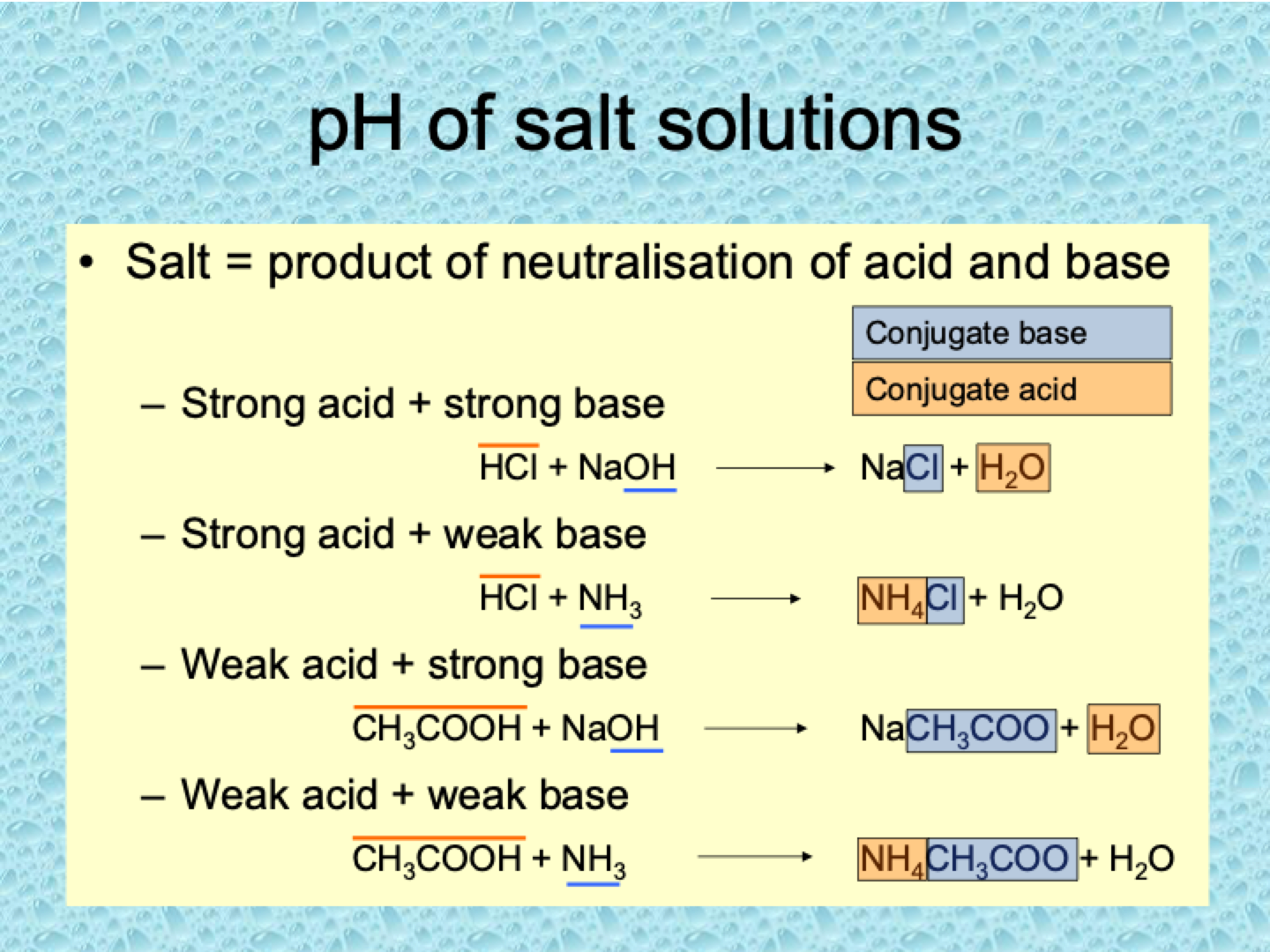
What is the pH of salt solutions?
Salt Formation: A salt is formed from the neutralization of an acid and a base.
Equivalence: A neutralized salt solution contains equivalent amounts of the acid and base used in the reaction.
pH of Salt Solutions: The pH of a salt solution is not always neutral. It depends on the strength of the acid and base that formed the salt.
What are ampholytes and how do their pH values behave?
Ampholytes: Compounds that can act as both acids and bases.
Examples:Salts of Weak Acids and Weak Bases: e.g., ammonium acetate, ammonium bicarbonate.
Hydrogen Salts of Di- or Triprotic Acids: e.g., sodium dihydrogen phosphate.
Compounds with Both Acid and Base Functional Groups: e.g., amino acids, which have both carboxyl (acidic) and amino (basic) groups.
pH Formula for Ampholytes:
pH = pKa1+pKa2/ 2
This applies to compounds with both acidic and basic functional groups, like amino acids.pH Behavior:
The pH of solutions containing ampholytes is independent of concentration and tends to be stable based on the pKa values of the acidic and basic functional groups.
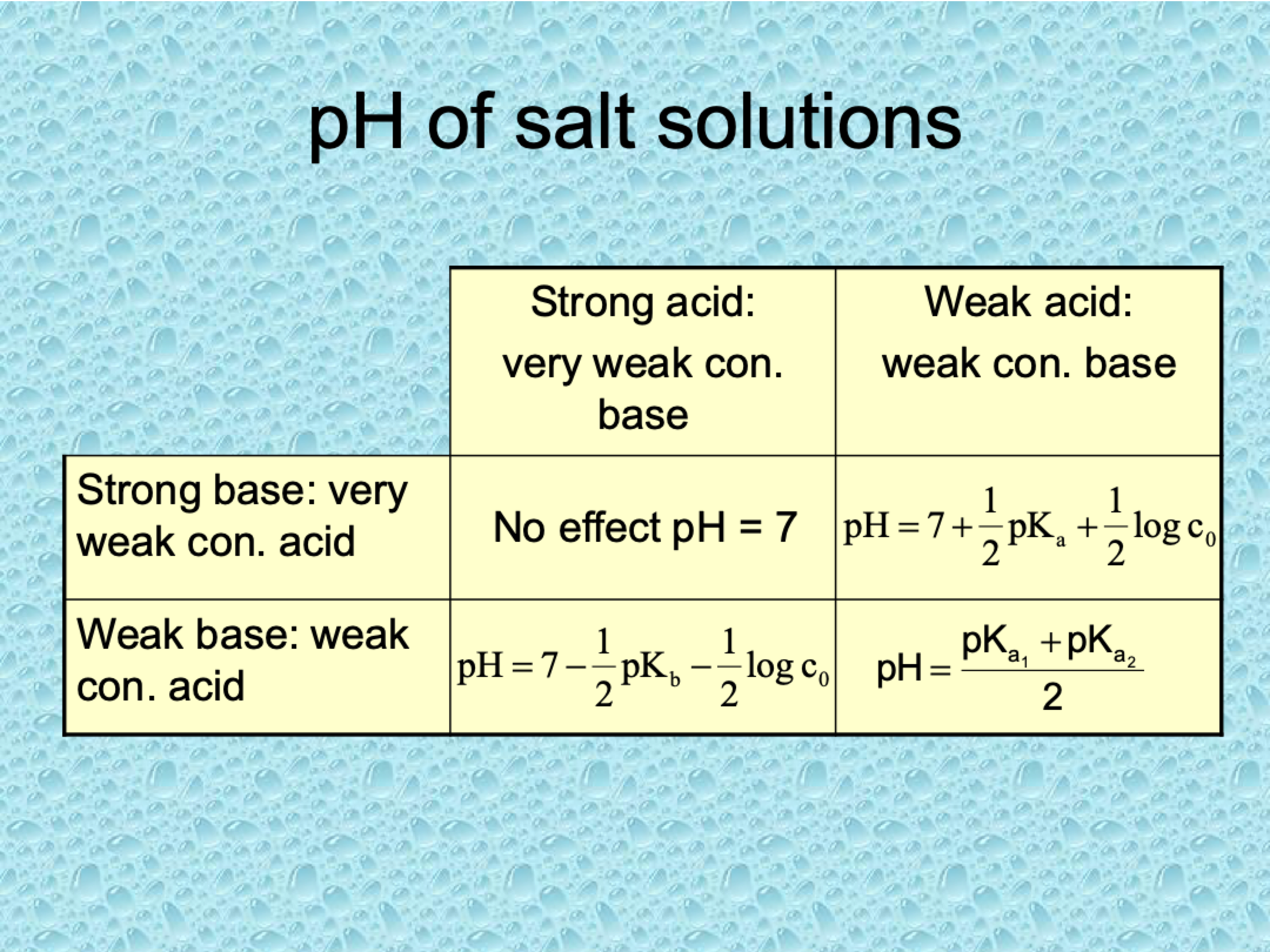
What are the four cases of pH for salt solutions?
pH equations of salt solutions
pH of acids and bases equations
What are buffers and examples of buffer solutions?
Definition: A buffer is a solution of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists pH changes.
Examples:
Acetic acid + Sodium acetate
Ammonia + Ammonium chloride
Solutions of ampholytes
NH₄CH₃COO (Ammonium acetate)
NaH₂PO₄ (Sodium dihydrogen phosphate)
What is buffer action?
Buffer action is the ability of a buffer solution to resist changes in pH when small amounts of acid (H⁺) or base (OH⁻) are added.
How does buffer action work?
When Acid (H⁺) is Added:
The conjugate base (A⁻) reacts with H⁺ to form the weak acid (HA), preventing a decrease in pH.
A⁻ + H⁺ ⇌ HAWhen Base (OH⁻) is Added:
The weak acid (HA) reacts with OH⁻ to form the conjugate base (A⁻) and water, preventing an increase in pH.
HA + OH⁻ ⇌ A⁻ + H₂O
What is buffering capacity?
Buffering Capacity refers to the ability of a buffer solution to neutralize added acid or base without significantly changing its pH.
It depends on:
Concentration of the buffer components (weak acid and its conjugate base).
The ratio of the acid and conjugate base concentrations.
Higher buffering capacity: Occurs when the concentrations of the acid and base are high and similar.
Effective range: Buffering capacity is strongest when the pH is close to the pKa of the weak acid/base pair.
How do you calculate the pH of a buffer solution?
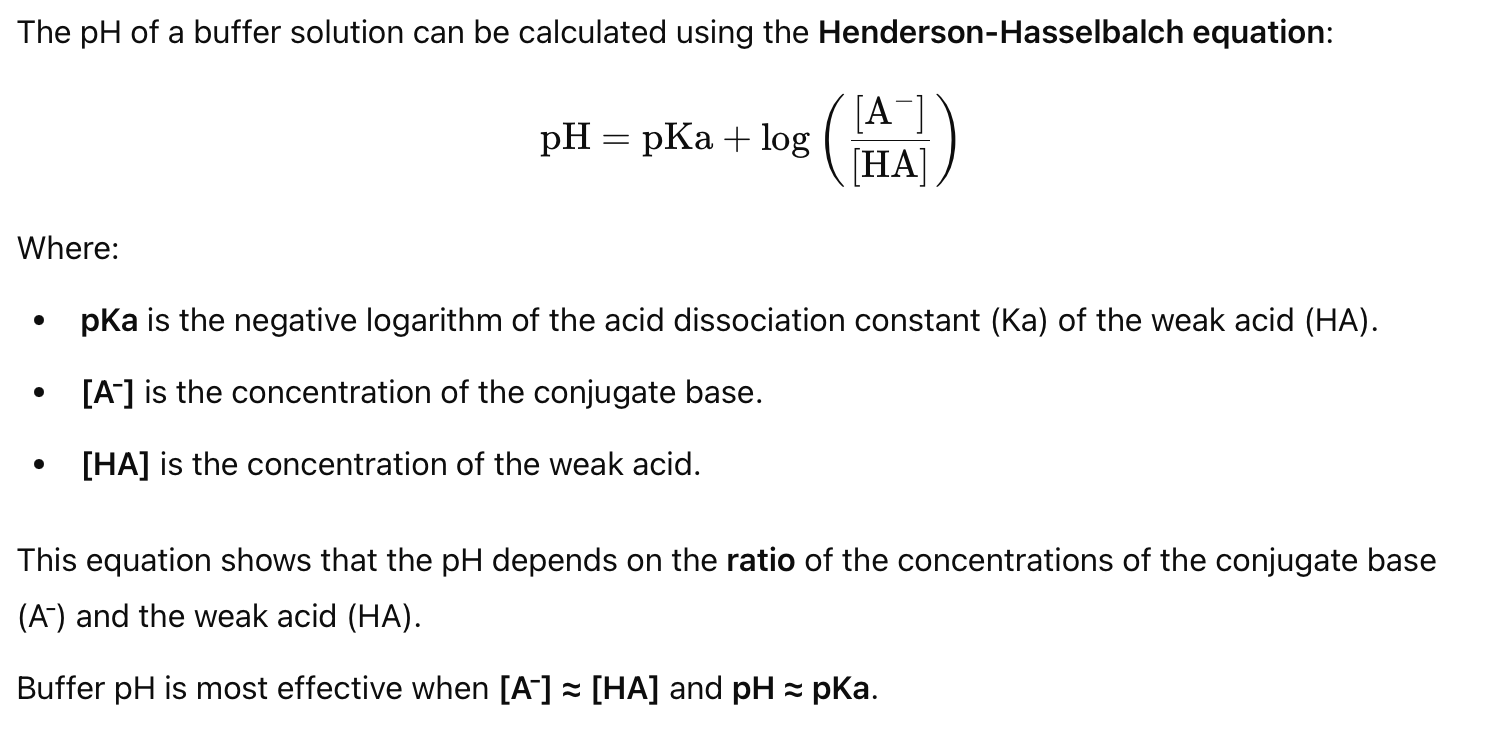
What are the key concepts regarding pH changes in buffers?
1 pH Change = 10-Fold Shift:
A change of 1 pH unit represents a 10-fold change in the concentration of H⁺ or OH⁻ ions.Buffer Curve:
The Henderson-Hasselbalch curve is the same for every weak acid/base but is shifted by the value of the pKa of the acid/base.Limitations:
The equation is not suitable for calculating the pH when there is 100% acid (i.e., [A⁻] = 0) or 100% base (i.e., [HA] = 0), because the buffer system doesn't work in these extreme cases.
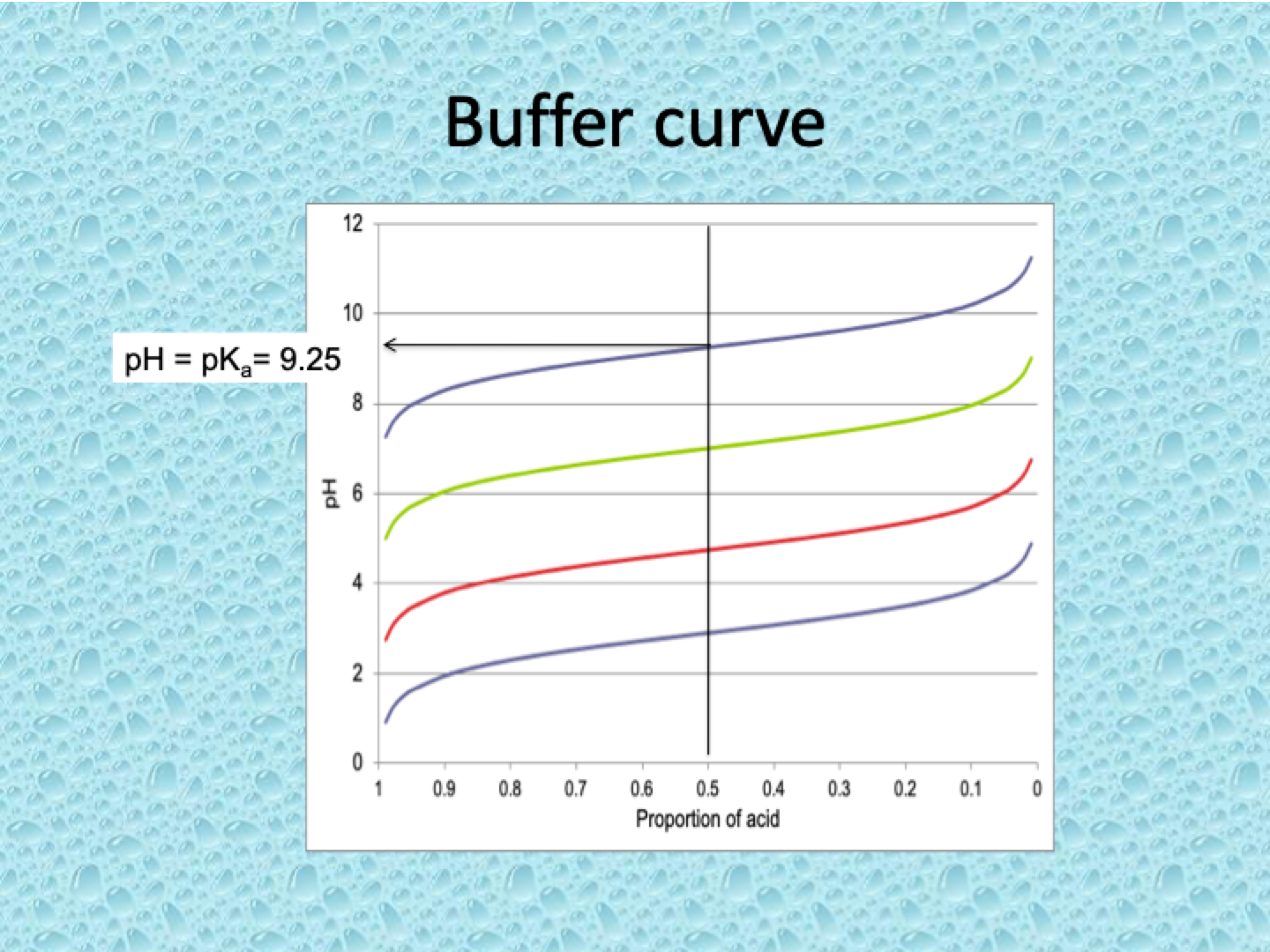
What is the buffer curve and how does it behave?
The pH vs. [A⁻]/[HA] ratio curve shows how the pH of a buffer solution changes as the ratio of the conjugate base ([A⁻]) to the weak acid ([HA]) changes.
Key Features:
Flat region: The buffer resists changes in pH when small amounts of acid or base are added. This is most effective when [A⁻] ≈ [HA].
pH near pKa: The pH of the buffer is closest to the pKa of the weak acid/base pair.
Sharp rise: When the ratio of [A⁻] to [HA] becomes very large or very small, the buffer's capacity diminishes, leading to sharp pH changes.
Shifted by pKa:
The curve for any weak acid/base will have the same shape but is shifted by the value of the pKa of the acid/base.
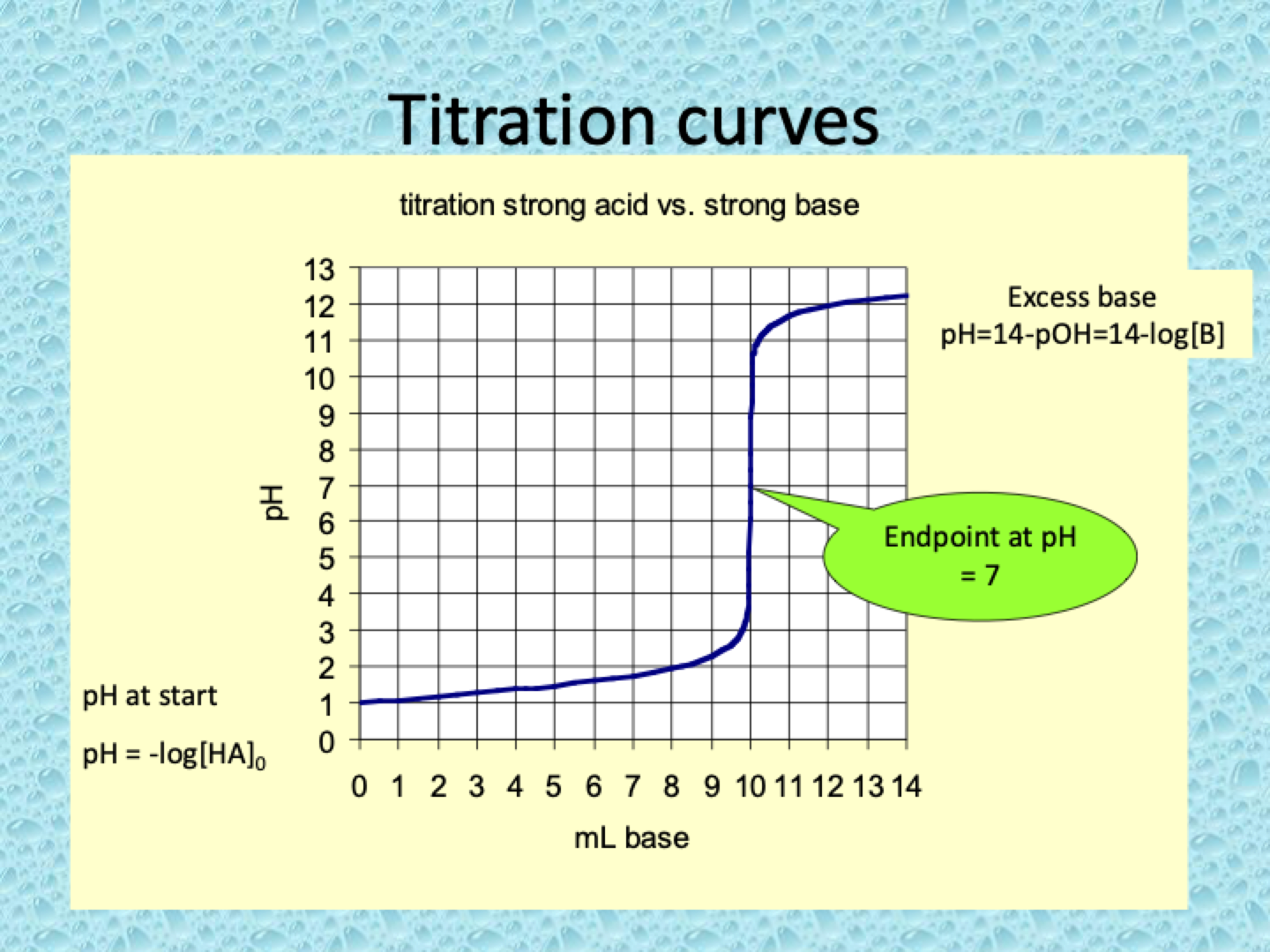
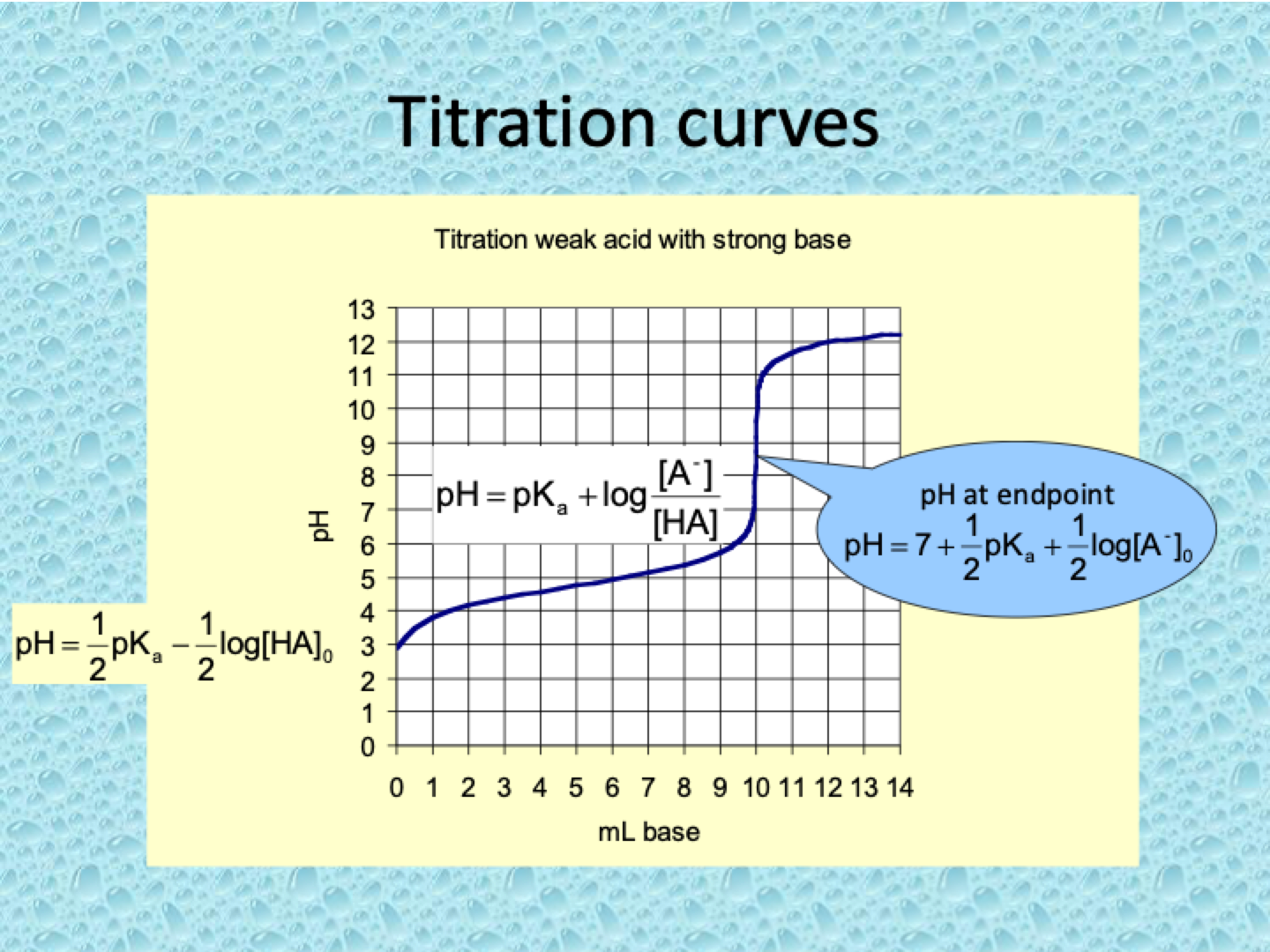
What are titration curves?
A titration curve shows how the pH of a solution changes as a titrant (acid or base) is gradually added during a titration.
Strong Acid - Strong Base Titration:
pH starts low (acidic) and increases sharply near the equivalence point, where pH rapidly rises to around 7 (neutral).
Weak Acid - Strong Base Titration:
Initial pH is higher (more acidic than strong acid), but pH rises more gradually.
Equivalence point: pH > 7 (basic).
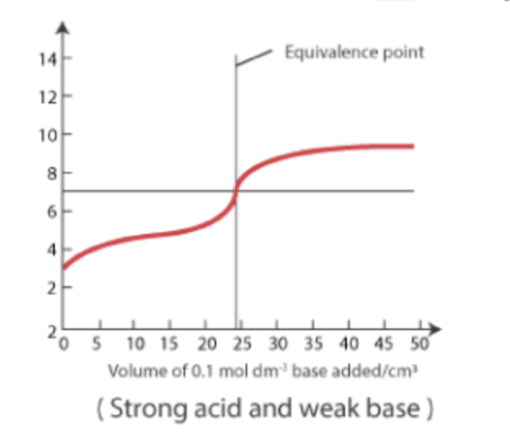
Strong Acid - Weak Base Titration:
Initial pH is very low, and the curve increases gradually but still remains below 7 at equivalence (acidic).
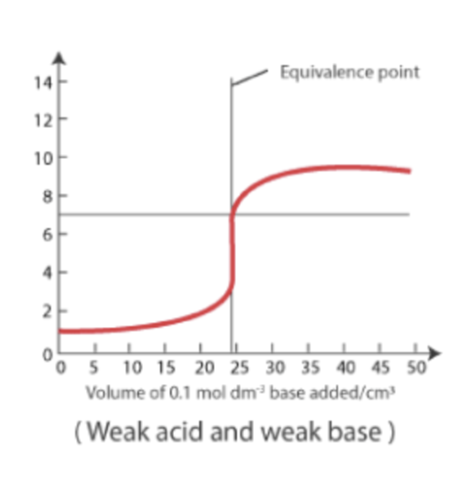
Weak Acid - Weak Base Titration:
Starts with a pH that’s higher than strong acid and lower than a strong base titration.
The curve shows a gradual increase, and the equivalence point pH is near 7.
What is the equivalence point in a titration?
The equivalence point occurs when the moles of acid equal the moles of base in a titration.
At this point, all of the acid or base has been neutralized.
What is the end point in a titration?
The end point is when the indicator changes color, signaling that the titration is complete.
It should be as close as possible to the equivalence point, but they are not always identical.