MCAT Chemistry: Electrochemistry and nuclear chemistry
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
reduction
gaining electrons by forming bonds to less electronegative atoms (hydrogen) or losing bonds to more electronegative atoms (oxygen)
oxidation
loosing electrons by losing bonds to less electronegative atoms (hydrogen) and gainging bonds to more electronegative atoms (oxygen)
the oxidation state of any element in its standard state is
0
The sum of the oxidation states of the atoms in a molecule or ion
must equal the overall charge
oxidation state of group 1 metals
+1
oxidation state of group 2 metals
+2
oxidation state of fluorine
-1
oxidation state of hydrogen when bonded to a more electronegative atom
+1
oxidation state of hydrogen when bonded to a less electronegative atom
-1
except for peroxides, oxygen has an oxidation state of
-2
oxidation state of halogens
-1
oxygen family oxidation state
-2
reduction potentials
is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. In a reduction table, the given potential is for the reduction of the reactants
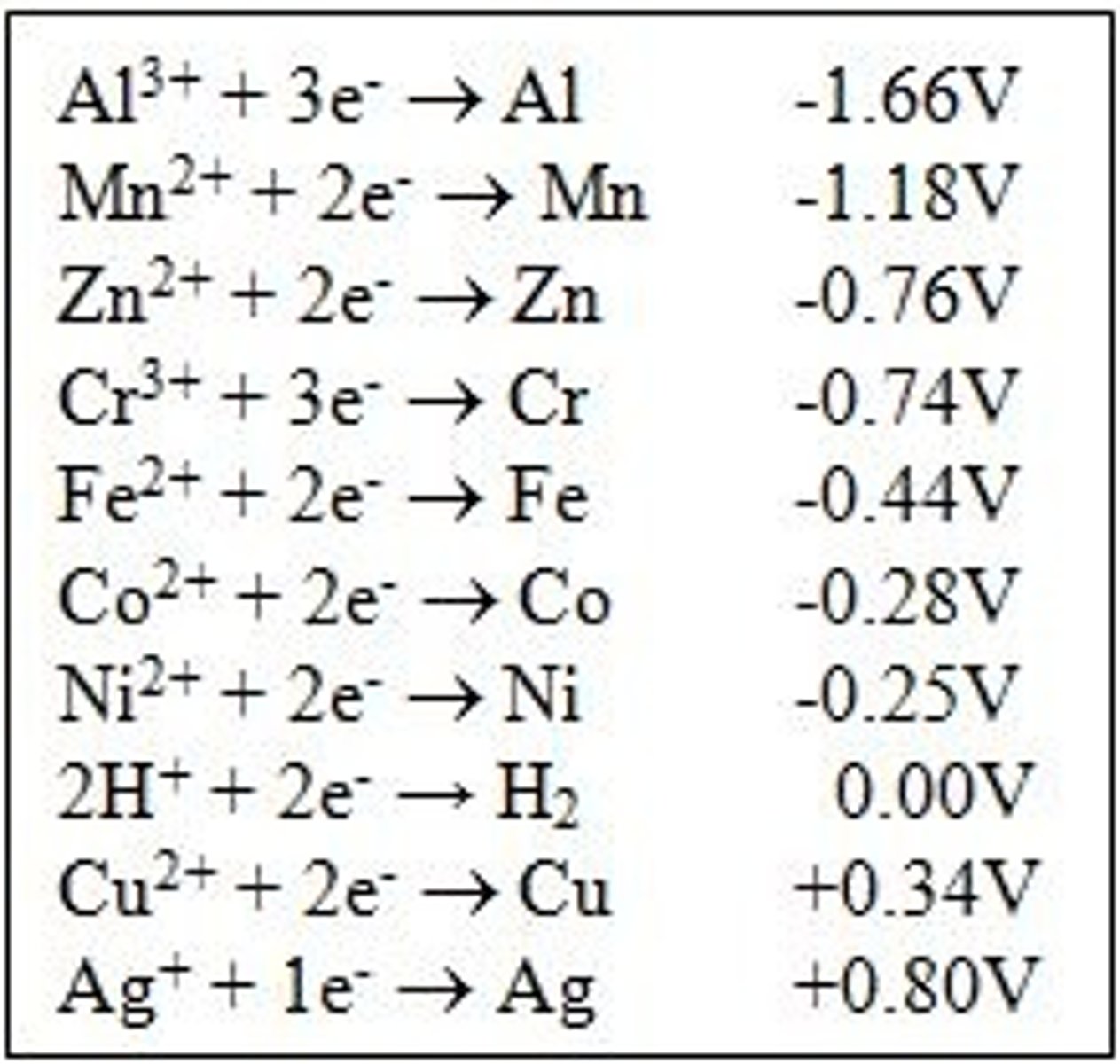
oxidation potentials
measure of the tendency of a chemical to lose electrons and be oxidized. In a reduction table, the products are being reduced and the oxidation potential is the negative of the reduction potential
Nonspontaneous reduction potentials are
negative
spontaneous reduction potentials are
positive
reducing agent
a compound that causes others to be reduced or gain electrons.
examples of reducing agents
H2, neutral metals, hydride reducing agents (LiAlH4, NaBH4)
examples of oxidizing agents
neutral nonmetals, oxides (MnO4-, CrO3)
strongest oxidizing agent
largest standard reduction potential
strongest reducing agent
most negative reduction potential
cell potential is not affected by
coefficients of the reaction
electrochemical cell notation
anode | electrolyte || electrolyte | cathode
cell potential is inversely proportional to
free energy
nerst equation
Delta G= -nFEcell
F = 96,500 C/mol
over time a quantity of electrons can be used to do work like
electroplating: coating an object with metal
all electrochemical cells consist of:
two conductive electrodes, an electrolyte bridging between the electrodes, a circuit to connect the electrodes (can have a resistor or power source)
galvanic or voltaic cell
part of a complete circuit with no external power source due to a positive cell potential. occurs when discharging a battery
electrolytic cell
part of a complete circuit with an external power source due to negative cell potential. occurs when recharging a battery
electroplating
An electrolytic process in which a metal ion is reduced and a solid metal is deposited on the surface of the cathode during battery discharge
Pitting
carrodes anode during discharging a battery
cathode in discharging battery
is reduced and is positive side of battery
Anode in a discharge battery
is oxidized and is the negative side of the battery
flow of electrons in a battery
anode to cathode ALWAYS; opposite current flow
anode in recharging battery
It is oxidized and is the positive side of the battery
cathode in recharging battery
is reduced and is the negative side of the battery
in an aqeous solution in an electrolytic cell, the cathode produces
H2
In an aqueous solution in an electrolytic cell, the anode produces
O2
rechargable batteries
car batteries/lead acid batteries
a cell in equilibrium has an actual cell potential equal to
0
actual cell potential equation
E = Estandard - (RT/nf)lnQ
n= number of electrons transferred
standard cell potential equation
Estandard = (RT/nF)lnK
n = number of electrons transferred
cerimetry
A redox titration method using ceric sulfate as the titrant.
half equivalence point of redox titration
Estandard of analyte (not titrant); analyte concentrations of reduced and oxidized form are in equilibrium
equivalence point of redox titration
occurs when all of the moles of reducing agent in the solution have been completely oxidized; actual cell potential
2x equivalence point of redox titration
E standard of the titrant; titrate concentration of two forms are in equilibrium
iodometry
a redox titration where the consumption of iodine indicates the endpoint
alpha decay
atom emits an alpha particle consisting of two protons and two neutrons. This decreases the atomic number by 2 and the mass number by 4. seen in atoms with large nuclei. least dangerous type of ionizing radiation
beta decay
An atom emits a beta particle consisting of -1 proton. This increases the atomic number by 1, seen in nuclei with high neutron/proton ratios. more dangerous
positron emission
An atom emits a positron consisting of one proton. This decreases the atomic number by 1, seen in nuclei with high proton/neutron ratios
electron capture
An inner orbital electron is captured by the nucleus of its own atom, causing a decrease in atomic number by 1. This occurs in nuclei with a high proton/neutron ratio
gamma decay
Nuclear decay that involves the release of gamma rays doesn't change the identity of the atom. accompanies nuclear reactions. most dangerous and most penetrable
half life
time it takes for a substance to decay to half of its original amount
to be considered safe, a radioactive species must
pass 10 half lives
nuclear reactions are always
exothermic
energy of decay equation
delta E = (bindng energy of parent) - (binding energy of daughter)
binding energy
The energy that holds the nucleus together; proportional to mass of the nucleus