MOD2 Leukocytes
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
leukopoiesis
production of leukocytes WBC
where are leukocytes produced
partially in bone marrow — neutro, eosino, baso, mono, few lymphos
partially in lymphatic tissue — lympho, plasma cells
synergy
when substances combine/act together their action is different/greater than the sum of their individual actions
what cytokines and hormones help w leukopoiesis
interleukins 1 to 19 — IL-1 to IL-19
GM-CSF — granulocyte/monocyte - colony stimulating factor
G-CSF — granulocyte - colony stimulating factor
M-CSF — macrophage - colony stimulating factor
major function of leukocytes
protection against foreign agents
phagocytosis — antigens labelled by antibodies
lysis — direct killing mechanisms
overall functions of immune system
encounters — lymphatic circulation, blood and tissues
recognition — receptor cells
activation — produce abs or chemical mediators
deployment — unable to destroy by themselves, cooperate in deployment
discrimination — btwn self and nonself antigens
regulation — shuts down immune response when cleared
B lymphocytes
primary source of humoral immune response (adaptive immunity)
transforms into plasma cells — produces antibodies
T lymphocytes
responsible for cellular immune responses (adaptive immunity)
regulation of antibody reactions — helping or suppressing activation of B lymphs
null cells
lacks B/T cell surface markers — innate immunity
killer cells/K
natural killer cells/NK
killer cells (K cells)
function via antibody-dependent cell-mediated cytotoxicity
recognize antibody-coated target cells via Fc receptors — induces lysis
dont require prior sensitization to antigen
natural killer cells (NK cells)
use direct cytotoxic mechanisms
recognize and kill virus-infected cells and tumor cells without prior sensitization
can also participate in antibody-dependent cell-mediated cytotoxicity
how do lymphs differ from leukos
resting cells, undergoes mitosis when stimulated to produce memory/effector cells
recirculate from blood to tissues back to blood
B cells can rearrange antigen receptor gene segments for antibodies — variety of abs
T cells can rearrange T cell receptor genes — variety of surface receptors
T cells develop and mature outside of bone marrow in thymus
which interleukins influence lymphocyte maturation
stem cells under influence of IL1 and IL6 differentiate into lymphoid stem cell CFU-L
primary lymphoid tissue
bone marrow and thymus
produces lymphocytes, promotes differentiation, doesnt require antigenic stimulation
stem cells in thymus > T lymphocytes
stem cells in bone marrow > B lymphocytes
secondary lymphoid tissue
lymph nodes, spleen, mucosal associated lymphoid tissues (peyers patches, tonsils)
main storage areas of already differentiated lymphocytes
stages of development of lymphocytes
lymphoblast
prolymphocyte
lymphocyte
characteristics of maturation in lymphocytes
decrease in size
chromatin condensation
lose nucleoli
cytoplasm changes from dark to light blue/clear
few azurophilic granules may be present (mature)
whats observed when theres a shift to the left with lymphocytes
first see increased number of lymphocytes
blasts/prolymphs — hard to differentiate under mic
what can be used to differentiate between lymphocytes
have specific antigenic receptors/markers on membrane and cytoplasm —help differentiate by type/maturity
flow cytometry is used to test the blood to determine what type of cell they are
what expresses CD marker 2
all T cells
what expresses CD marker 3
all peripheral (post-thymic, mature) T cells
what expresses CD marker 4
T helper cells
what expresses CD 5
all T cells
what expresses CD 8
cytotoxic T cells
what expresses CD 10
developing B cells
what expresses CD 19
developing and mature B cells
what expresses CD 20
developing and mature B cells
adult normal value of lymphocytes in the peripheral blood
20-44%
approx 60-80% are T cells, 20-35% are B cells
morphological characteristics of plasma cells
typically not seen in peripheral blood — maybe one
has bluish cytoplasm
nucleus is typically on the side/edge of cell
has perinuclear halo
lymphocytosis
increase in lymphocytes
lymphopenia
deficiency in lymphocytes
variant/reactive lymphocytes
account for approx 5-6% of lymphs in peripheral blood
represent normal immune system
inc numbers may be found in viral disorders
represent stimulated lymphs w inc DNA and RNA activity
how to differentiate from reactive lymph and monocytes
nucleoli — sometimes present in lymph, absent in monos
chromatin — variable clumping
granules — few prominent azurophilic granules
neutrophils
most numerous leukocyte in blood and marrow — 50-70% of all circulating WBC
not most numerous in body
stays in blood for 8-10 hours, 3-5 days in tissue — cant go back into blood like lymphs
function: locate and destroy through phagocytosis
neutrophilia
increased number of neutrophils (greater than 70% in PBS)
seen when acute bacterial infections
neutropenia
deficiency of neutrophils
seen with leukemia
what cells shift to the left most commonly
neutrophils
maturation series of neutrophils
myeloblast > promyelocyte > myelocyte >metamyelocyte > band > segmented neutrophil
first stage of neutrophil maturation where we can differentiate that its a neutrophil
myelocyte (neutrophilic)
granules throughout neutrophil maturation
myeloblast — no granules
promyelocyte — azurophilic (nonspecific) granules
myelocyte — both azurophilic and neutrophilic (specific) granules
rest — abundance of fine violet-pink neutrophilic granules, non specific still there but harder to see through the neutro grans
what stage do nucleoli disappear in neutrophil maturation
metamyelocyte
changes in cell size during neutrophil maturation
gets smaller as matures EXCEPT promyelocytes (2) can be larger than myeloblasts (1)
contents and functions of primary/azurophilic/nonspecific granules in neutrophils
lysozyme — enzyme breaks down the peptidoglycan in bacterial cell wall; acts more against GPO
myeloperoxidase — produces acid from H2O2 > makes bleach, cytotoxic to org
acid phosphatase — provides acidic environment to kill org
elastase — enzyme breaks down outer membrane of GNO
contents and functions of secondary/specific granules in neutrophils
lysozyme
NADPH oxidase — byproduct of O2, used in respiratory burst
cytochrome b — generates ATP
lactoferrin — antimicrobial
tertiary granules
can only see using electron microscopy
contain plasminogen activator, alkaline phosphate and gelatinase
neutrophil membrane
contains receptors for most common opsonins
has coating of glycoproteins — adherence
high concentrations of cytoskeletal proteins — actin, myosin, tubulin for movement, shape changes, engulfing bacteria
antigenic receptors (markers)
antigenic receptors/markers on neutrophils
CD 13 — granulocytes and monocytes
CD 15 — granulocytes
CD 33 — myeloid precursors and monocytes
migration sequence
margination, adherence, anchoring
diapedesis
migration (directed or random)
margination
about 50% of neutros in blood at all times
cells roll along in contact with endothelial cells while patrolling inside walls of blood vessels
adherence
flattening of the neutrophil against endothelial wall
response to chemical mediators released from area of inflammation
if small amounts of mediators, may be temporary
anchoring
result of adherence when high concentrations of chemical mediators are present in vessels
diapedesis
movement of neutrophils from bloodstream to tissues through junctions in btwn cells of vessel wall in response to chemotaxins from area of inflammation
assisted by vasodilators released from infected site
migration
movement of neutrophils towards area of infection in the tissue
chemotaxins
induces chemotaxis — directed movement of a cell or organism in response to a chemical stimulus
naturally occurring substances produced by body’s activated coag, kinin, and complement systems or that are released from infected/injured tissues from lymphocytes and other leukocytes
when do neutrophils have vacuoles
after phagocytosis, neutro is degranulating (exocytosis of undigested material)
leaves behind holes — shows that the neutro is actively working
killing cascade (phagocytosis)
immune adherence (recognition)
endocytosis (engulfment)
lysosome fusion
killing and digestion
exocytosis
when does the phagocytic process begin
when neutrophil arrives at site of inflammation by chemotaxis
endocytosis
extension of actin-rich pseudopods of membrane
surrounds bacterium and forms phagocytic vacuole (phagosome)
lysosome fusion
activated azurophilic and neutrophilic granules (lysosomes) attach to walls of phagosome and empty their contents into vacuole
respiratory burst
peroxide/peroxidase/halide system — major killing mechanism used by neutrophils
activated by NADPH oxidase, converts O2
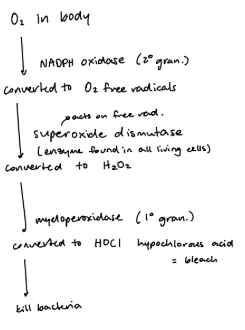
what are some non-oxygen dependent methods for neutrophilic killing
acidic pH 5.7 in phagosome kill pneumococcus
microbicidal enzymes
H+ ions on cationic granular proteins kill e.coli
lysozyme hydrolyzes mucopolysaccharide wall in some bact
what happens after neutrophilic killing takes place
dead microorg is digested by hydrolytic enzymes from lysosomes
useful components are absorbed into cytoplasm of neutrophil
waste components in phagosome ejected from cell via exocytosis
what happens after neutrophils die and lyse
converted into pus — resulting release of lysosomal enzymes and pyrogens amplifies inflammatory response
maturation stages of monocytes
monoblast
promonocyte
monocyte
characteristics of monoblasts
nucleus is round, oval, slightly folded or convoluted, with fine, thread-like pale, red-purple chromatin and up to 5 nucleoli
cytoplasm is moderately basophilic with greyish colouration, no granules
* non motile
* non phagocytic
characteristics of promonocytes
nucleus is folded or convoluted with chromatin creases, red-purple
chromatin and 0-5 nucleoli
cytoplasm is abundant, blue-grey, often with pseudopods and vacuoles
* slightly motile
* can phagocytize, but rarely
monocyte granules
only azurophilic (primary) granules
monocytic maturation in blood and tissues
red marrow — monoblast > promonocyte
blood — monocyte for 36-72hr; immature cells, relatively inactive
tissue — immature macrophage > macrophage
macrophage vs monocyte
inc energy level
inc metabolic rate
produces hydrolases (lysozymes) in ER, package in lysosomes until cytoplasm filled with small azurophilic granules
inc size — up to 50um diameter
function of macrophages
phagocytic response
immune recognition
secretory effector cell
iron metabolism
preservation of youthful/healthy cell population
monocyte phagocytosis
responsible for phagocytosis of dead neutrophils and body tissue in aftermath of acute infections
predominate in chronic infections
interleukin 1
best known monokine
activation of CD4 T cells by foreign antigen — activates tissue macrophages
stimulates hepatocytes to secrete acute phase proteins
stimulates GM-CSF production
acts as endogenous pyrogen
difference in response to inflammation btwn neutrophils and macrophages
macs arrive slower in smaller numbers
takes longer to exert cumulative effects
macs designed to survive combat w foreign microorganisms
functions of mono/macrophage secretions
removal of old blood cells
stimulation of self-defense against tumor cells
modulation of immune function
regulation of hematopoiesis
stimulation of inflammatory reactions
removal of infectious organisms by phagocytosis
where are there alot of eos and basos
little in peripheral blood, lots in tissues
eosinophils
0-4% of peripheral blood
have numerous specific eosinophilic cytoplasmic granules
eosinophilia
increase in eosinophils
seen in allergic reactions
40% or more when parasitic infections
eosinophilic granules
released to kill parasites and during hypersensitivity reactions
has major basic protein — enzyme against parasitic worms and bacteria
has eosinophil peroxidase
stimulates release of histamine
degranulates/deactivates mast cells and basophils
eosinophil membrane receptors
H1 histamine receptor
H2 histamine receptor
IgE receptor
H1 histamine receptor
produces symptoms of allergic reactions
acted against by antihistamines
H2 histamine receptor
negative feedback — turns off inflammatory reaction caused by basophils/mast cells
IgE receptor
binds IgE antibodies to cell — activates response from eosinos and basos
what stage of granulopoiesis can you determine the difference between granulocytes
myelocytes
basophils
0-2% of peripheral blood
immature form when in blood > turns into mast cells in tissues
basophilic granules
sulphated glycosaminoglycans
histamine
enzymes
eosinophil chemotactic factor ECF-A
histamines in basophils
almost all synthesized and stored in mast cells
eosinophil chemotactic factor ECF-A
specific tetra peptides which attract eosinophils — induces eosinophils to go to site
what happens as basophils use their granules
can synthesize more, causes anaphylaxis symptoms
basophil function
capable of ingesting foreign particles and produces heparin and histamine (induce inflammation)
what happens to parasites that are too big to phagocytose
eosinophils release cytotoxic substances from their granules onto surface of parasite
what cell increases during viral infections
lymphocytosis
what cell increases during acute bacterial infections
neutrophilia
what cell increases during chronic bacterial infections
monocytosis
what cell increases during allergies and parasitic infections
eosinophilia
what cell increases during inflammation and allergies
basophilia
whats the most chemotactic chemical factor that signals neutrophil activation
leukotriene B