L1: Amino acids and proteins
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
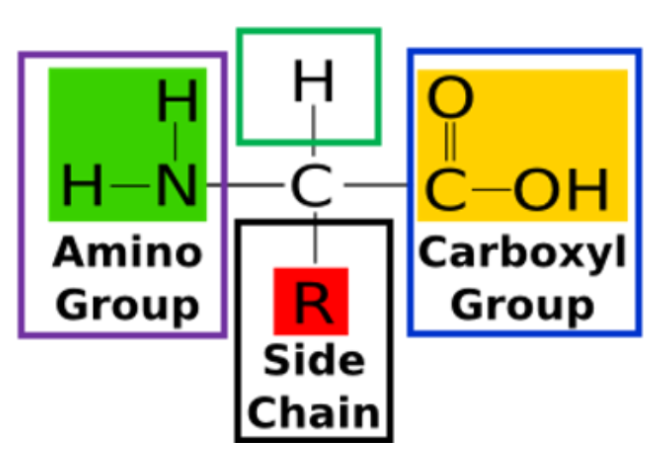
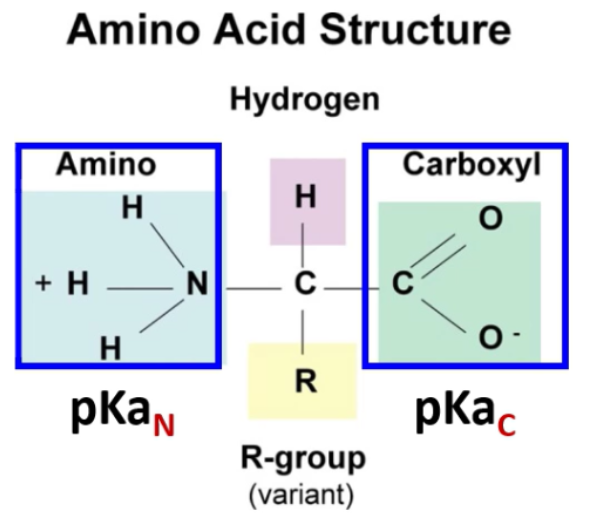
What are the 4 different groups covalently bonded to the a-carbon of an amino acid?
Amino group (-NH2)
Carboxyl group (-COOH)
Radical group (R-)
Hydrogen atom (H-)
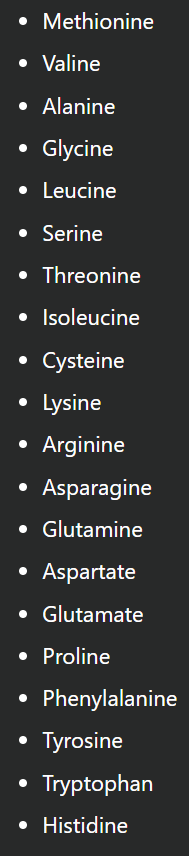
State the 3 letter sequence and 1 letter code for each amino acid
Methionine (Met) [M]
Valine (Val) [V]
Alanine (Ala) [A]
Glycine (Gly) [G]
Leucine (Leu) [L]
Serine (Ser) [S]
Threonine (Thr) [T]
Isoleucine (Ile) [I]
Cysteine (Cys) [C]
Lysine (Lys) [K]
Arginine (Arg) [R]
Asparagine (Asn) [N]
Glutamine (Gln) [Q]
Aspartate (Asp) [D]
Glutamate (Glu) [E]
Proline (Pro) [P]
Phenylalanine (Phe) [F]
Tyrosine (Tyr) [Y]
Tryptophan (Trp) [W]
Histidine (His) [H]
Which is the only amino acid without a chiral carbon?
Glycine
What is physiological pH?
7.4
What are the ways to classify amino acids?
Based on side chains:
non-polar aliphatic side chains
aromatic side chains
sulfur-containing side chains
side chain with hydroxyl (OH) group
charged basic side chains
charged acidic side chains + derivatives
side chain with amide groups
Describe non-polar aliphatic side chains. State which amino acids have them (GLaciers in ALAska VALiantly Locate ISOlated Prowlers)
Consists of H and C
Hydrophobic, non-polar (uncharged)
Chemically non reactive
R groups only contain inert methylene (CH2) or methyl (CH3) groups
Longer hydrocarbon chain = Amino acid is more hydrophobic
Solely aliphatic: Glycine, Alanine, Valine, Leucine, Isoleucine
Aliphatic + others: Proline
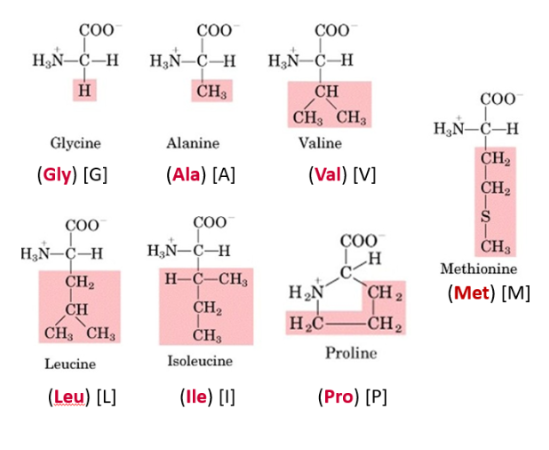
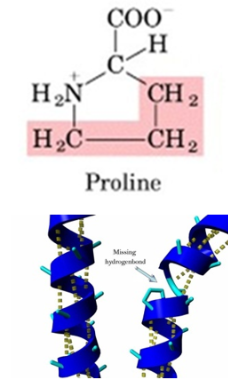
State which amino acid has an aliphatic cyclic group. Explain its how it affects the structure of the amino acid
Proline
Proline has its aliphatic side chain bonded back onto the α-amino group and does not fit nicely into any class because it is cyclic.
Proline is conformationally rigid. This rigidity of the ring plays a critical role in protein structure. Proline is usually found at bends in the polypeptide
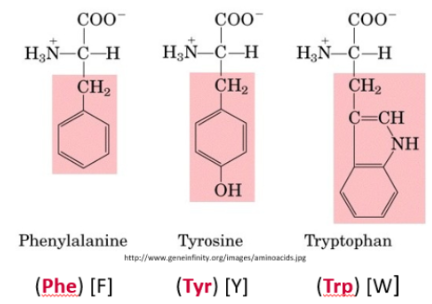
Describe aromatic side chains. State which amino acids have them
Hydrophobic ring
Strongly absorb UV light at 280nm
R-group consists of a phenyl ring, which is responsible for most of the UV properties of proteins
Phenylalanine, Tyrosine, Tryptophan
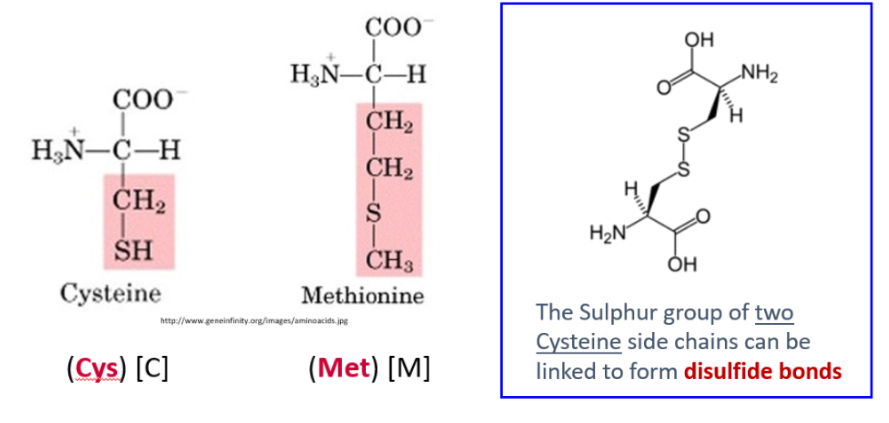
Describe sulfur-containing side chains. State which amino acids have them
Has sulfur atom in structure
2 Cysteine molecules can form disulfide bond (S-S) from sulfur group
Cysteine, Methionine
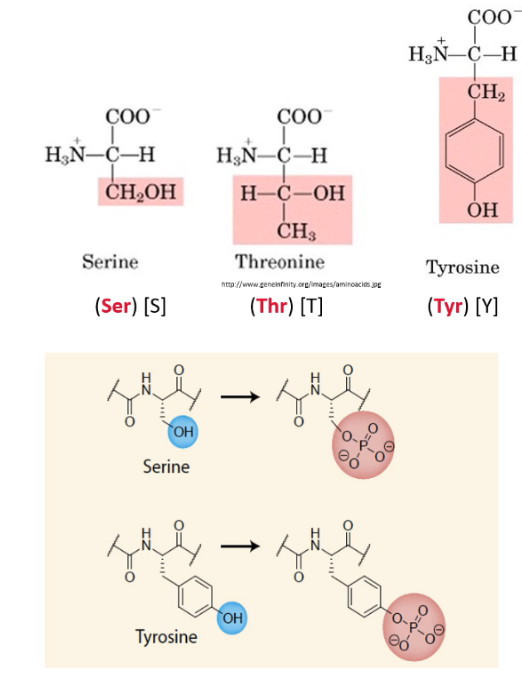
Describe side chains with hydroxyl groups (alcohols). State which amino acids have them
OH side chain can participate in the formation of hydrogen bonds
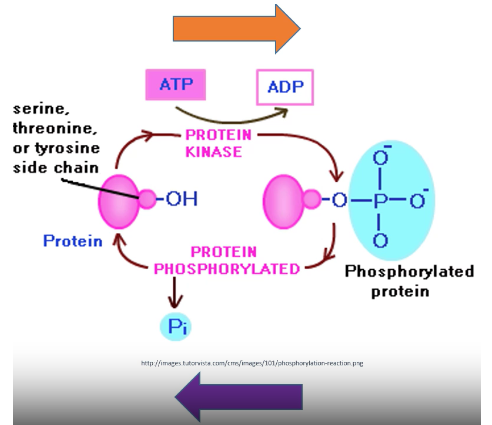
Amino acids with hydroxyl groups are important in Phosphorylation reactions
Phosphorylation → When a phosphate group is added to the O on a hydroxyl group. A H is loss. Phosphorylation reactions are catalysed by protein kinases
Serine, Threonine, Tyrosine
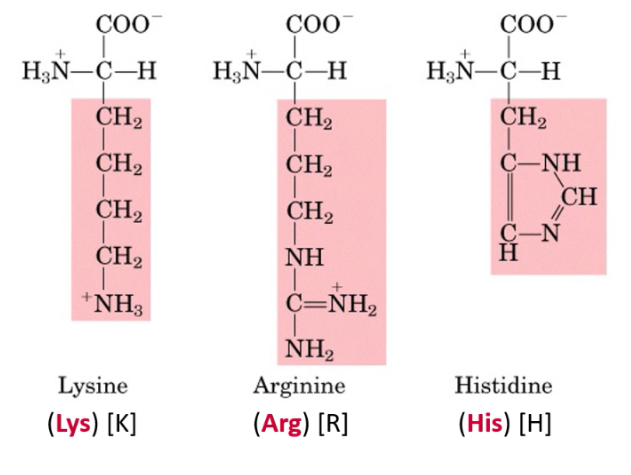
Describe charged basic side chains (-NH2 and =NH), basic amino acids. State which amino acids have them. (HLA)
At neutral pH, amino group side chains are protonated → Positively charged
Histidine, Lysine, Arginine
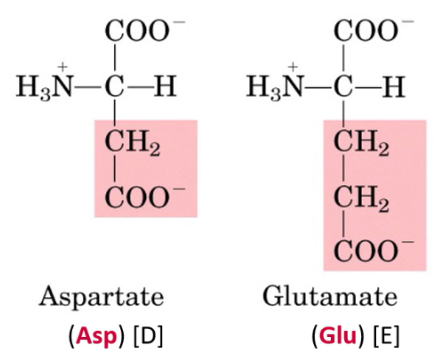
Describe charged acidic side chains (-COO-) Acidic amino acids. State which amino acids have them
At neutral pH, carboxyl group side chains are not protonated → Negatively charged
Glutamate, Aspartate
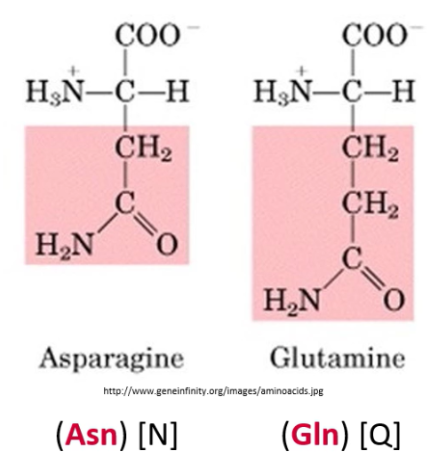
Describe amide side chains (-CONH2). State which amino acids have them
Amide side chains (-CONH2) are uncharged, but can form Hydrogen bonds
Glutamate, Aspartate
What is the difference between essential and non-essential amino acids?
Essential: Cannot be synthesised in the body, has to be obtained from diet
Non-essential: Can be synthesised in the body
Which amino acids are essential (LAMP LIT HTV) and non-essential (AAACGGGPST)?
Essential | Non-essential |
LAMP LIT HTV - Lysine - Arginine* - Methionine - Phenylalanine - Leucine - Isoleucine - Threonine - Histidine - Tryptophan - Valine | AAACGGGPST - Alanine - Aspartate - Asparagine - Cysteine - Glutamine - Glutamate - Glycine - Proline - Serine - Tyrosine |
Arginine → Because mammals cannot synthesise enough arginine to meet the metabolic needs of infants and children, it is classified as an essential amino acids
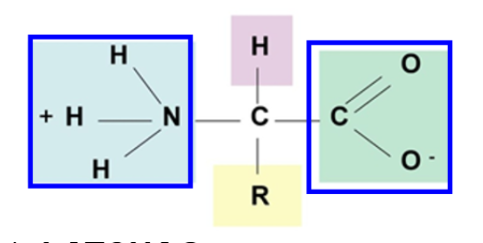
What are the properties of amino acids?
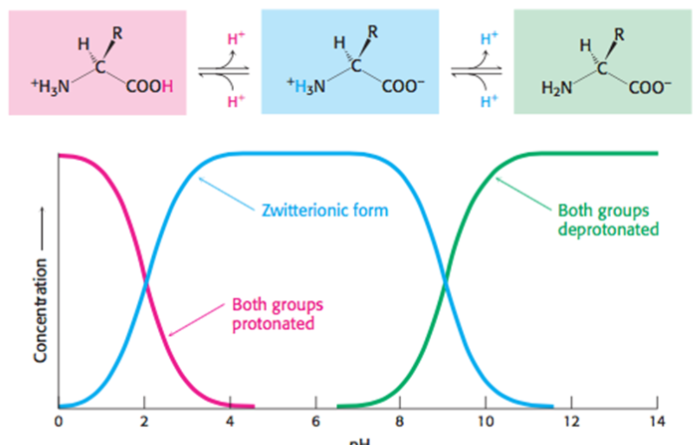
At physiological pH (pH 7.4), amino acids are dipolar ions (always charged)
Amino group is protonated (-NH3+)
Carboxyl group is unprotonated (-COO-)
What are the 7 ionisable amino acids? (Triprotic amino acids) LATCHAG
Triprotic → Contains 3 groups that can be ionized (Amino group, carboxyl group, side chain)
Lysine (NH2), Arginine (NH), Tyrosine (OH), Cysteine (SH), Histidine (NH), Aspartic acid (COOH), Glutamic acid (COOH)
What is the charge of an ionizable group on an amino acid dependent on?
The charge of an ionizable group on an amino acid is dependent on the pKa of the group
What is pKa?
pKa → pH at which concentrations of protonated and unprotonated forms are equal.
pKa = -log(Ka)
pKa <2 means strong acid
pKa >2 but <7 means weak acid
pKa >7 but <10 means weak base
pKa >10 means strong base
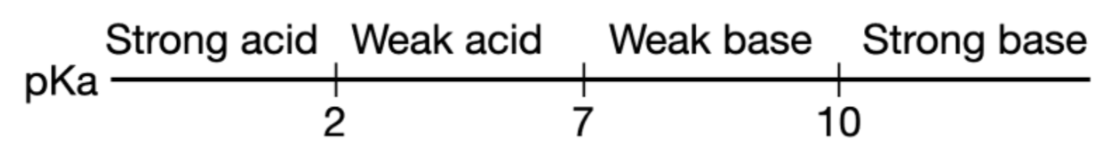
Does protonation/deprotonation occur at low/high pH?
How to determine charge on an ionisable group
To determine the charge on an ionizable group, we need
pKa of ionizable group
pH
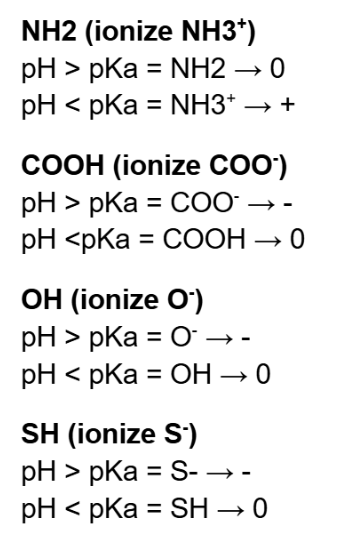
State how many H+ ions there are in the solution and the charge when,
pH > pKa
pH < pKa
pH > pKa means have very little H+ ions (protons) in the solution
pH < pKa means have a lot of H+ ions (protons) in the solution
pH > pKa -- negative charge (if have)
pH < pKa -- positive charge (if have)
What is the isoelectric point (pI)?
Isoelectric point (pI) → pH at which overall charge of molecule = 0
The overall charge of a protein or amino acid is dependent on the pI
Same logic as pKa (E.g pH > pKa: 0 or -. pH > pI: -)
pH > pI → -
pH < pI → +
pH = pI → 0
What is the formula for calculating the Isoelectric point for diprotic amino acids?
pI = (pKaC + pKaN )/2
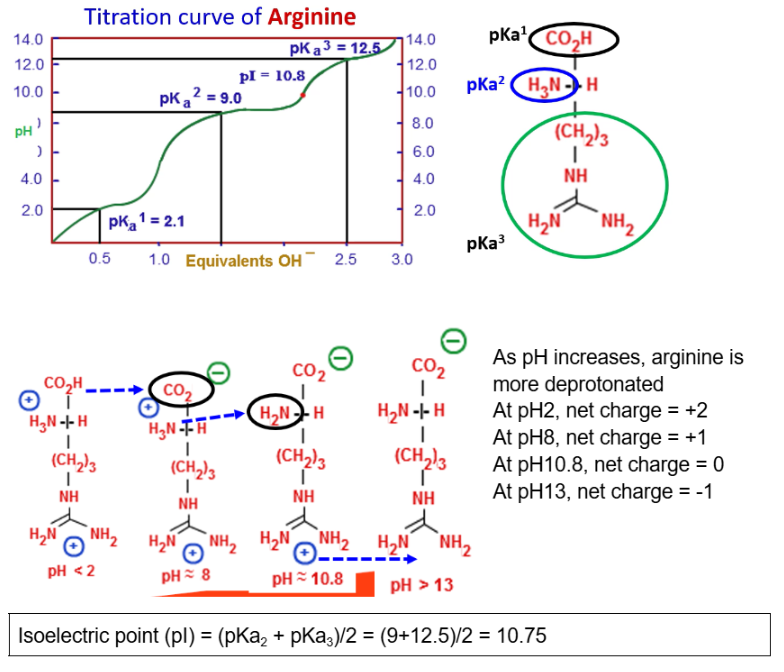
How to calculate isoelectric point for triprotic amino acids?
To determine pI, need to perform a titration
At low pH, ______ protons are present, ______ the chance of protonation.
At high pH, ______ protons are present, ______ the chance of deprotonation
At low pH, more protons are present, increasing the chance of protonation
At high pH, less protons are present, increasing the chance chance of deprotonation
What are the functions of proteins? (DESCC) Give examples.
Defense / immunity
Immunoglobins, antibodies
Energy production
ATP
Structure/ Mechanical function
Collagen, keratin
Catalyst
Enzymes
Contraction/movement
Actin, myosin, tubulin
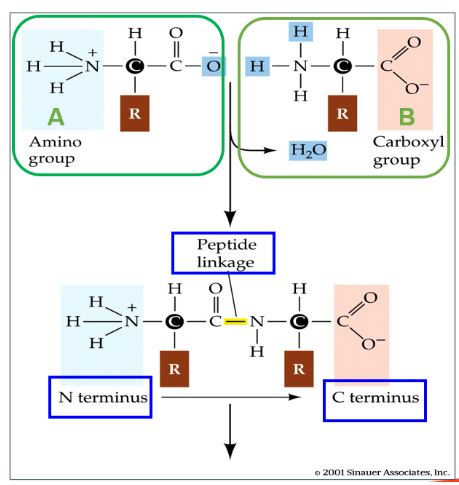
What is the N terminus and C terminus?
N terminus: free amino end of a peptide
C terminus: free carboxyl end of a peptide. Where more amino acids are added on.
All proteins have free amino group at N terminus and free carboxyl group at C terminus.
What type of reaction is peptide bond formation?
Condensation reaction
How do amino acids form proteins?
Carboxyl group of amino acid A reacts with amino group of amino acid B, forming a peptide bond, releasing one molecule of water.
What is a Dipeptide, Tripeptide, Polypeptide?
Dipeptide → two amino acids joined together
Tripeptide → three amino acids joined together
Polypeptide → many amino acids joined together
What are the types of protein structure?
Globular
Hydrophobic interior, hydrophilic exterior
e.g. enzymes, carrier proteins, regulatory proteins
Fibrous
Provide mechanical support
e.g. collagen, keratin
What are the 4 levels of protein structure?
Primary structure
Secondary structure
Tertiary structure
Quaternary structure
Describe primary structure. Give examples
Sequence and number of amino acids held together by peptide bonds in a polypeptide chain
E.g Ala-Arg-Asp-Gly
Describe secondary structure (alpha helix)
Alpha-helix
Regular, repeating motif in protein
In globular, water-soluble proteins
Composed of right-handed spiral amino acid chain stabilised by H bonds parallel to helix axis
In an alpha-helix, the carboxyl group of residue n forms H bond with NH group of residue n +4
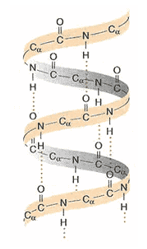
Describe secondary structure (beta pleated sheets)
Beta-pleated sheets
Regular, repeating motif in protein
In rigid, insoluble proteins
Made of polypeptide chains running parallel or anti-parallel to one another
Stabilized by H bonds formed perpendicular between adjacent chains
Describe secondary structure (Loops and turns)
Loops and turns
Cause directional change in the polypeptide backbone
About 5 a.a
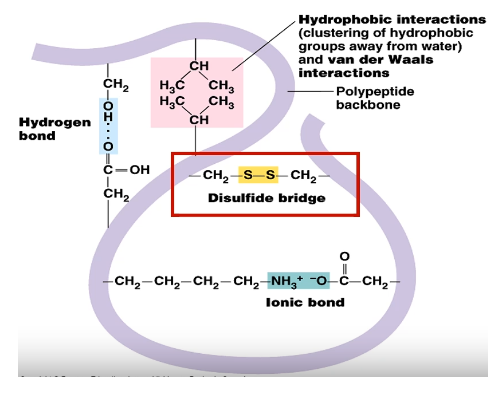
Describe tertiary structure by stating the bonds.
Protein folding (bonds)
Disulfide bonds (-S-S-): Between two Cysteine amino acids, Strong covalent bonds
Hydrogen bonds: Between O and H, weak bond
Ionic electrostatic bonds: Between COO- and -NH3+, weak bond
Hydrophobic/Hydrophilic interactions: Between R groups, weak bond
Explain what is a domain in tertiary structure
A structurally independent region of polypeptide that folds independently of other domains
Have specific function
Can be removed & added to other proteins by genetic engineering
Proteins can have 1 to many domains depending on protein size
Describe quaternary structure
Multi-subunit proteins - composed of several protein subunits grouped together.
E.g Hemoglobin (Consists of four tightly packed chains: Two alpha chains, Two beta chains)
What is protein folding dependent on?
Primary structure
Bonds
Disulfide, Hydrogen, Ionic (electrovalent), Hydrophobic / Hydrophilic interactions
Polar vs. Non-polar R groups
Chaperone proteins (heat-shock proteins)
What are chaperone proteins?
Large multi-subunit protein
Facilitate folding of newly synthesised proteins to prevent incorrect folding
Over-produced when cells exposed to heat to prevent protein denaturation
Explain Post translational modification
Post translational modification → When newly synthesized polypeptide chains are modified immediately after translation
Post translational modifications are critical for the functional capability of the protein
What are the types of post translational modification of proteins
Remove part of amino acid chain
N terminal or C terminal residue
Part of polypeptide (e.g. insulin)
Signal sequence
Remove/add functional group
Add carbohydrate group
Form complex with metal ion
Phosphorylation / dephosphorylation
Insulin is produced by ____ to ____. When is insulin secreted? What are the effects of insulin?
Produced by pancreas to control blood sugar levels
Secreted when blood glucose levels are high
Has the following effects:
Increases glucose uptake into muscle & fat cells
Stimulates glycogen synthesis
Describe the post translational modification for insulin
Removal of part of a polypeptide sequences (E.g Post translational modification for Insulin)
Signal sequence removed from preproinsulin, producing proinsulin
Proinsulin undergoes further modifications, where a peptide sequence within proinsulin is removed
This leaves behind 2 fragments linked by a disulfide bond, thus forming insulin
Briefly describe and give an example of the post translational modification process of attaching carbohydrate groups
Carbohydrate + polypeptide = glycoproteins
E.g Blood ABO antigens
Briefly describe and give an example of the post translational modification process of complexing with metal ions
Haemoglobin made of 4 polypeptide chain forming 4 heme subunits. 4 iron atoms required for each heme
Briefly describe the post translational modification process of phosphorylation and dephosphorylation
Kinases → enzymes that phosphorylate proteins
Phosphatases → enzymes that remove phosphate from protein
Phosphorylation: Uses ATP to add phosphate to protein (ATP → ADP)
Dephosphorylation: Phosphatase removes phosphate group