Organic Chemistry - Unit 4 AOS 1 - Edrolo
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
Organic Substance
Any molecule containing carbon (except CO₂ and carbonates); central to organic chemistry.
Carbon Valence
Carbon has four valence electrons (in group 14), so it typically forms four covalent bonds to complete its octet.
Carbon-Carbon Bond
Strong covalent bond; carbon's tetravalency allows catenation (chain formation) leading to many possible organic molecules.
Tetrahedral Geometry
The 3D arrangement when carbon is single-bonded to four atoms: bond angles ≈109.5°, arranged like a pyramid with triangular base.
Bond Length vs. Strength
Shorter bonds (e.g., C≡C) are stronger; longer bonds (e.g., C-I) are weaker because atomic orbitals overlap less effectively.
Electronegativity Difference
The larger the difference (e.g., C-F), the more polar the bond; can influence bond strength and reactivity.
Atomic Size Effect
Larger atoms (e.g., I) have more diffuse valence orbitals, making their bonds (e.g., C-I) weaker than those with smaller atoms (e.g., C-F).
FONCl Mnemonic
Reminds that among common elements, electronegativity order is F > O > N > Cl, followed by C, H, etc.
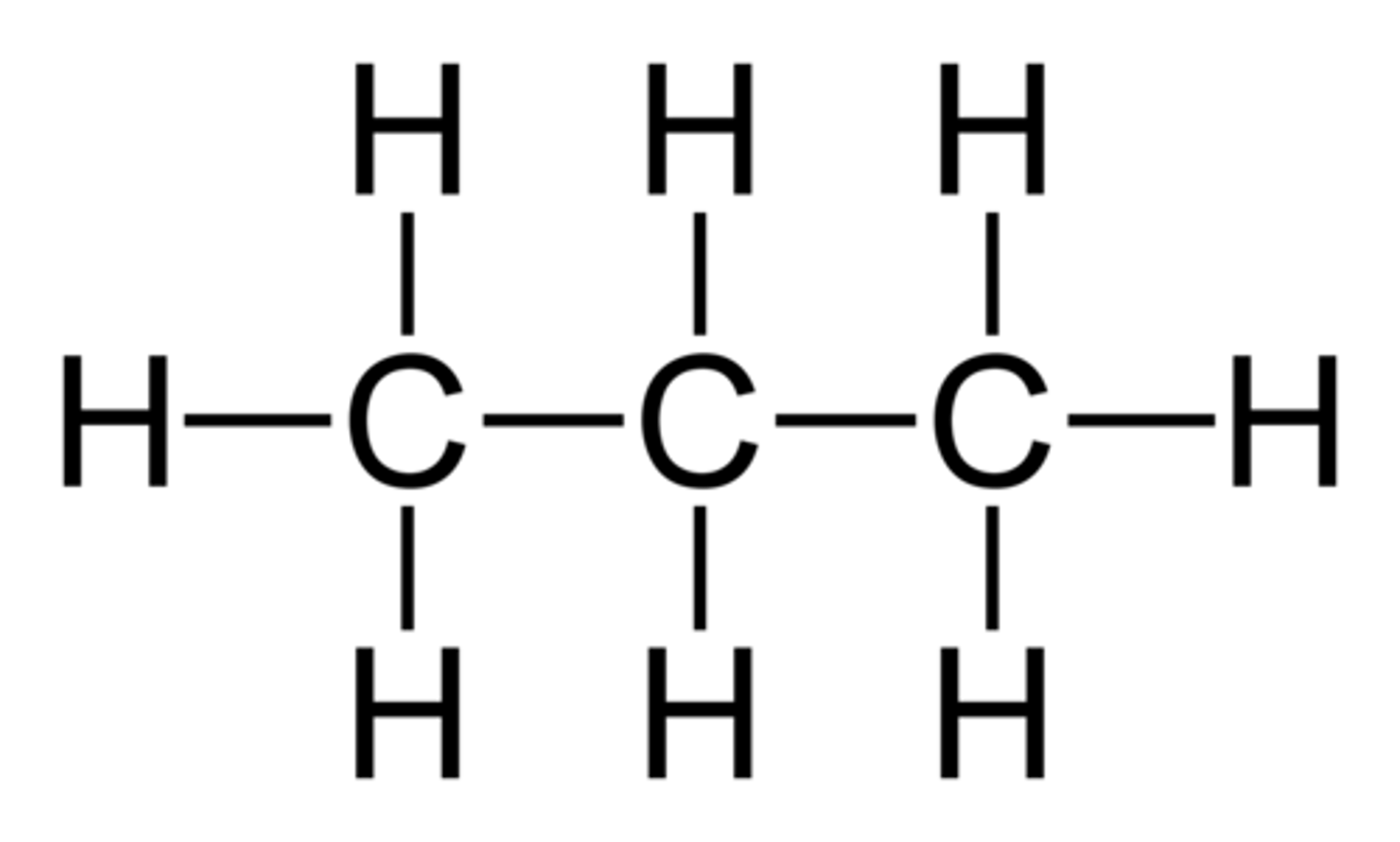
Structural Formula
Draws each atom and bond explicitly, showing connectivity in full detail (e.g., H-C-C-O-H).
Condensed (Semi-Structural) Formula
Writes atoms in sequence with implied bonds (e.g., CH₃CH₂OH for ethanol).
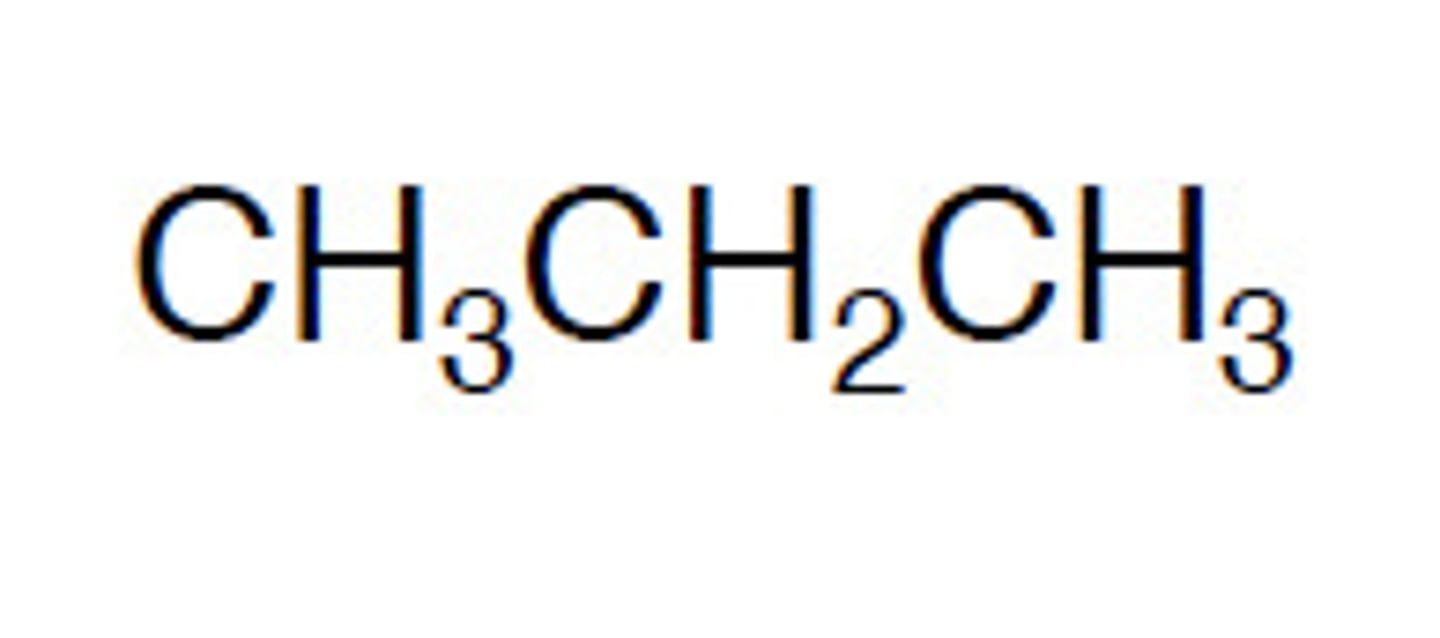
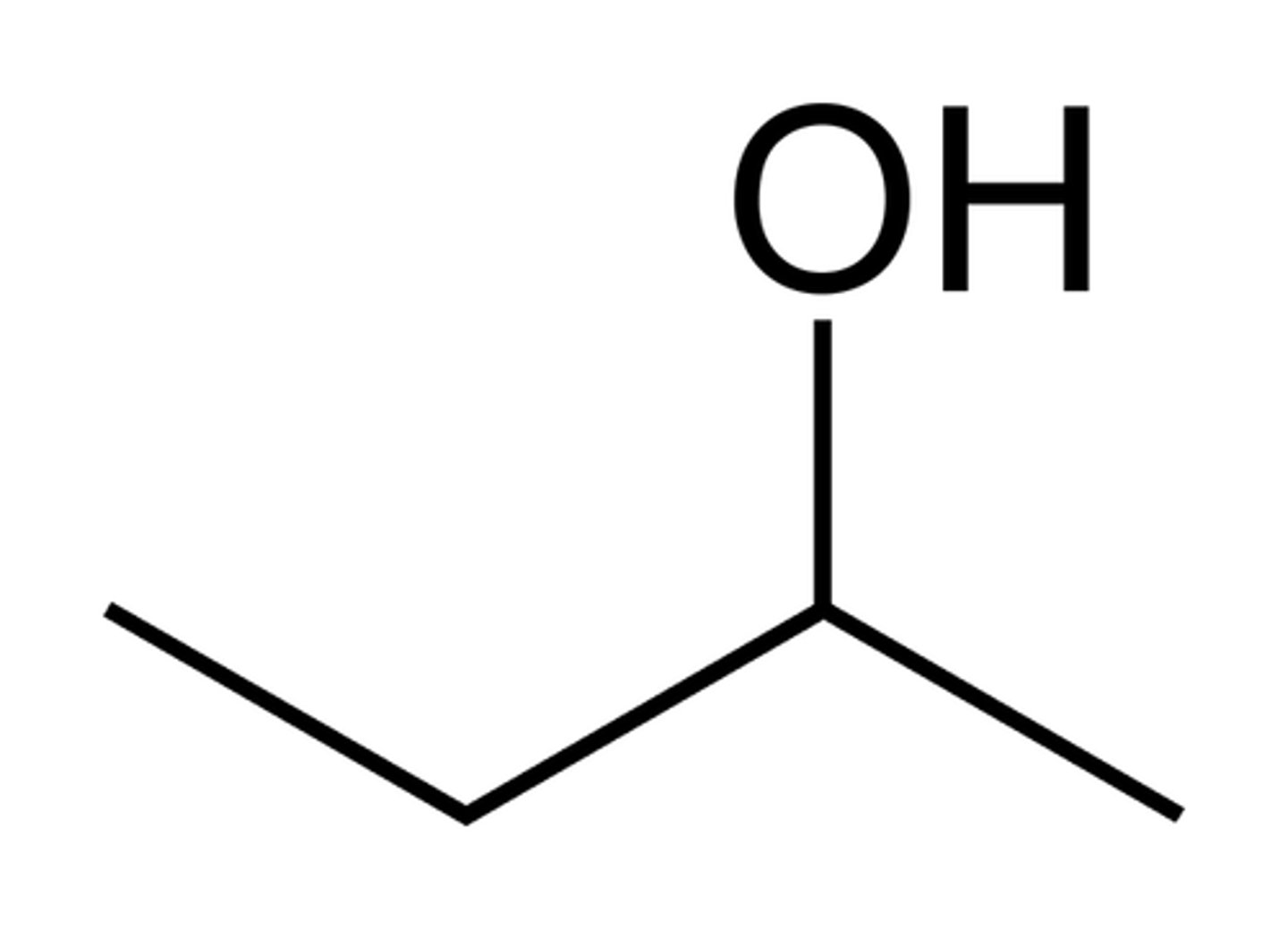
Skeletal (Line) Formula
Abbreviates carbon backbone as zigzag lines; carbons are implicit at vertices; H's on C are omitted; heteroatoms shown.
Molecular Formula
Lists total atom counts (e.g., C₂H₆O) but gives no bonding/connectivity information.
Empirical Formula
Simplest integer ratio of atoms in a compound (e.g., CH₂O for C₆H₁₂O₆).
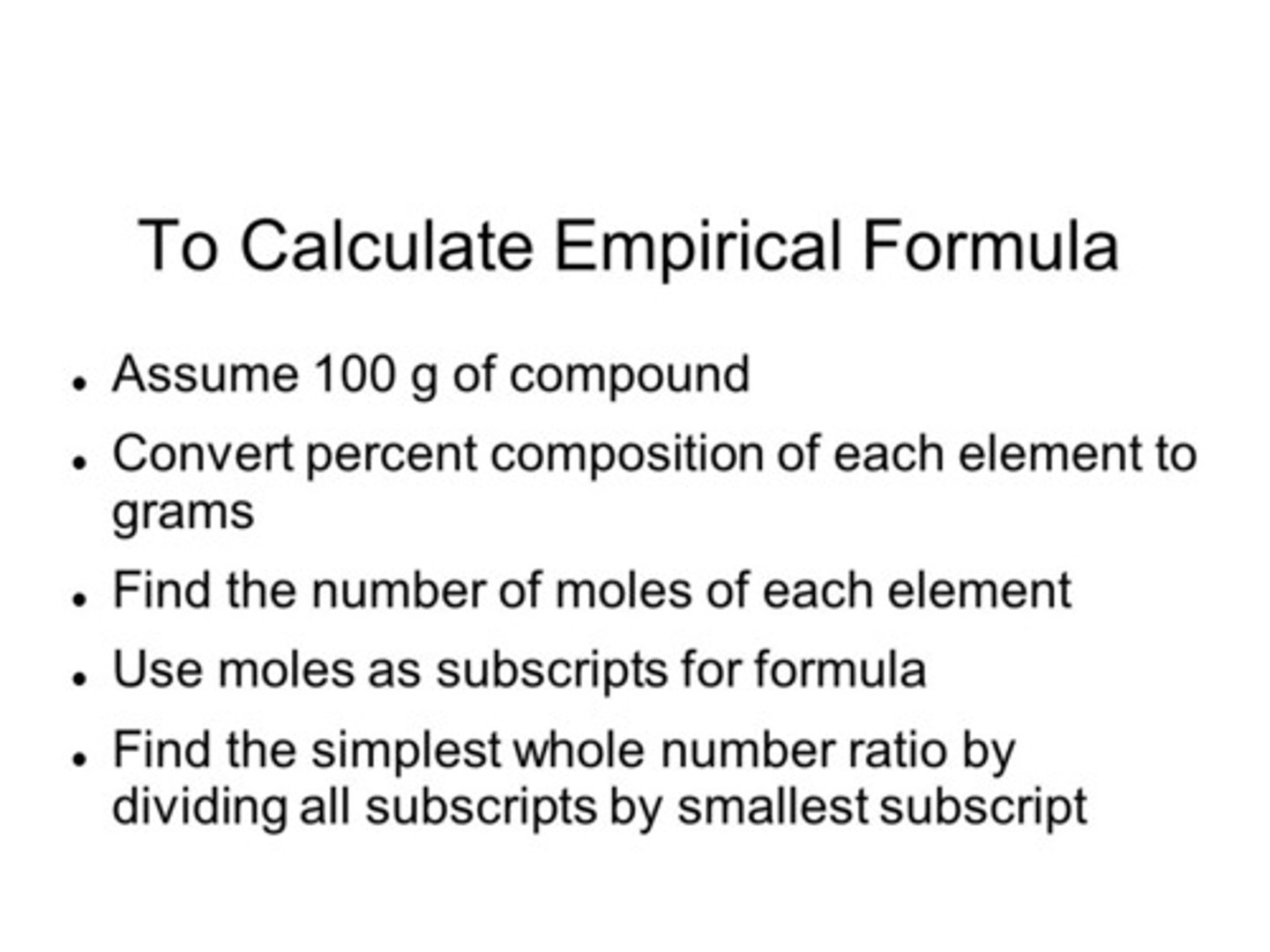
Alkane
Saturated hydrocarbon with only C-C single bonds; general formula CₙH₂ₙ₊₂.
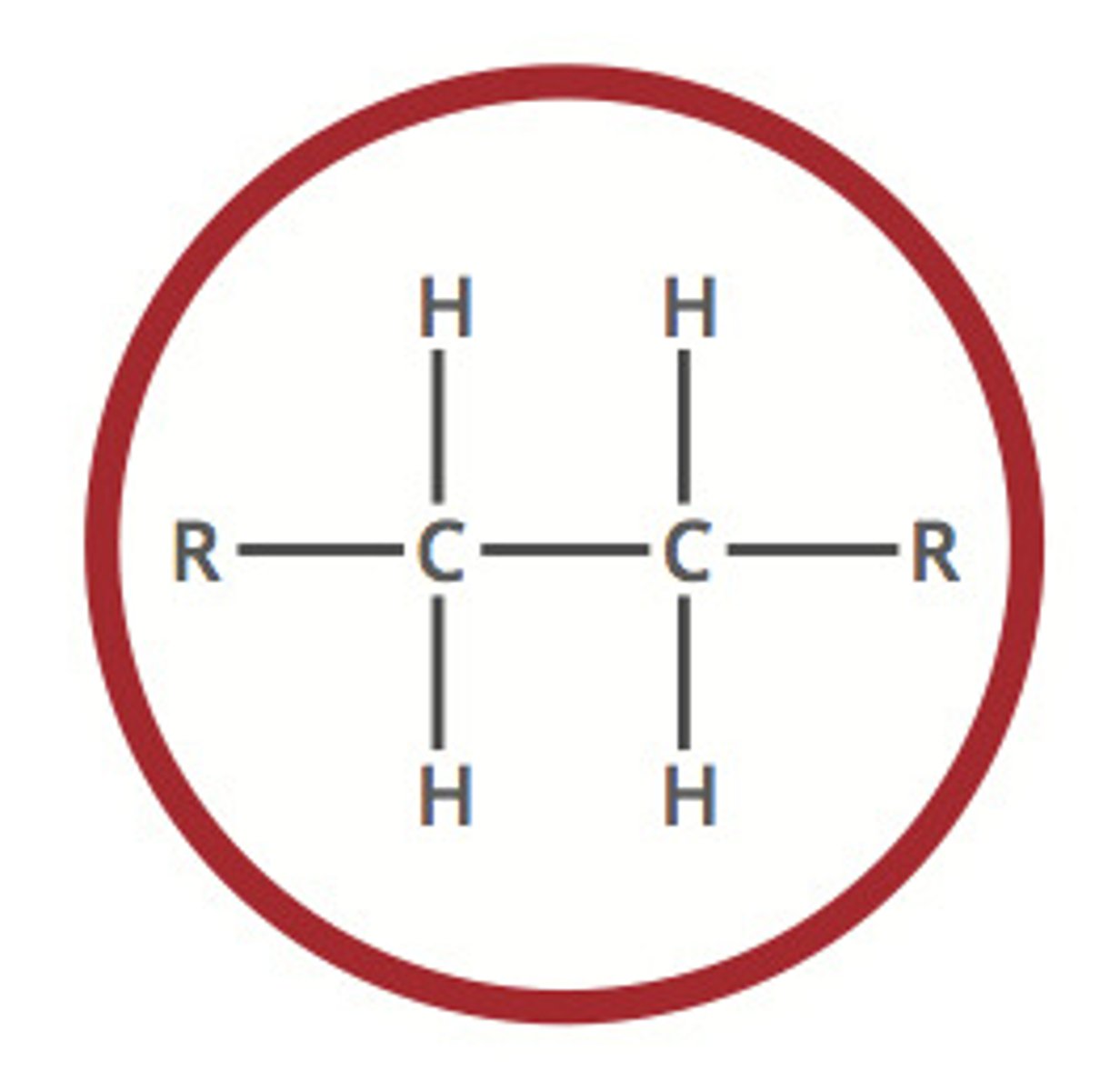
Alkene
Unsaturated hydrocarbon containing at least one C=C double bond; general formula CₙH₂ₙ.
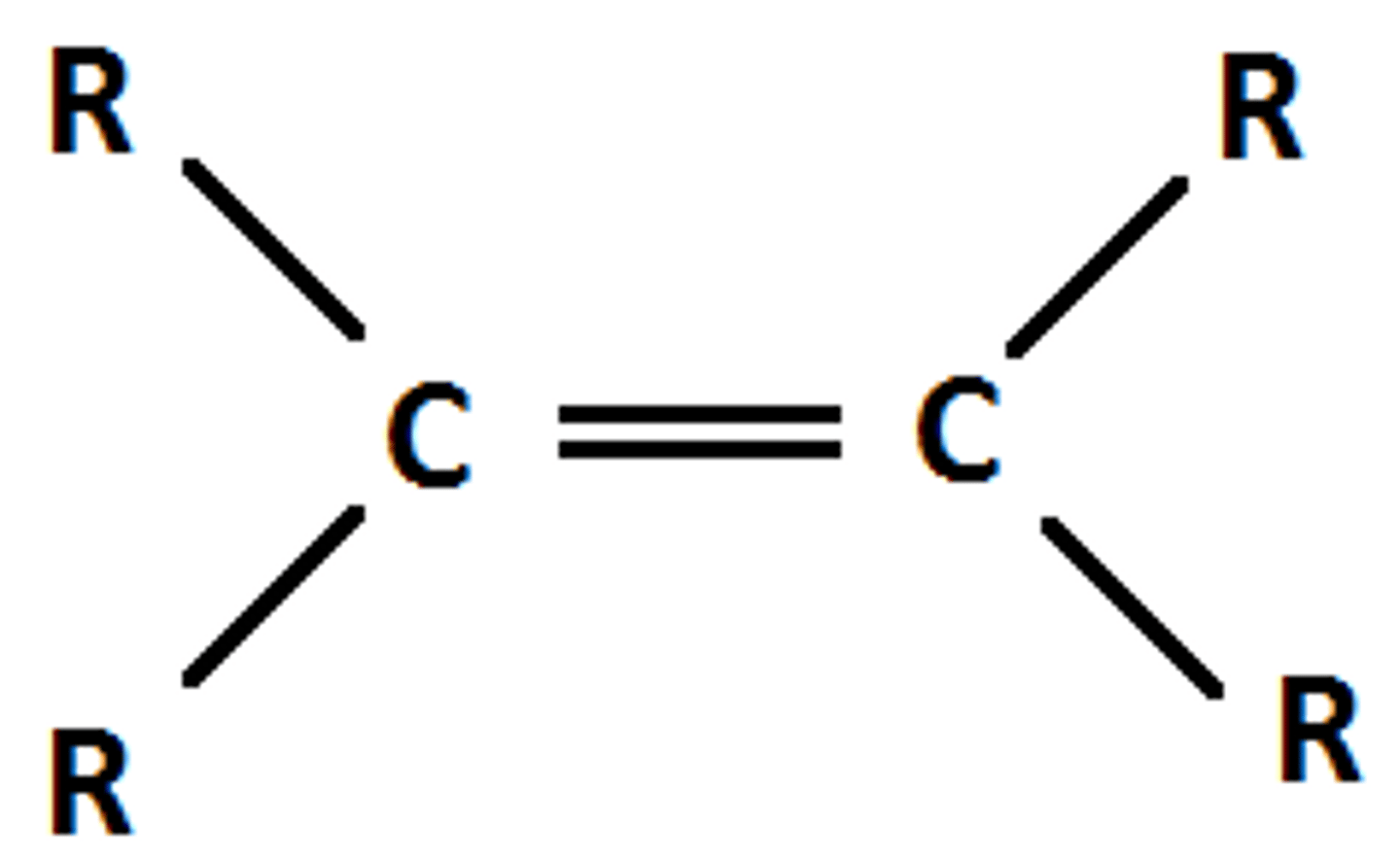
Cycloalkane
Ring hydrocarbon (no double bonds); general formula CₙH₂ₙ (same as alkene formula but cyclic).
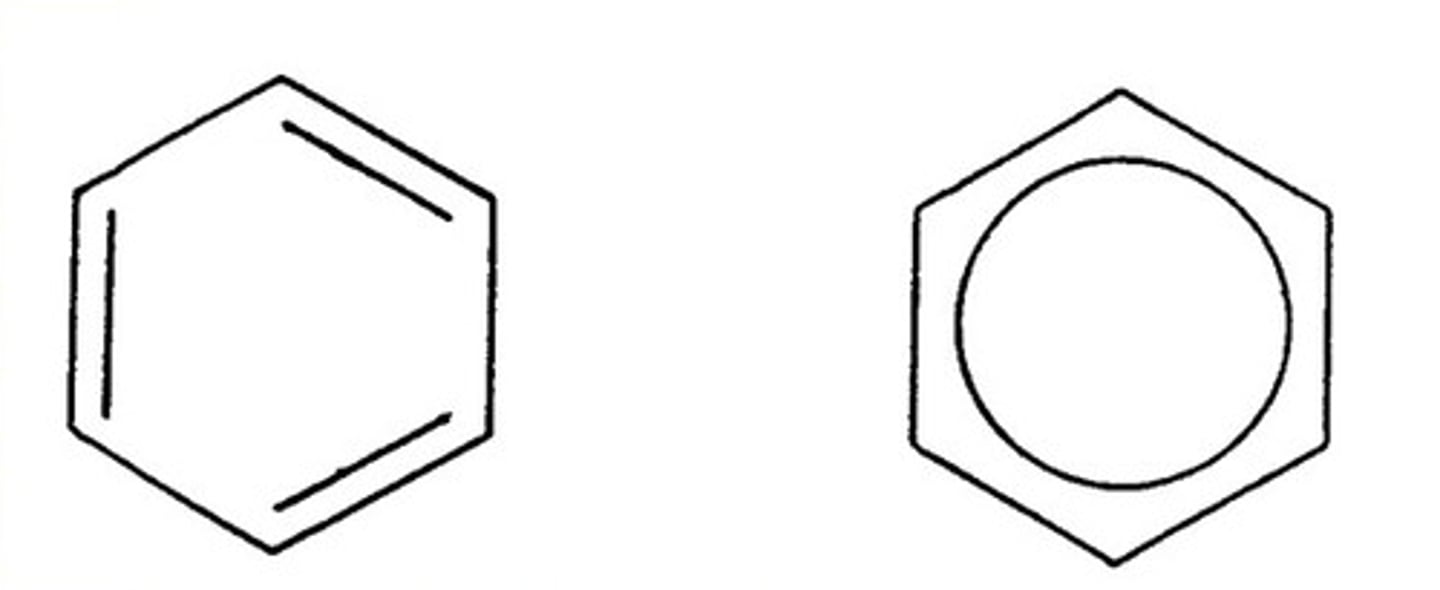
Benzene
Aromatic hydrocarbon C₆H₆ with six delocalized π electrons in a planar ring; unusually stable.
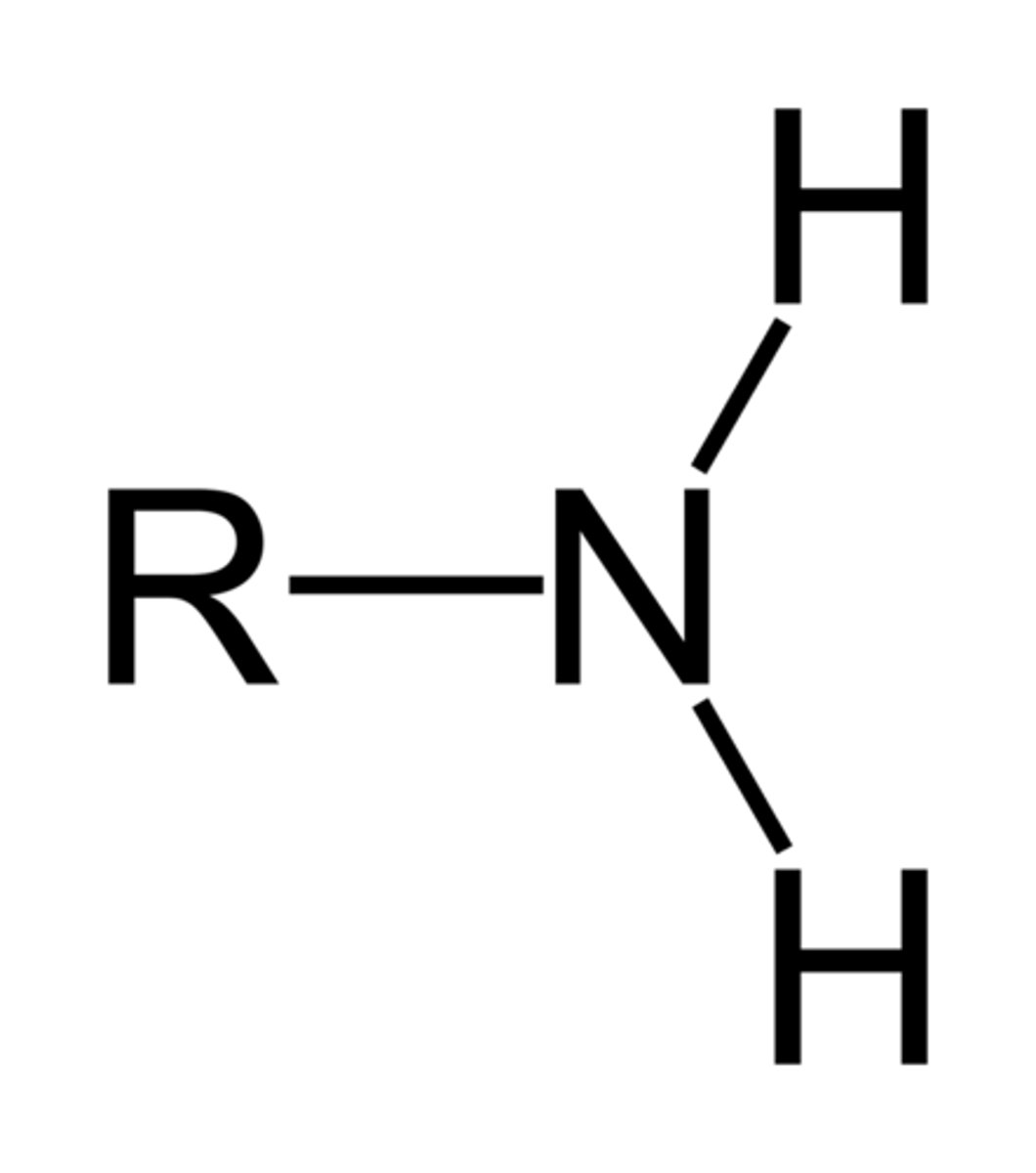
Primary Amine
Functional group -NH₂ attached to a carbon; derived from ammonia (NH₃).
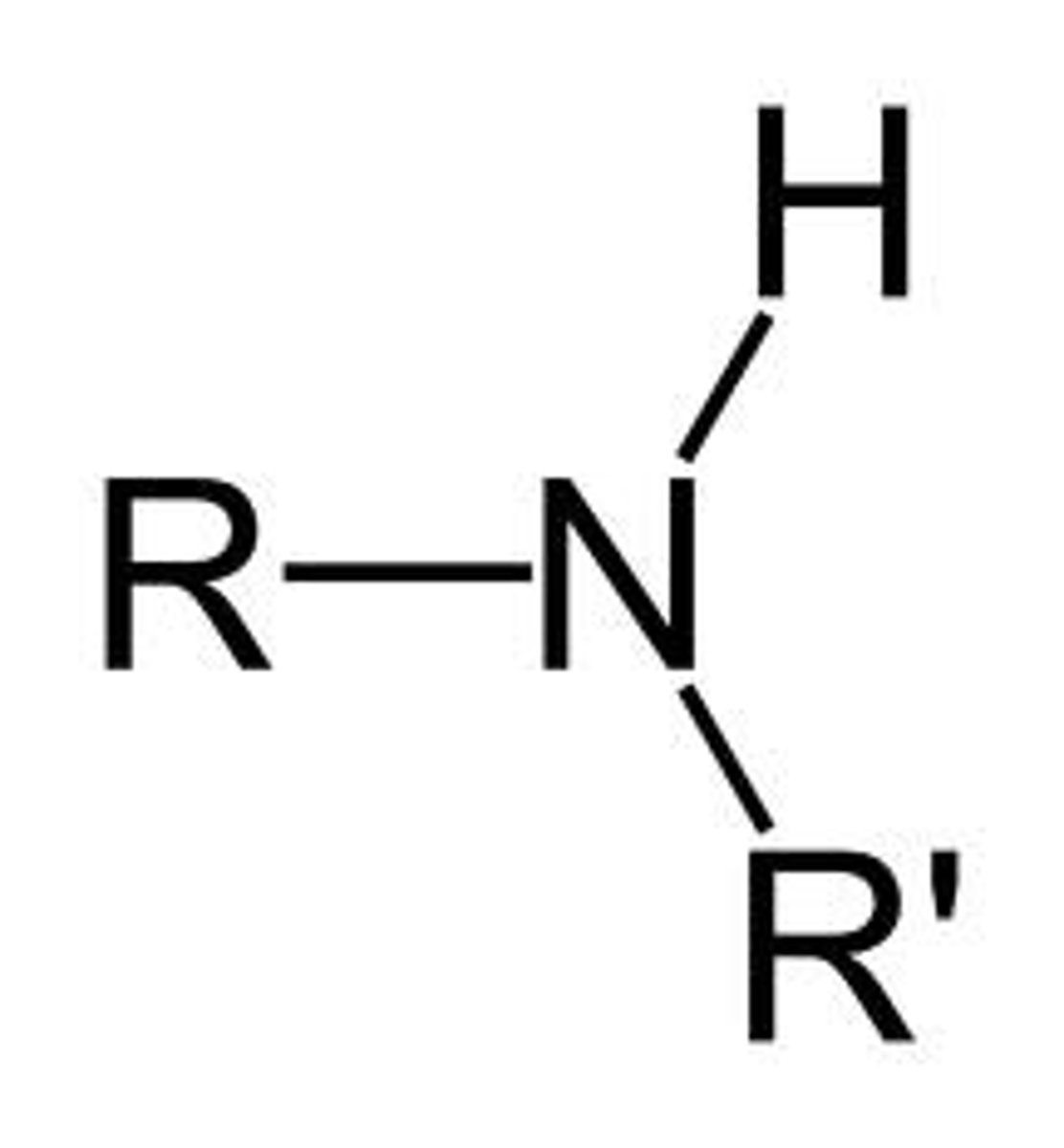
Secondary Amine
Functional group R-NH-R′; nitrogen bonded to two carbon groups and one hydrogen.
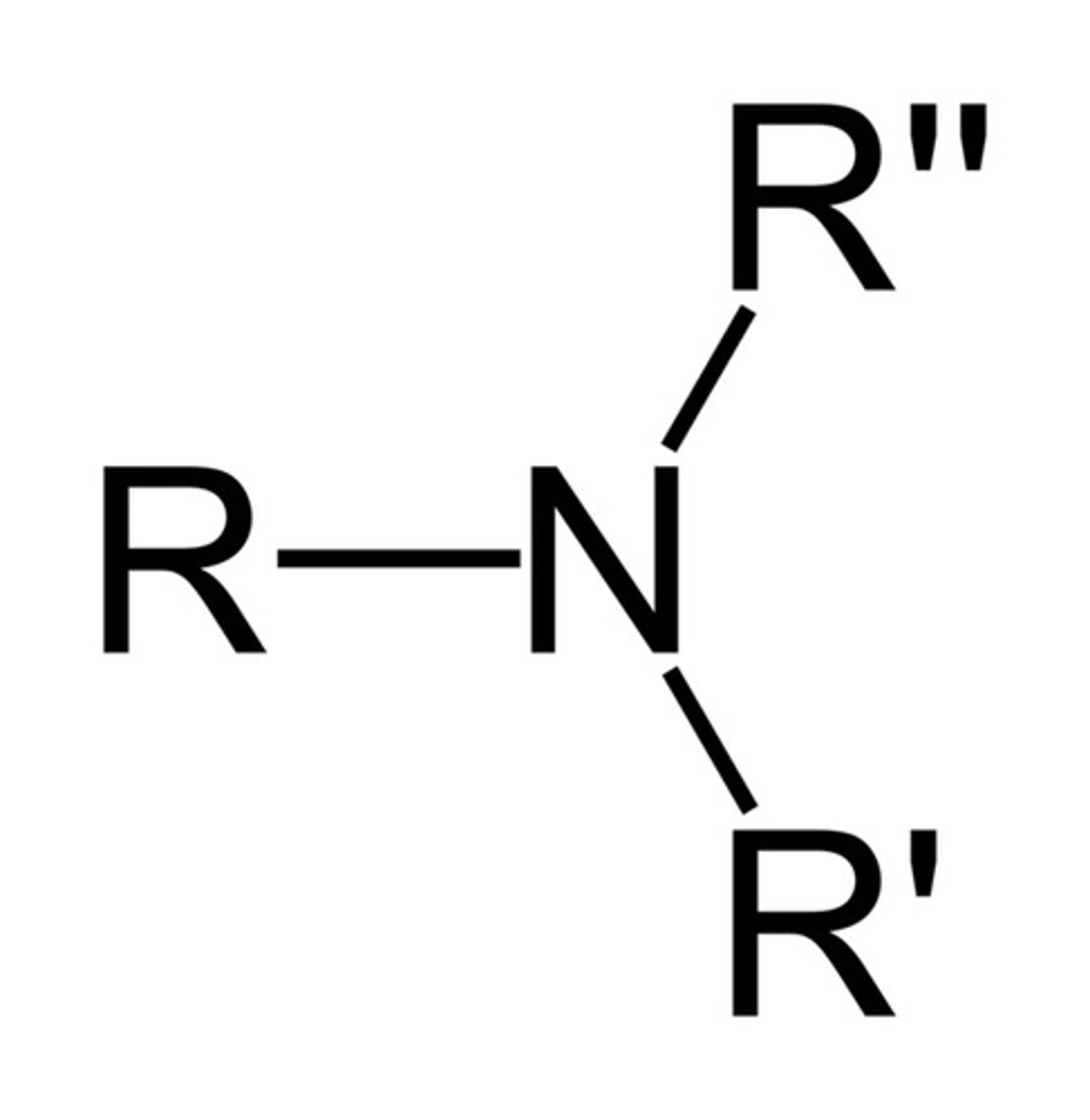
Tertiary Amine
Functional group R-N-R′-R″; nitrogen bonded to three carbon groups; no N-H bond.
Primary Amide
Functional group -CONH₂; carbonyl (C=O) bonded to nitrogen with two H's (peptide bond in proteins is amide).
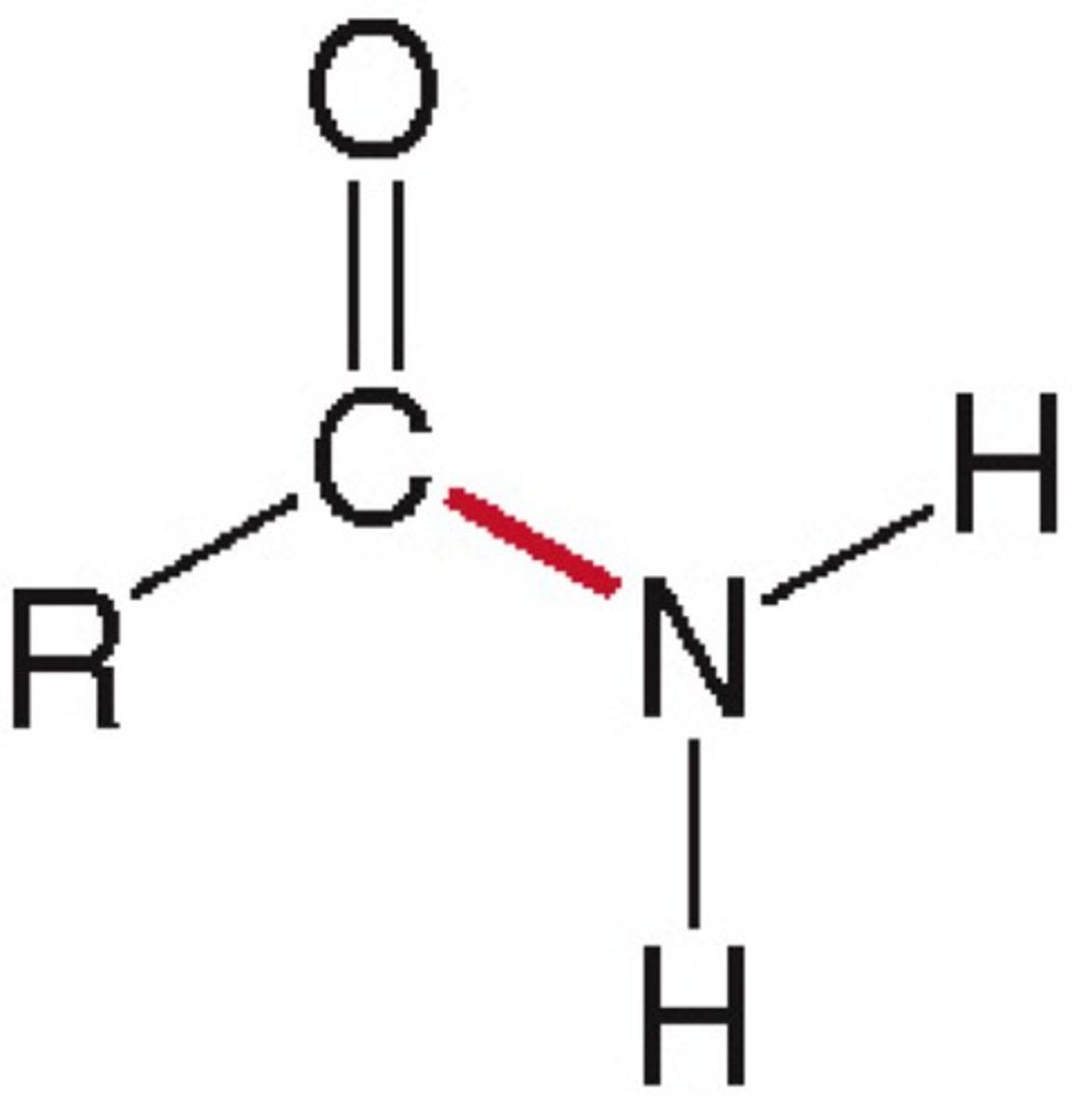
Aldehyde
Functional group -CHO; a carbonyl (C=O) at chain end bonded to one hydrogen.
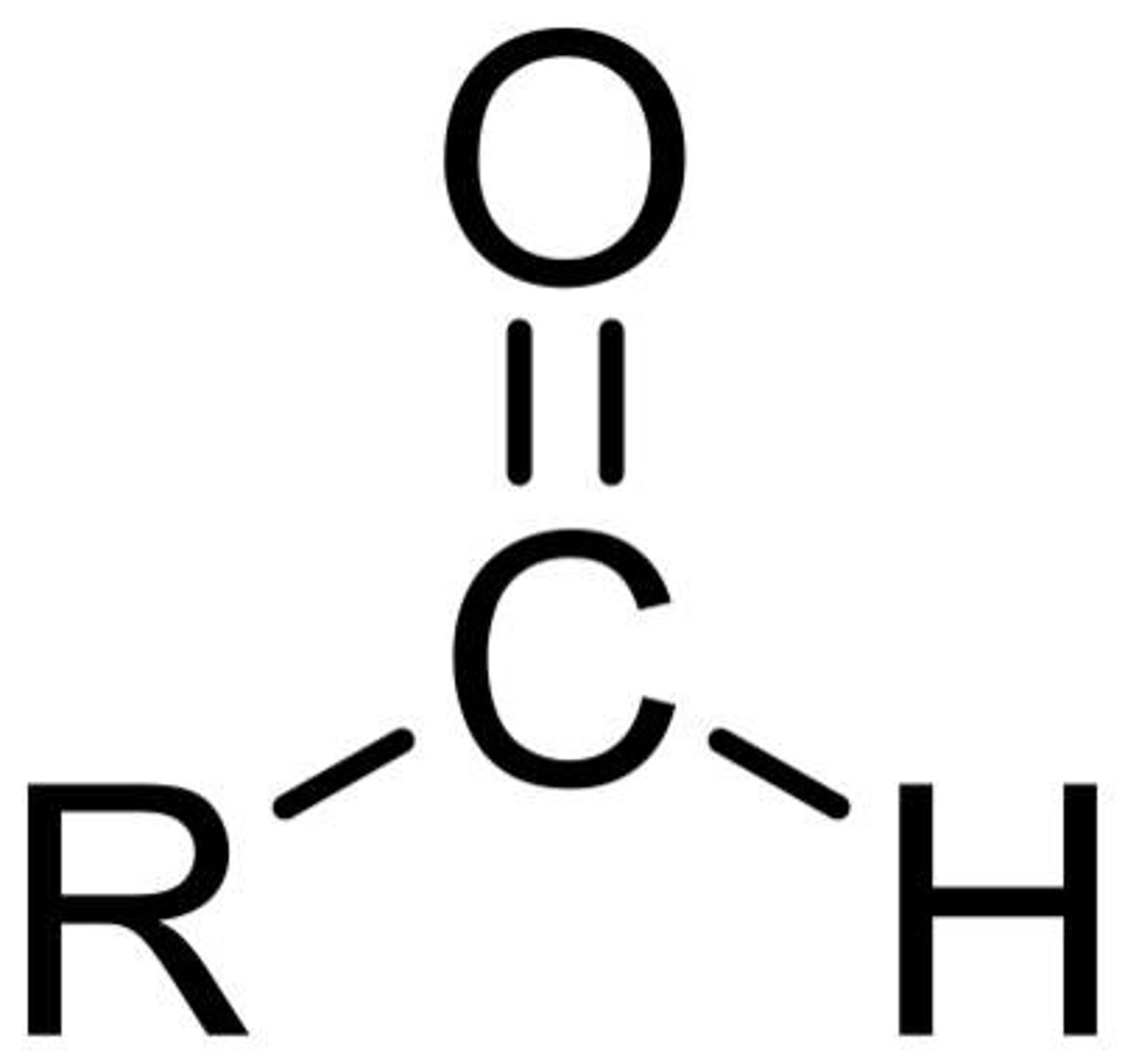
Ketone
Functional group R-C(=O)-R′; carbonyl in the interior of a carbon chain; two alkyl groups attached.
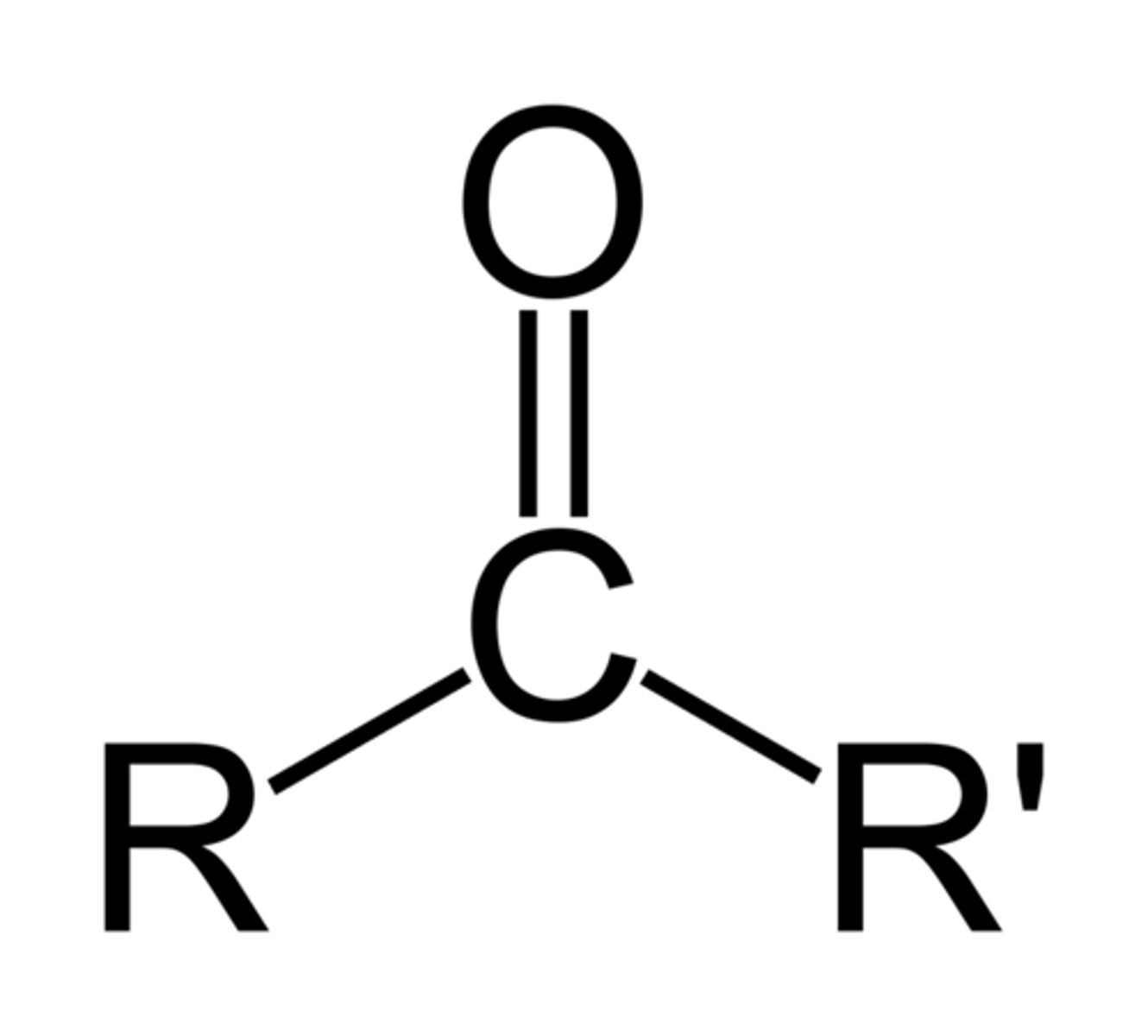
Carboxylic Acid
Functional group -COOH; carbonyl C bonded to -OH; acidic because the proton can dissociate, stabilized by resonance.
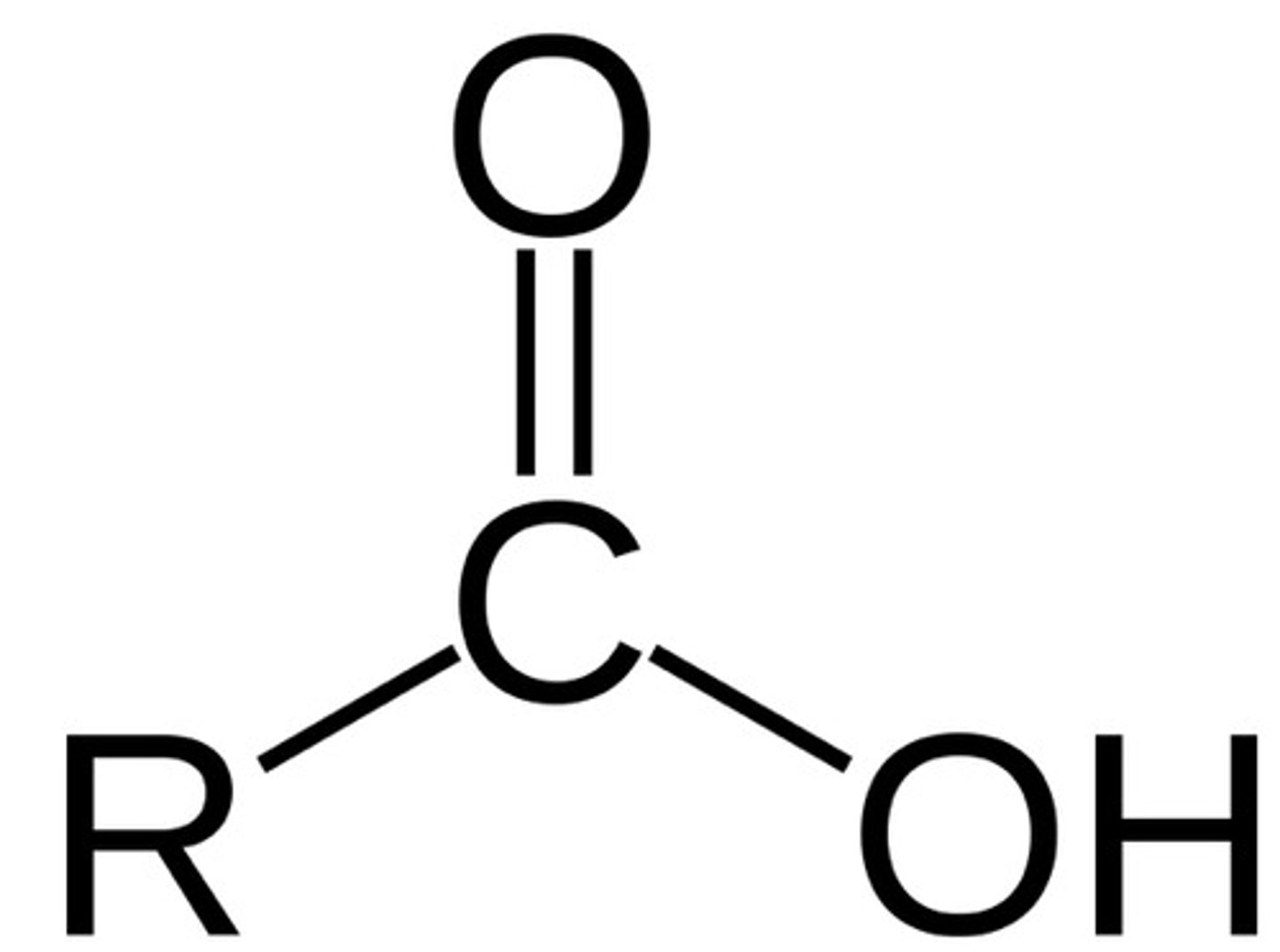
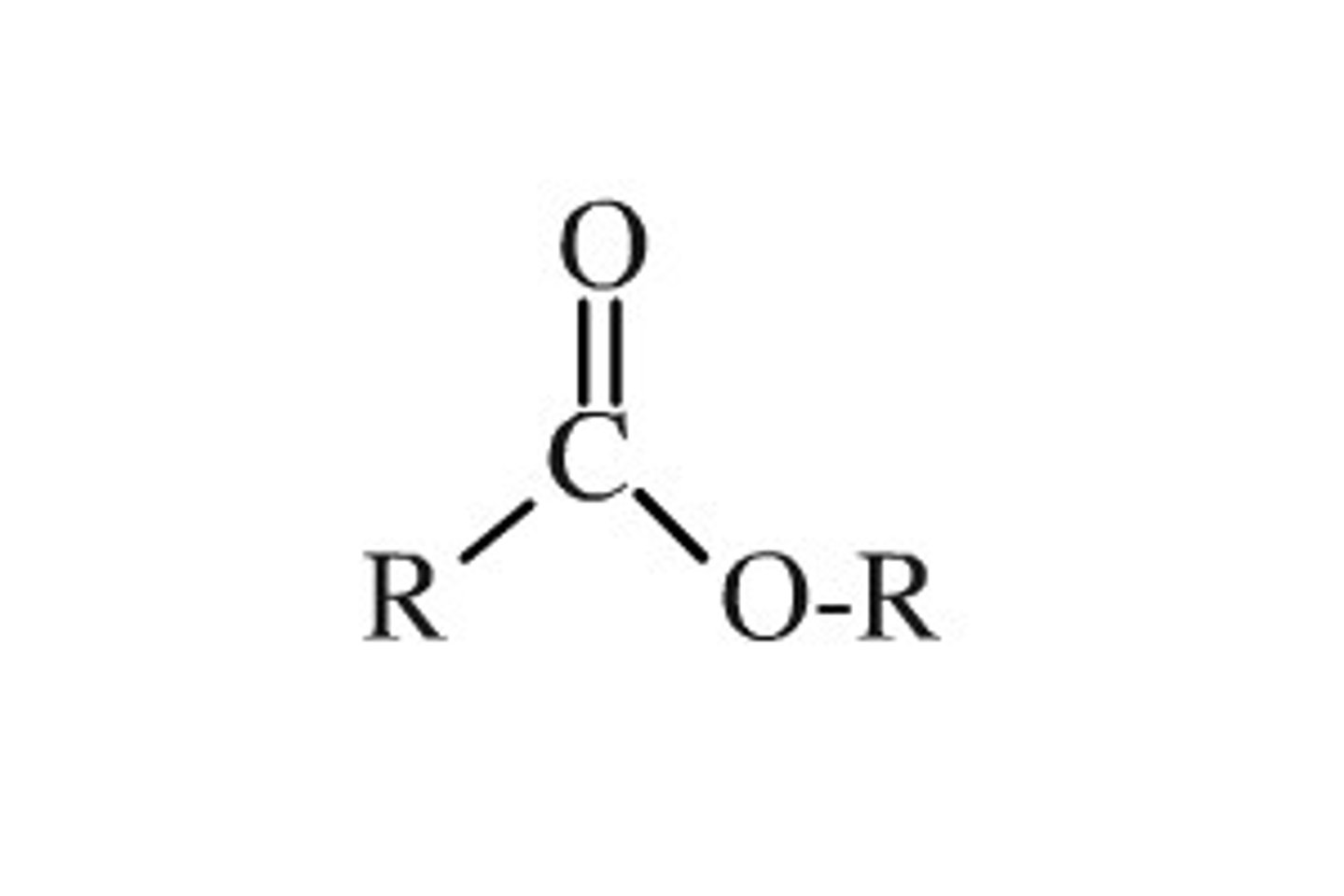
Ester
Functional group R-C(=O)-O-R′; formed by condensation of a carboxylic acid and an alcohol.
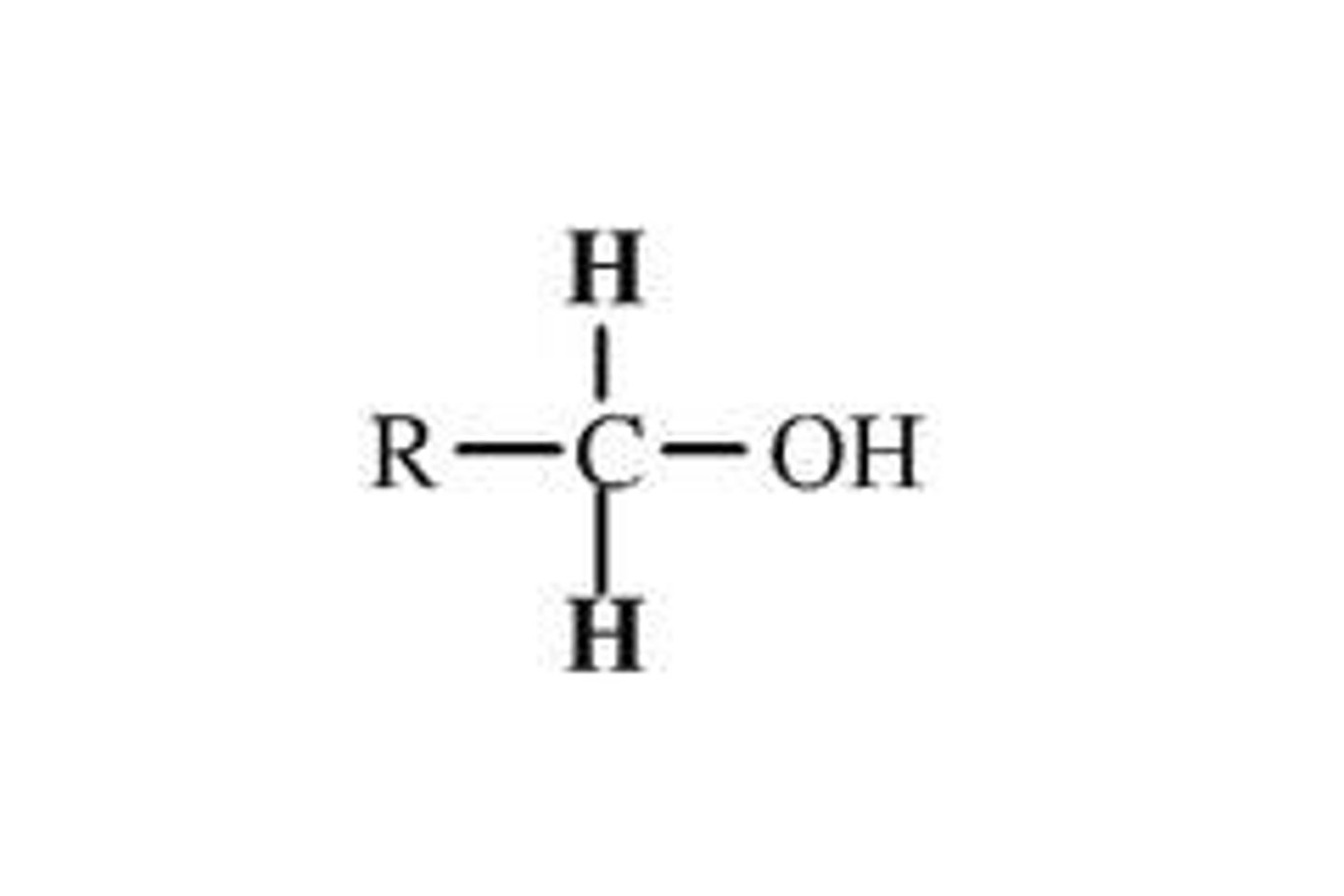
Alcohol
Functional group -OH attached to a carbon: primary (1°) carbon bonded to one carbon, secondary (2°) to two, tertiary (3°) to three.

Primary Alcohol
R-CH₂-OH; carbon bearing -OH bonded to only one other carbon.
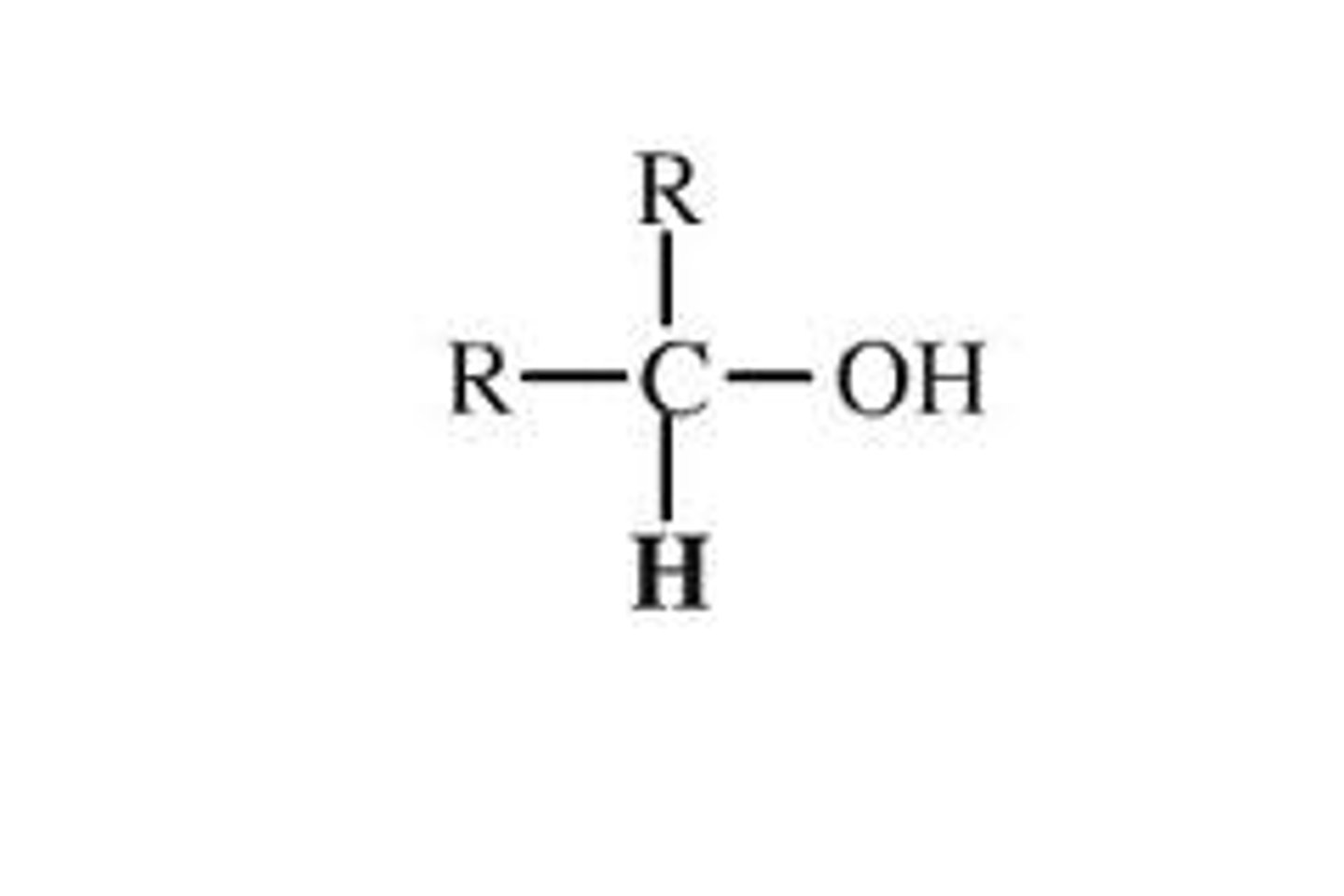
Secondary Alcohol
R-CH(OH)-R′; carbon bearing -OH bonded to two other carbons.
Tertiary Alcohol
R-C(OH)-R′-R″; carbon bearing -OH bonded to three other carbons.
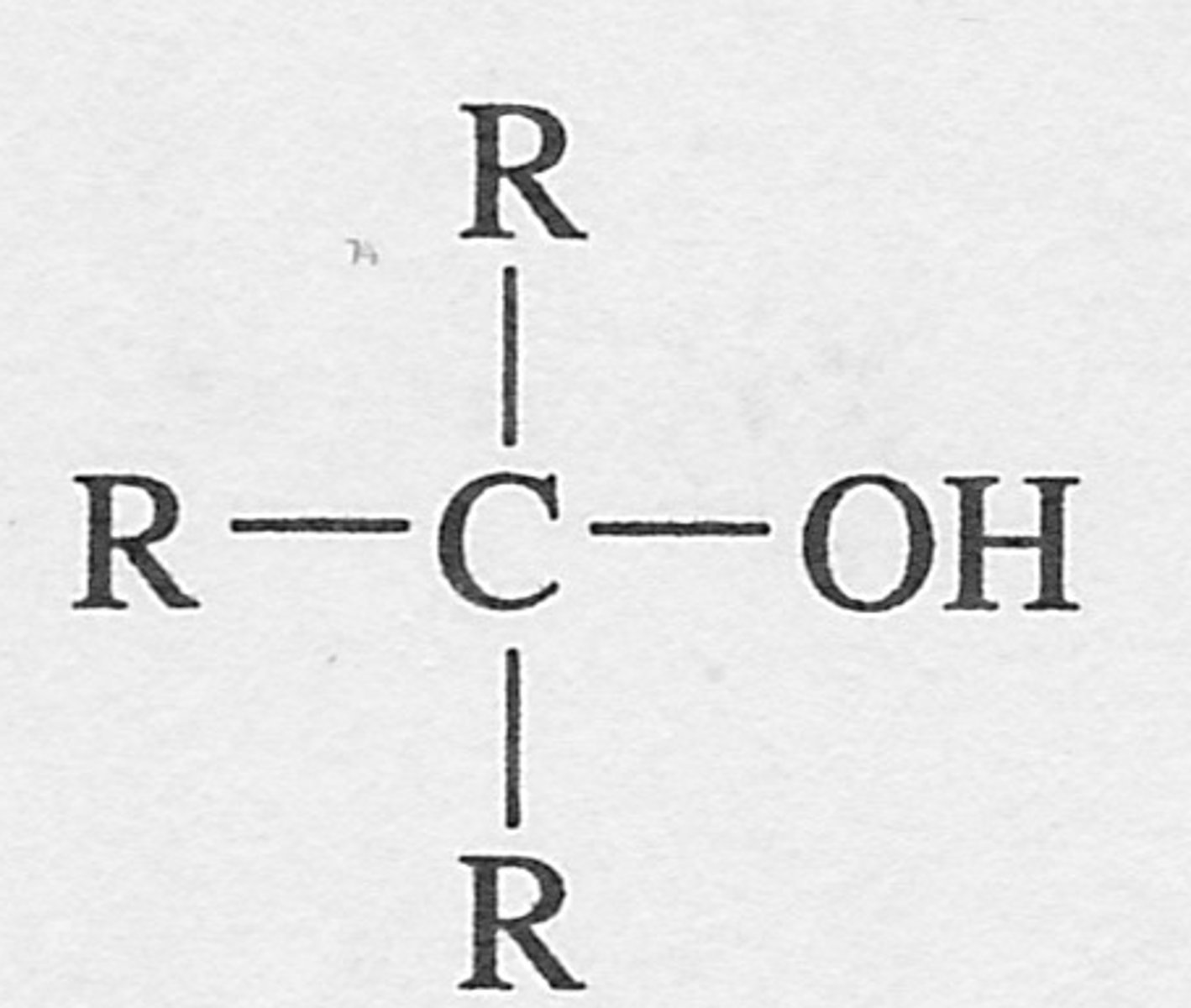
Substitution Reaction
A reaction in which one atom or group in a molecule is replaced by another (e.g., R-Cl + OH⁻ → R-OH + Cl⁻).
Sₙ2 Mechanism
A bimolecular nucleophilic substitution: nucleophile attacks backside of C bearing leaving group; inversion of configuration.
Sₙ1 Mechanism
A unimolecular nucleophilic substitution: leaving group departs first (forming carbocation), then nucleophile attacks.
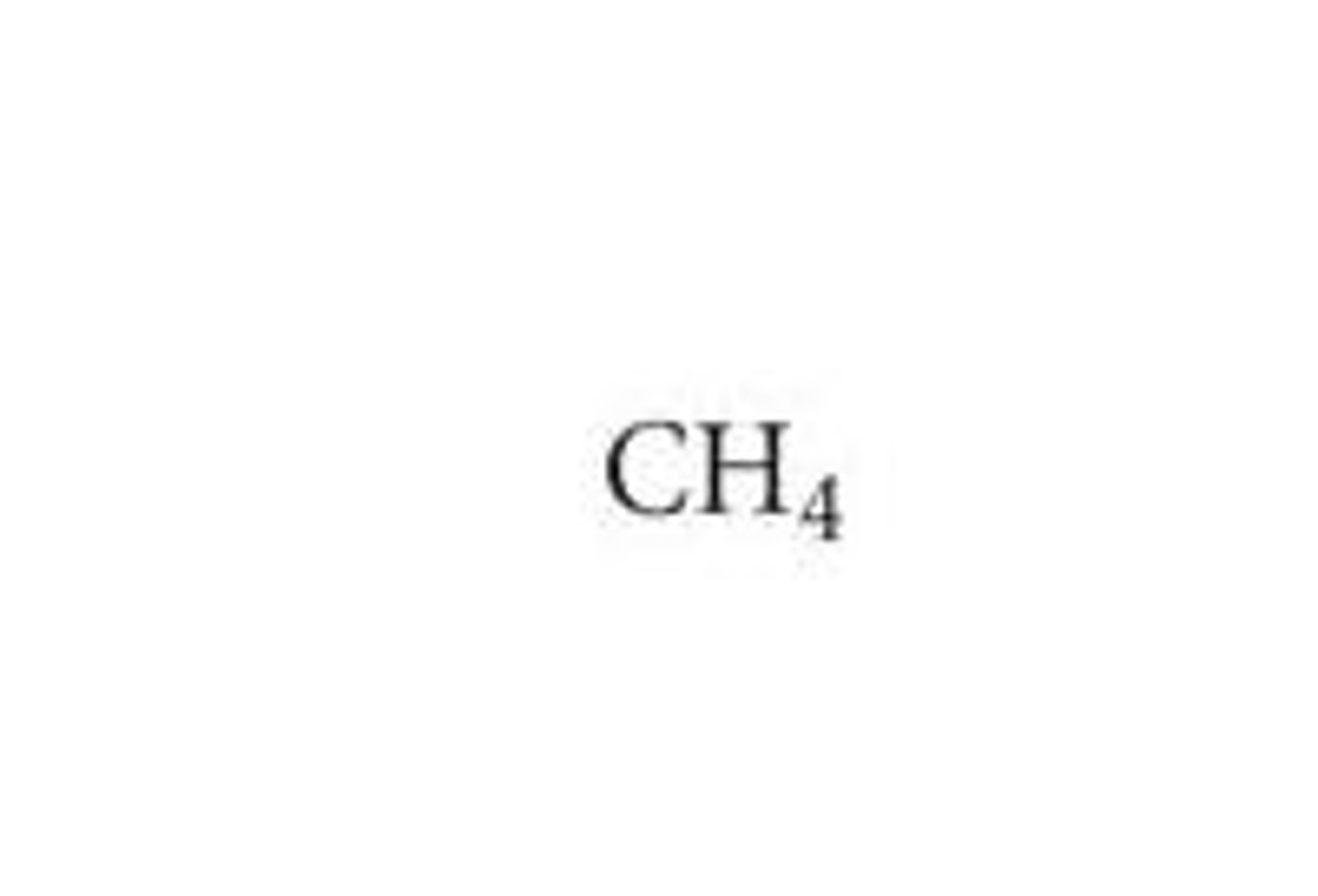
Alkane Halogenation
Radical chain reaction where an alkane (e.g., CH₄) reacts with Cl₂ (UV light) to yield R-Cl + HCl, then can form RCl₂, RCl₃, RCl₄.
Alcohol to Haloalkane (RX)
Substitution: R-OH + HCl (or PCl₃, SOCl₂) → R-Cl + H₂O. Often requires acid catalyst to convert -OH into a better leaving group.
Haloalkane to Alcohol (R-OH)
Substitution: R-Cl + OH⁻ (aqueous) → R-OH + Cl⁻ (Sₙ2 if primary).
Haloalkane to Amine
R-Cl + NH₃ → R-NH₂ + NH₄Cl. Excess NH₃ ensures primary amine formation.
Alcohol to Amine (Indirect)
First convert R-OH to R-Cl (using SOCl₂ or PCl₃), then R-Cl + NH₃ → R-NH₂.
Alkene Hydrogenation
Addition: R-CH=CH-R′ + H₂ (Pd/Pt/Ni catalyst) → R-CH₂-CH₂-R′ (alkane).
Alkene Halogenation
Addition: R-CH=CH-R′ + Br₂ → R-CHBr-CHBr-R′ (vicinal dihalide).
Alkene Hydrohalogenation
Addition: R-CH=CH₂ + HBr → R-CH₂-CH₂Br (Markovnikov addition: H to carbon with more H's).
Alkene Hydration
Addition: R-CH=CH₂ + H₂O (acid catalyst) → R-CH₂-CH₂OH (alcohol).
Addition Polymerization
Monomers with C=C link to form long chains (e.g., n CH₂=CH₂ → -[CH₂-CH₂]ₙ- polyethylene).
Primary Alcohol Oxidation (to Aldehyde)
R-CH₂-OH + [O] → R-CHO (requires mild oxidizer like PCC).
Primary Alcohol Oxidation (to Acid)
R-CH₂-OH + 2[O] → R-COOH (strong oxidizer like K₂Cr₂O₇/H₂SO₄).
Secondary Alcohol Oxidation (to Ketone)
R-CH(OH)-R′ + [O] → R-C(=O)-R′ (oxidation stops at ketone).
Tertiary Alcohol
No easy oxidation under mild conditions (no H on C-OH to remove); strong conditions may crack it.
Esterification (Acid + Alcohol)
R-COOH + R′-OH ⇌ R-COO-R′ + H₂O (acid-catalyzed; reversible condensation).
Transesterification
R-COO-R′ + R′′-OH → R-COO-R′′ + R′-OH (used in biodiesel: triglyceride + methanol → methyl ester + glycerol).
Ester Hydrolysis (Acidic)
R-COO-R′ + H₂O → R-COOH + R′-OH (acid-catalyzed; reverse of esterification).
Ester Hydrolysis (Basic, Saponification)
R-COO-R′ + OH⁻ → R-COO⁻ + R′-OH (irreversible under basic conditions; makes soap from fats).
Peptide Bond
Amide linkage (-CO-NH-) between amino acids; formed by condensation (-COOH + -NH₂ → -CO-NH- + H₂O).
Peptide Hydrolysis
Rupture of amide bond with water (and acid or enzyme catalyst) → amino acids.
Amino Acid
Contains both -NH₂ (amino) and -COOH (carboxyl) on the same carbon (plus an R side chain).
Protein (Polypeptide)
Long chain of amino acids joined by peptide (amide) bonds; structure determined by primary sequence and folding.
Monosaccharide
A single sugar unit (e.g., glucose C₆H₁₂O₆) with multiple -OH groups and a carbonyl (aldehyde or ketone).
Disaccharide
Two monosaccharides joined by a glycosidic C-O-C bond (e.g., sucrose = glucose + fructose).
Polysaccharide
Long polymer of monosaccharide units (e.g., starch, glycogen); linked via glycosidic bonds.
Glycosidic Bond
Ether linkage (C-O-C) between sugar monomers formed by condensation (-OH + -OH → -O- + H₂O).
Starch
Plant storage polysaccharide made of α-glucose units (amylose + amylopectin); digestible by animals.
Glycogen
Animal storage polysaccharide of α-glucose with more branching than starch; rapidly mobilized.
Triglyceride
Glycerol backbone (three -OH) esterified with three fatty acids; major form of dietary fat.
Fat
Solid triglyceride at room temperature; contains more saturated fatty acids → straight chains pack tightly.
Oil
Liquid triglyceride at room temperature; contains unsaturated fatty acids → kinks prevent tight packing.
Lipid Hydrolysis (Saponification)
Triglyceride + 3OH⁻ → glycerol + 3 fatty acid salts (soap).
Dispersion (London) Forces
Weak attractions arising from instantaneous dipoles; present in all molecules; increase with molecular size.
Dipole-Dipole Interaction
Attraction between permanent molecular dipoles in polar molecules; stronger than dispersion.
Hydrogen Bonding
Special dipole-dipole when H bonded to F/O/N interacts with lone pair on F/O/N; strongest intermolecular force.
Boiling Point
Increases with stronger intermolecular forces; more atoms or more polarity → higher boiling point.
Melting Point
Temperature at which a solid becomes liquid; influenced by intermolecular forces and crystal packing.
Viscosity
A measure of a fluid's resistance to flow; higher when stronger intermolecular forces (e.g., H-bonding) hinder movement.
Structural Isomers
Molecules with the same molecular formula but different connectivity (e.g., ethanol vs. dimethyl ether).
Positional Isomer
Differ only in the location of a functional group on the same carbon skeleton (e.g., 1-propanol vs. 2-propanol).
Functional Isomer
Differ in the type of functional group with the same formula (e.g., C₂H₆O = ethanol or dimethyl ether).
Green Solvent Replacement
Using water, bioethanol, supercritical CO₂, or recyclable ionic liquids to reduce hazardous VOCs.
Biodegradable Packaging
Packaging made from materials that microbes can break down into harmless substances (e.g., PLA from corn starch).
Seaweed-Derived Polymer
Polymers (e.g., agar, alginate, carrageenan) from seaweed; used for biodegradable plastics or edible films.
Solvent Toxicity Reduction
Designing processes to avoid carcinogenic or organ-toxic solvents (e.g., replacing benzene with ethanol).
Biofuel Generation
Classification of biofuel feedstocks by "generations" (1st-4th) based on origin and sustainability.
First-Generation Biofuel
Feedstock: edible crops (e.g., corn, sugar cane); Process: transesterification of crop oil under alkaline conditions; Pro: renewable; Con: competes with food supply.
Transesterification
A reaction in which a triglyceride's glycerol portion is replaced by an alcohol (e.g., methanol) to yield biodiesel (methyl esters) and glycerol.
Second-Generation Biofuel
Feedstock: nonfood biomass (e.g., waste cooking oil, forest residues); Process: pretreat biomass, extract oils or ferment sugars, then transesterify; Pro: avoids food vs. fuel; Con: energy-intensive pretreatment.
Third-Generation Biofuel
Feedstock: algae/microalgae; Process: cultivate algae (using CO₂) → harvest → extract algal lipids → transesterify to biodiesel; Pro: high productivity, CO₂ capture; Con: costly scale-up.
Fourth-Generation Biofuel
Feedstock: genetically engineered cyanobacteria; Process: modify metabolism so cyanobacteria excrete lipids/biofuels while fixing CO₂; Pro: potentially carbon-negative; Con: R&D stage, high cost, biosafety.
Catalyst
A substance that increases reaction rate or selectivity without being consumed; provides an alternative lower‐energy pathway.
Catalyst Selectivity
The ability of a catalyst to direct reactants toward a desired product while minimizing side-products.
Catalyst Energy Reduction
Using catalysts often allows reactions to proceed at lower temperatures/pressures, saving energy and reducing emissions.
Catalyst for Renewable Feedstocks
Catalysts can enable efficient conversion of biomass‐derived materials into target chemicals, making use of renewable raw materials.
Catalyst Safety
Running reactions at milder conditions (lower T, gentler reagents) reduces risk of thermal runaway, toxic by-products, or explosions.
Catalyst Longevity
A good heterogeneous catalyst can be recovered and reused many times, minimizing waste of catalytic material.