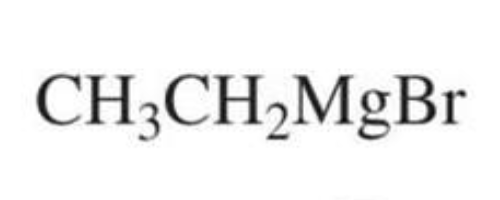Reactions Chapter 12
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
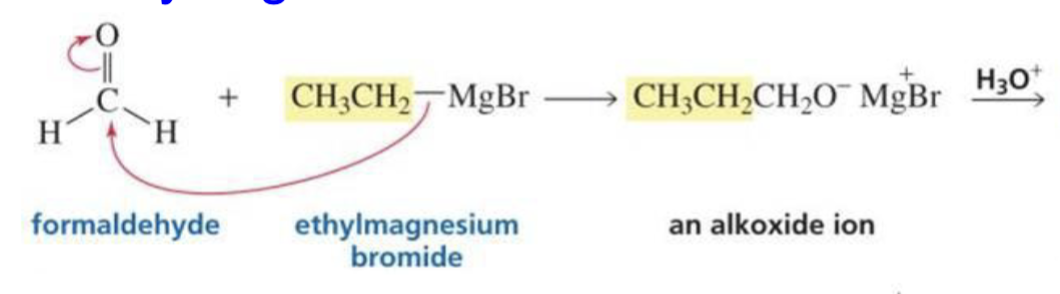
What is formed with:
Formaldehyde + Grignard + H3O+
Primary alcohol
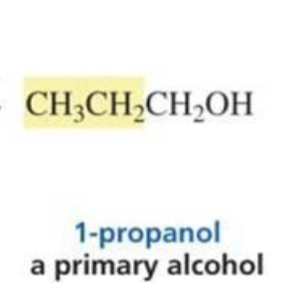
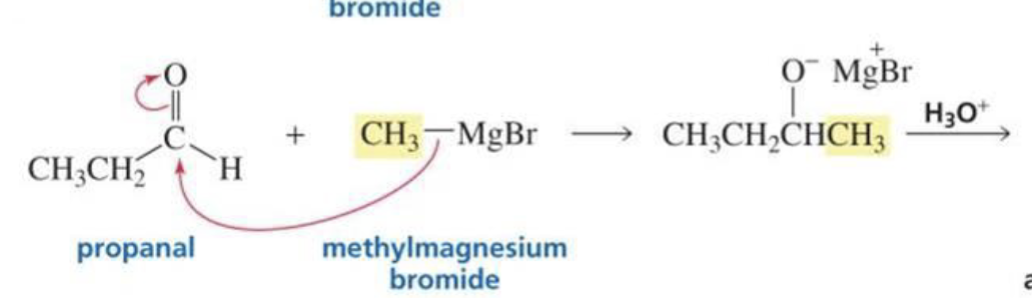
What is formed with:
Aldehyde (other than formaldeyde) + Grignard + H3O+
Secondary alcohol
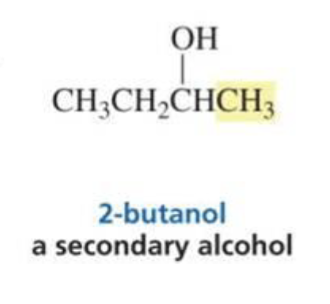
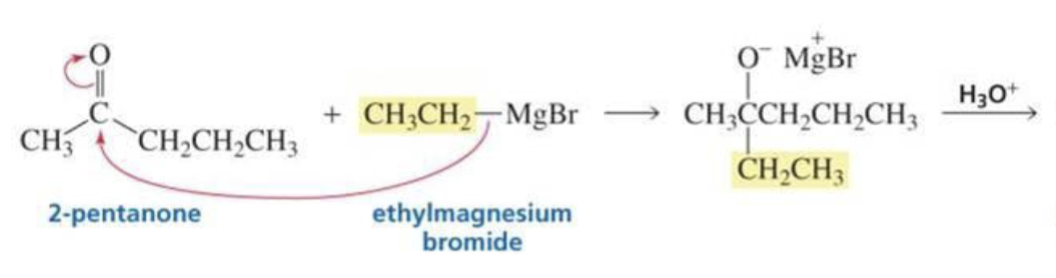
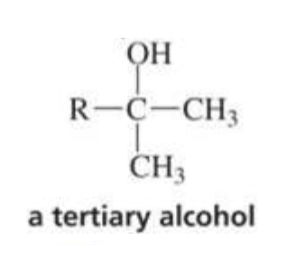
What is formed with:
Ketone + Grignard + H3O+
Tertiary alcohol
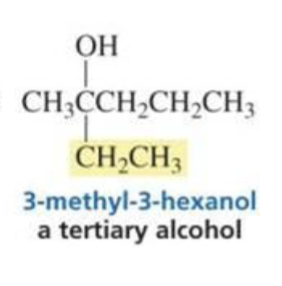
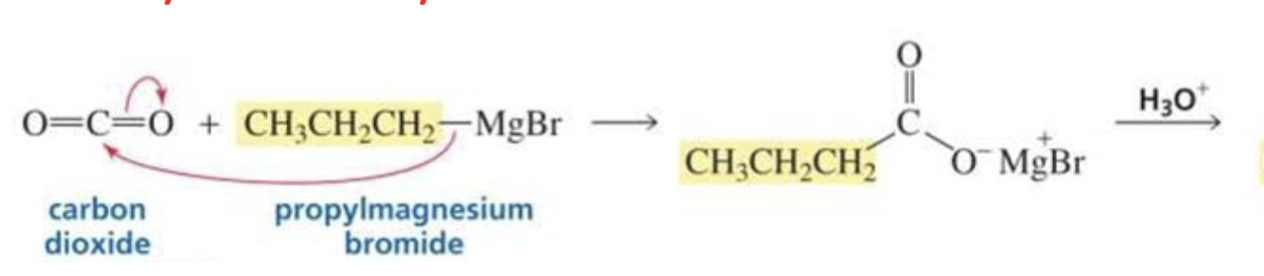
What is formed with:
CO2 + Grignard
Carboxylic acid
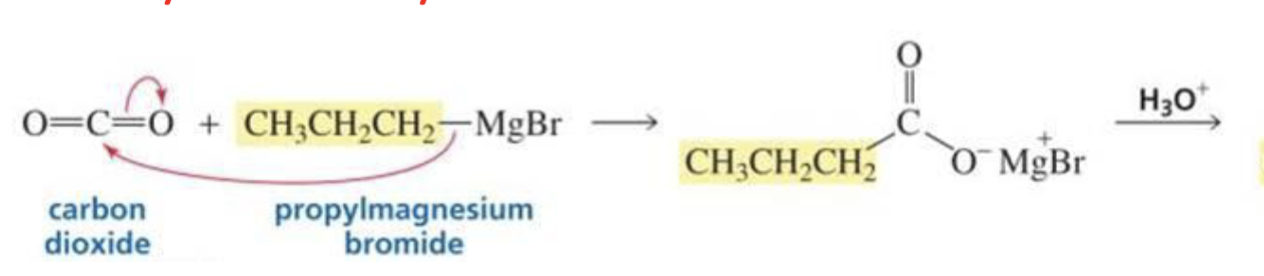
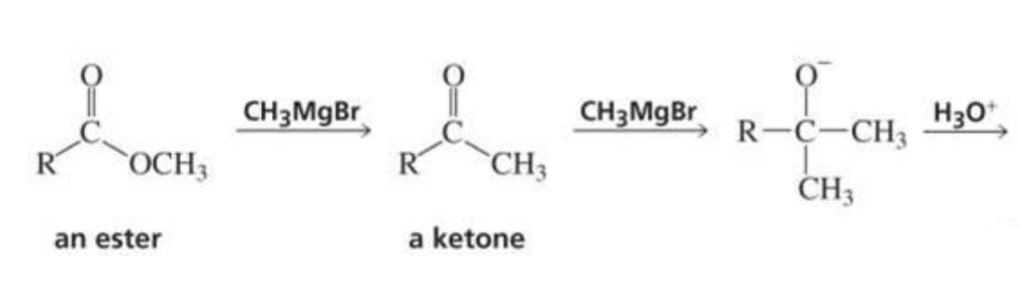
What is formed with:
Ester + Grignard
and special note?
Tertiary alcohol
Has two of the same R groups!
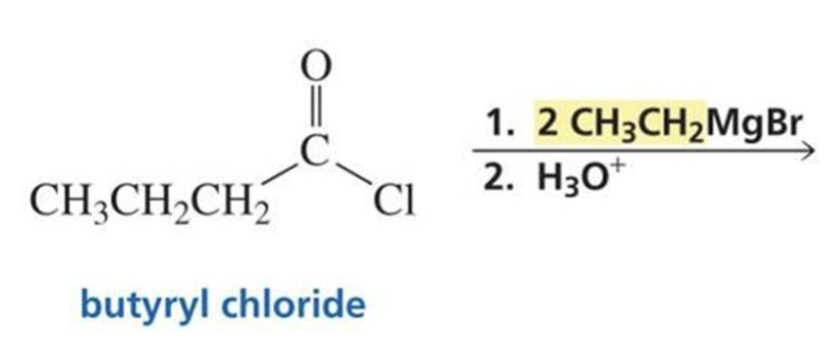
What is formed with:
Acyl chloride + Grignard
and special note?
Tertiary alcohol
Has two of the same R groups!
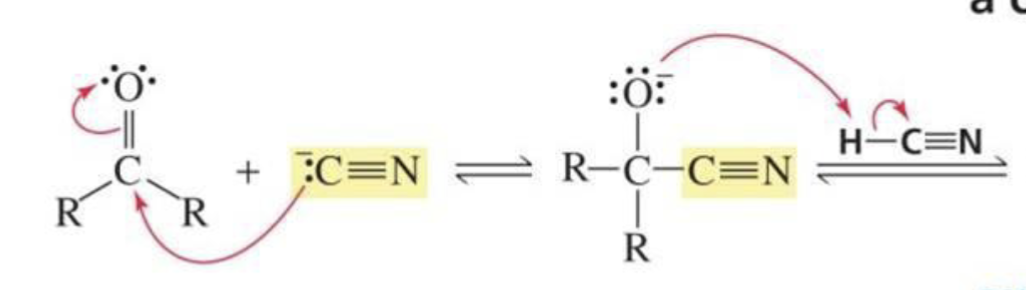
What is formed with:
Aldehyde/ketone + cyanide ion
Cyanohydrin
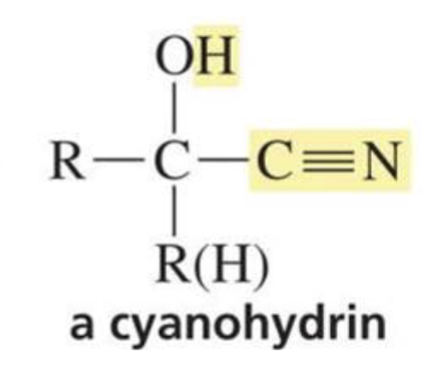
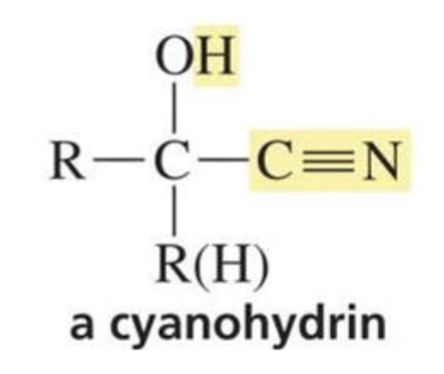
Reactions of cyanohydrins:
Can be used to make α-hydroxycarboxylic acid or reduced to a primary amine with an OH group on the β carbon
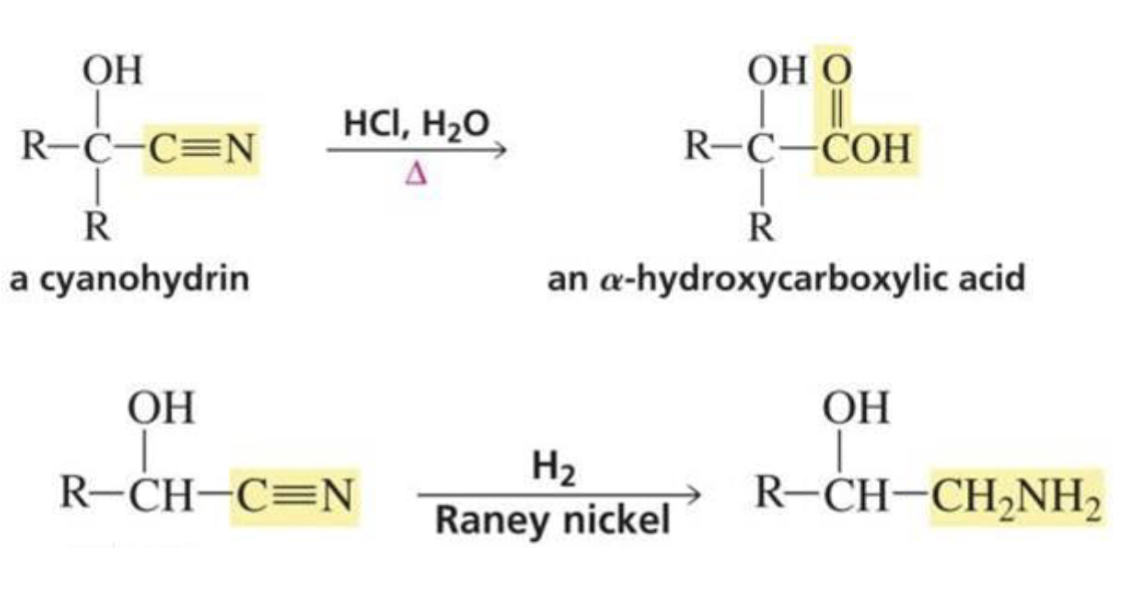
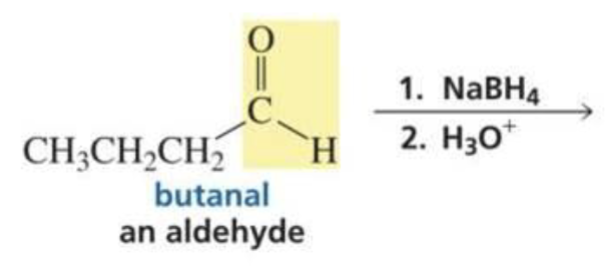
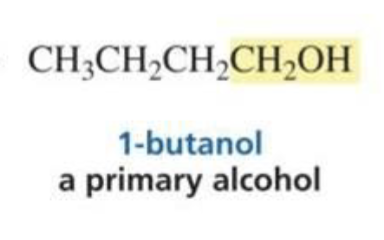
What is formed with:
Aldehyde + hydride ion (BH4)
Primary alcohol
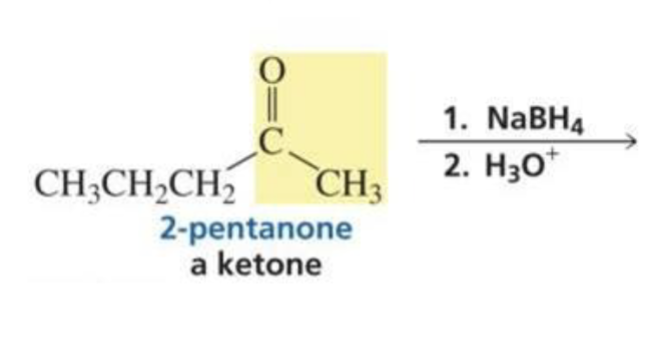
What is formed with:
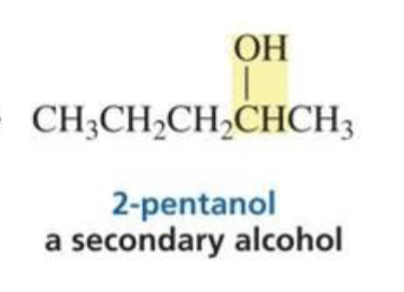
Ketone + hydride ion (BH4) + H3O+
Secondary alcohol
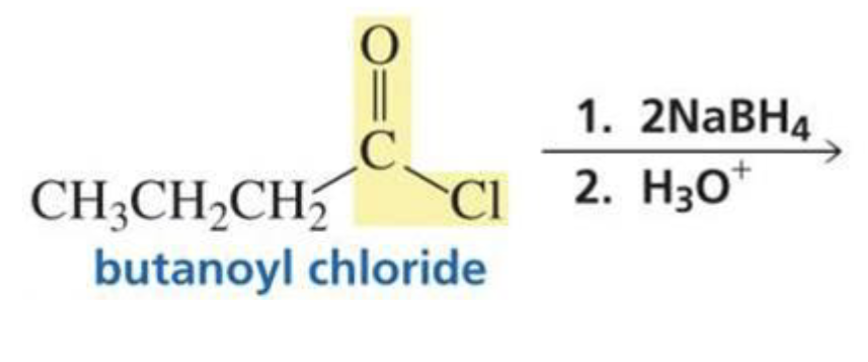
What is formed with:
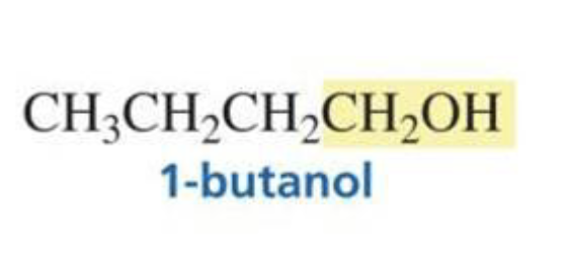
Acyl chloride + hydride ion (BH4) + H3O+
Primary alcohol
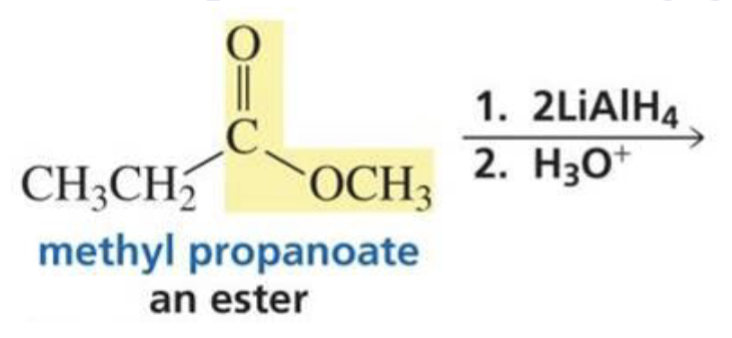
What is formed with:
Ester + hydride ion (LiAlH4) + H3O+
2 primary alcohols
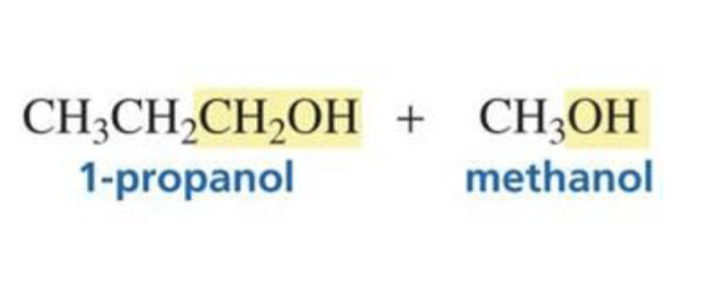
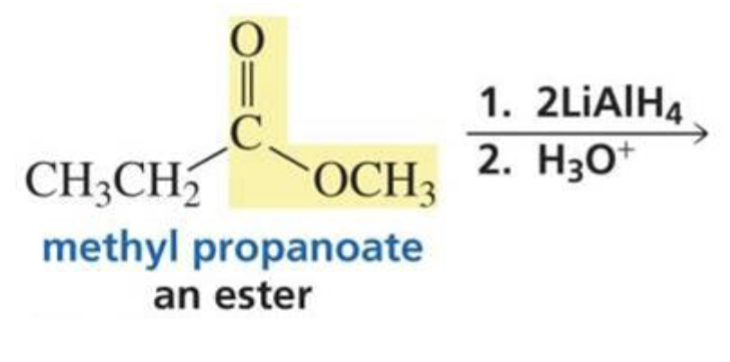
What is formed with:
Carboxylic acid + hydride ion (LiAlH4) + H3O+
Primary alcohol
(same # of C as the carboxylic acid)
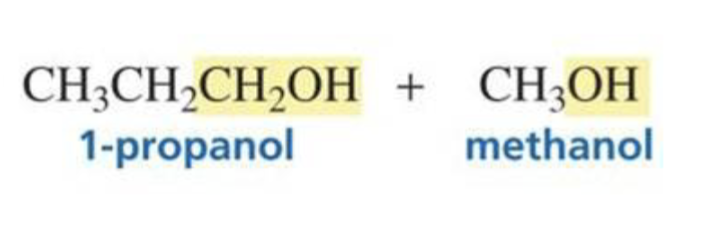
What is formed from:
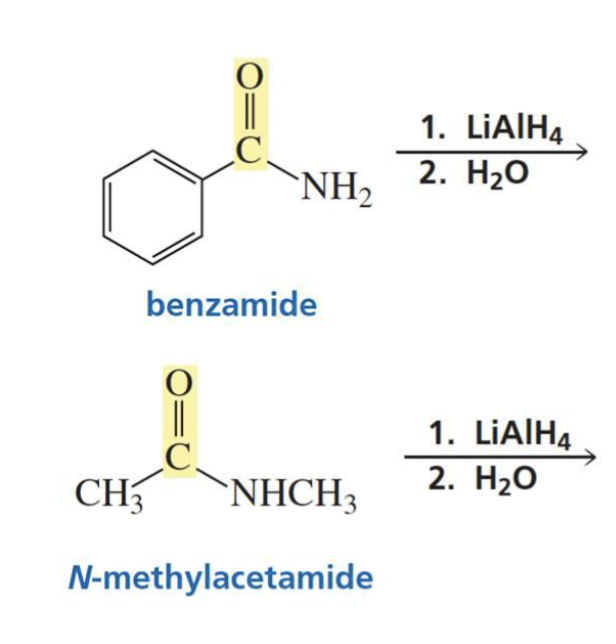
Amide + hydride ion (LiAlH4) + H2O
and special note?
Primary, secondary, or tertiary amine (depends)
H2O is used instead of H3O+
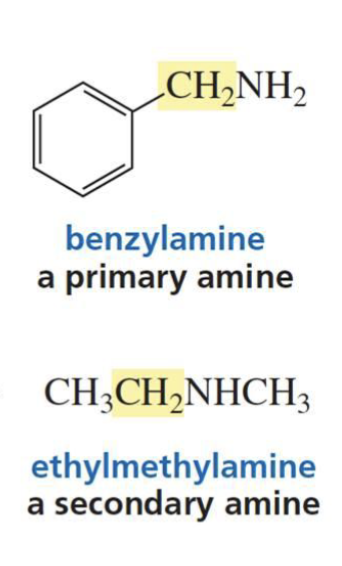
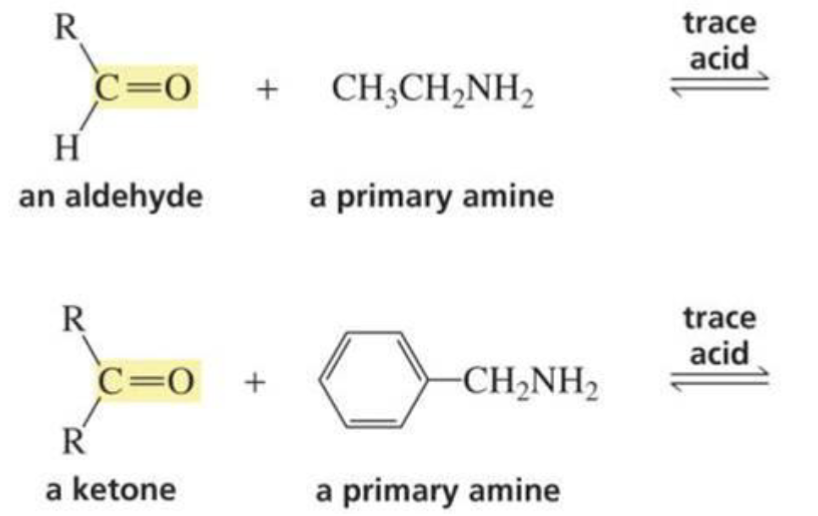
What is formed with:
Aldehyde/ketone + amine + trace acid
Imine
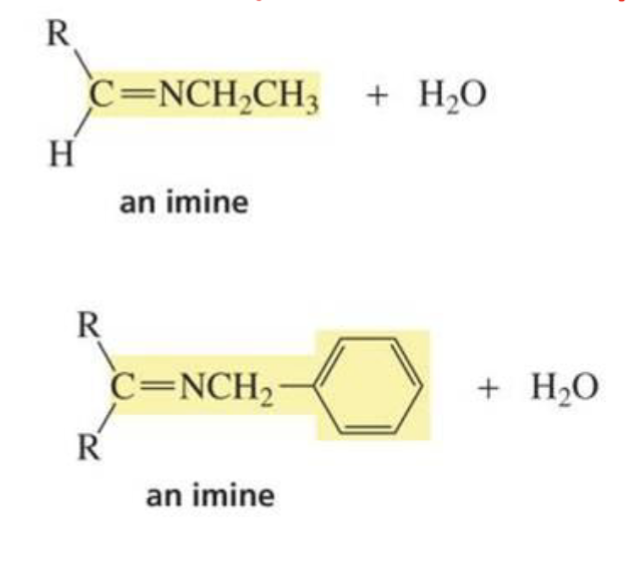
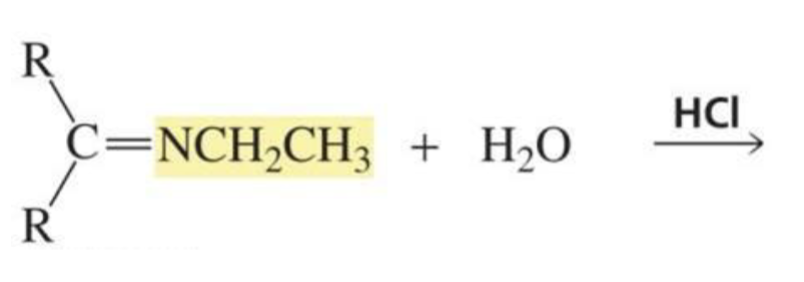
What is formed with:
Imine + H2O + trace acid
Aldehyde/ketone + protonated amine
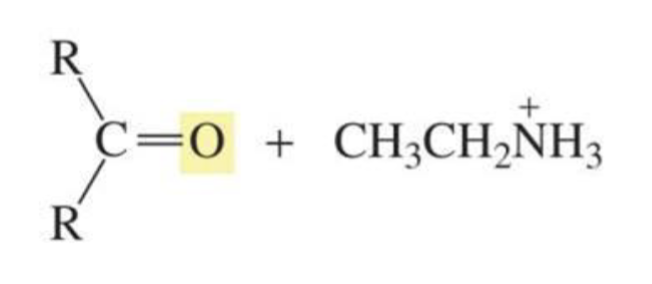
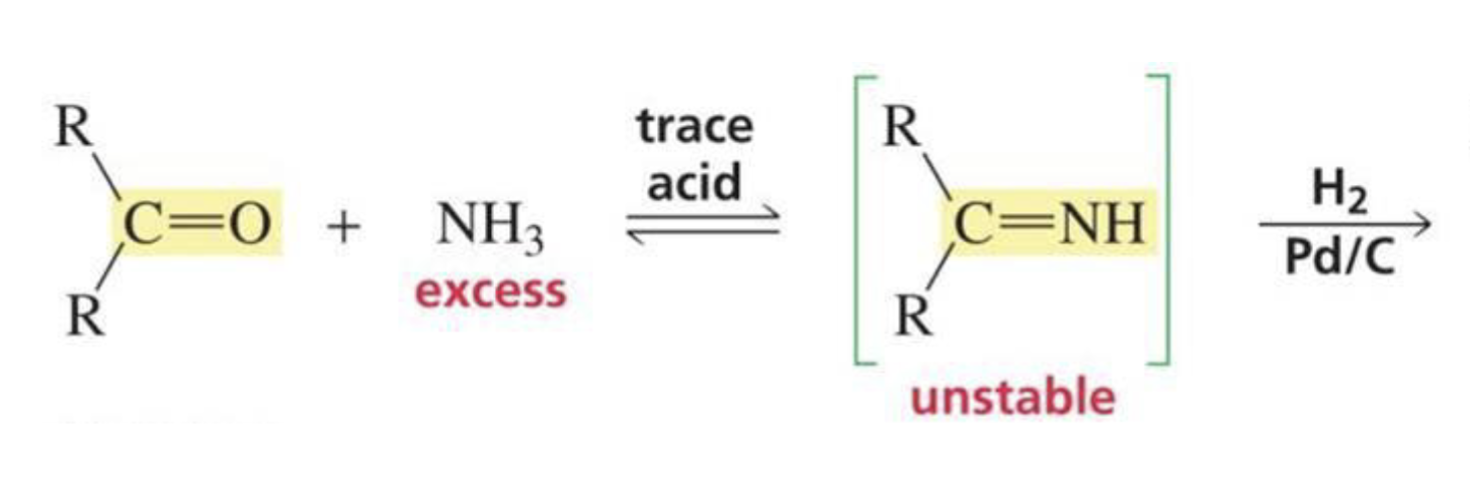
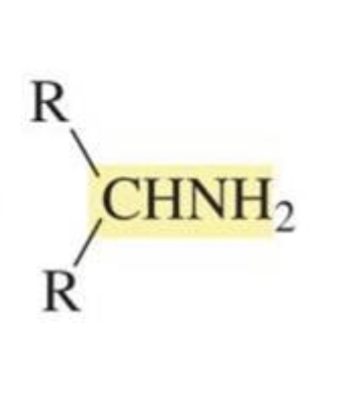
What is formed with:
Aldehyde/ketone + ammonia + trace HCl + H2 + Pd/C
Amine
(reductive amination)
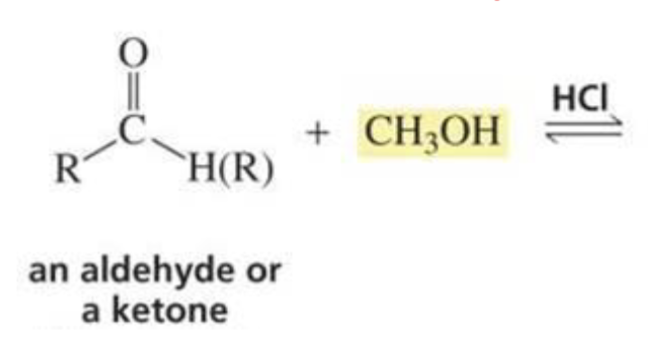
What is formed with:
Aldehyde/ketone + alcohol + HCl
Acetal
(reactions of aldehydes and ketones w/ alcohol)
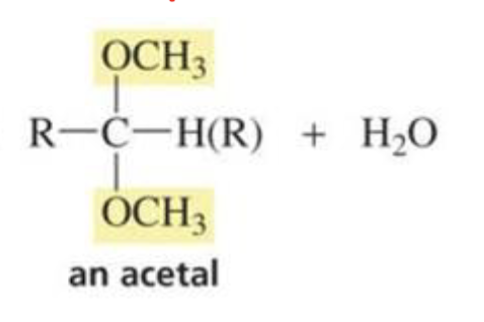
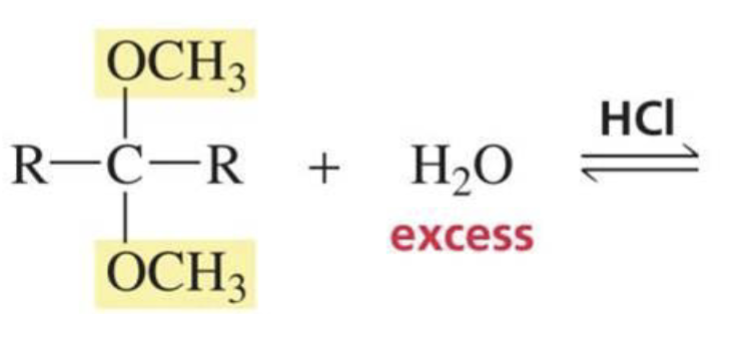
What is formed with:
Acetal + H2O + HCl
Aldehyde/ketone + alcohol
(reverse reaction, reactions of aldehydes and ketones w/ alcohol)
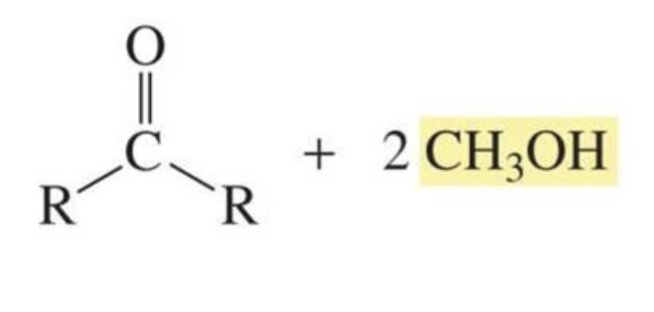
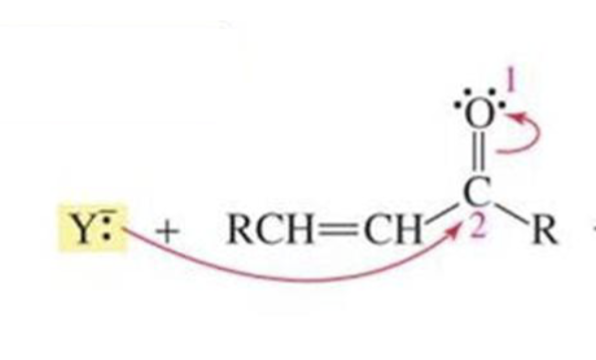
What is formed with:
α,β-unsaturated aldehyde/ketone + STRONG nucleophile + H2O
(can have 1) NaBr4 then 2) EtOH
1,2 direct addition
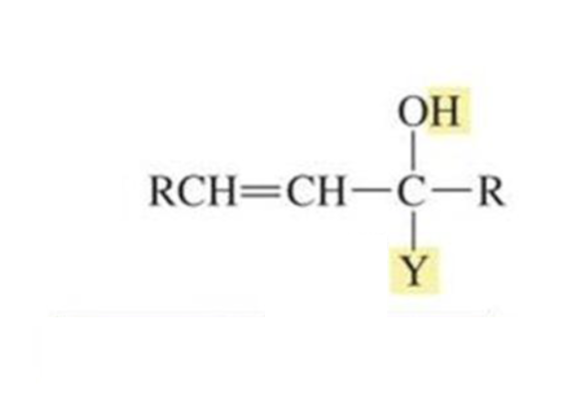
What is formed with:
α,β-unsaturated aldehyde/ketone + WEAK nucleophile + H2O
1,4 conjugate addition
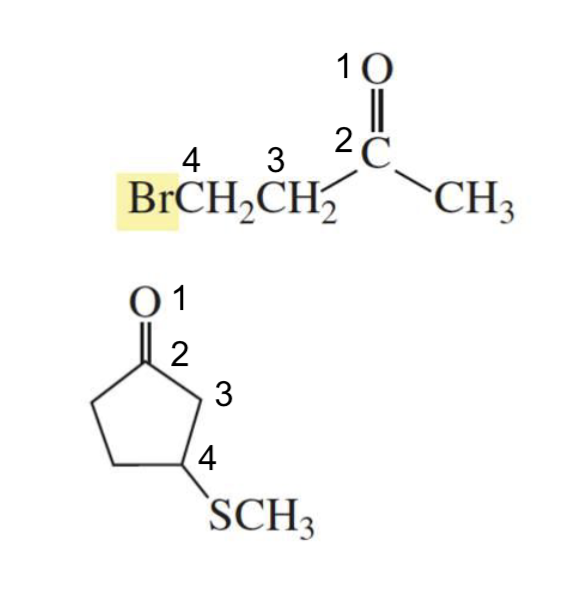
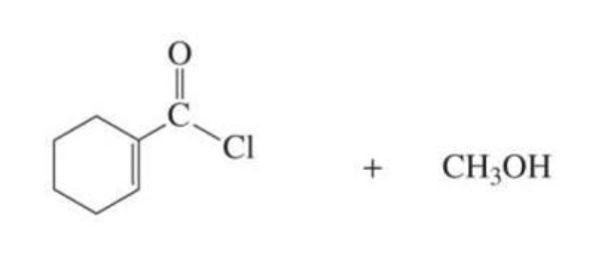
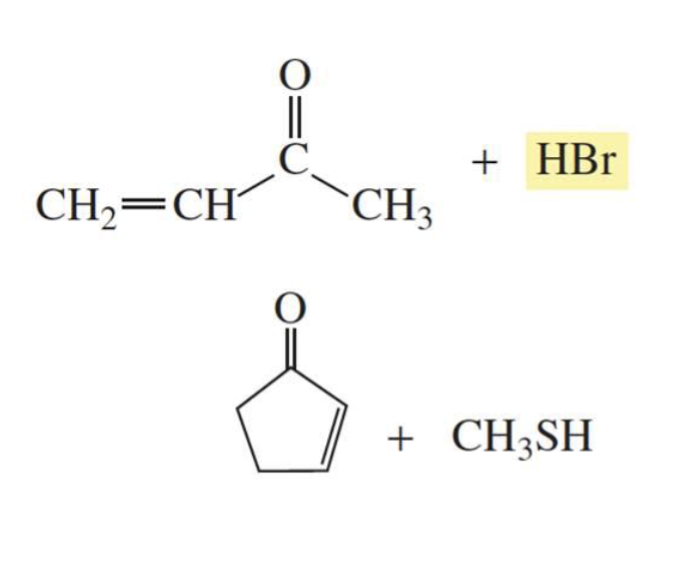
What is formed with:
REACTIVE α,β-unsaturated carboxylic acid derivative + nucleophile
Nucleophilic addition-elimination products
reactive: acyl chlorides
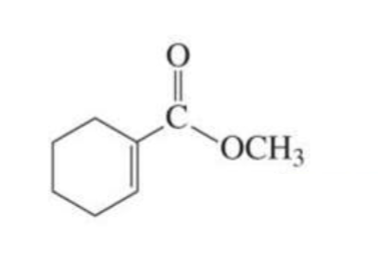
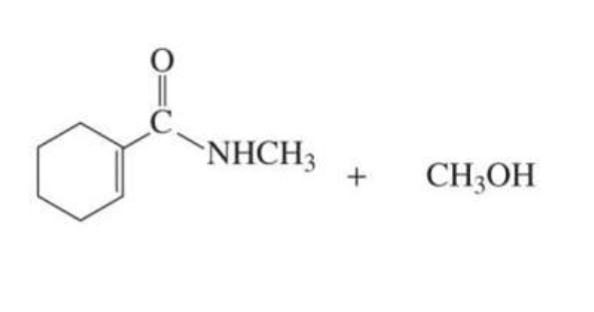
What is formed with:
LESS REACTIVE α,β-unsaturated carboxylic acid derivative + nucleophile
1,4 conjugate addition
less reactive: esters and amides
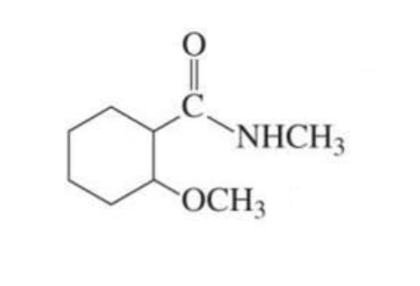
Carboxylic acid derivatives undergo…
Nucleophilic acyl substitution (Y- can leave)
Aldehydes + ketones undergo…
Nucleophilic addition (neither alkyl nor hydride groups can leave)
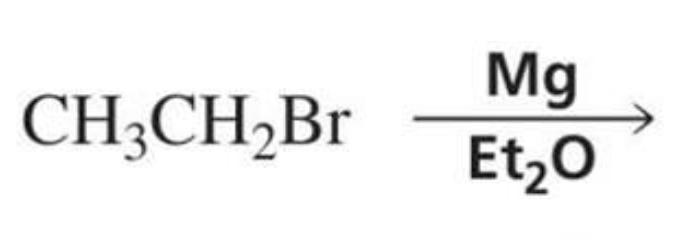
What is formed with:
Alkyl halide + Mg + Et2O (diethyl ether)
Grignard reagent