Bonding, structure & properties of atoms topic 2
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
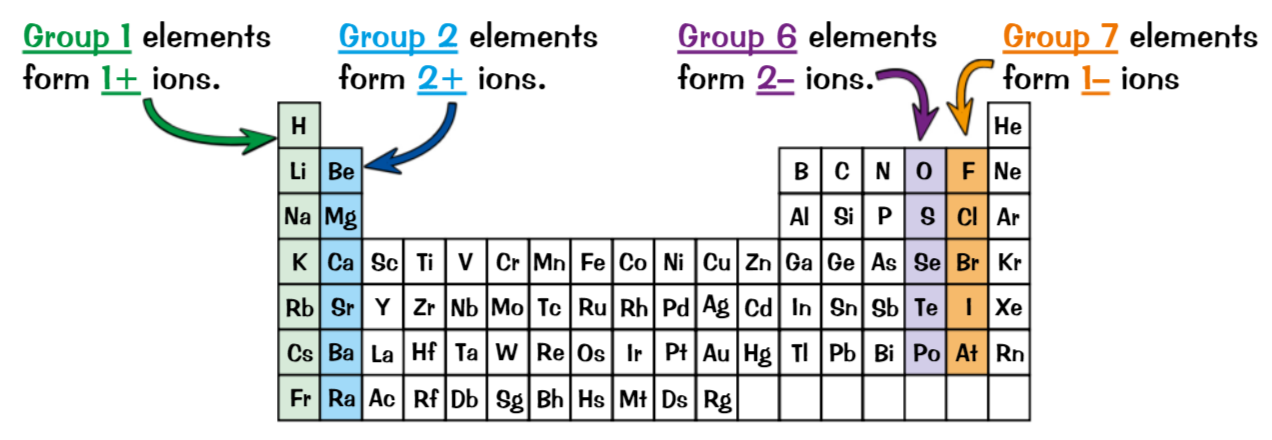
What groups make cations, what groups make anions, group no. is same as…
Group 1 & 2 elements = metals, lose electrons = positive ions (cations)
Group 6 & 7 elements = non-metal, gain electrons = negative ions (anions)
group number = number electrons in outer shell, form ions with same charges
Examples of group 1,2,6,7 elements (sodium, magnesium, chlorine, oxygen)
Sodium (Na) group1 → loses electron to form sodium ion (Na+), same electronic structure as neon: Na →Na++e-
magnesium atom (Mg) group 2 → loses 2 electrons, (Mg2+), same electronic structure as neon: Mg → Mg2+2e-
Chlorine atom (Cl) group 7 → gains 1 electron, (Cl-), same electronic structure as argon: Cl +e → Cl-
oxygen atom (O) group 6 → gains 2 electrons (O2-), same electronic structure as neon: O+2e- → O2-
what reacts tgther, what forms positive ions/ negative ions, oppositely charged ions are…
Metal & non-metal react together
metal loses electrons = positive ion,
Non-metal gains these electrons = negative ion
oppositely charged ions → strongly attracted to one another by electrostatic forces (ionic bond)
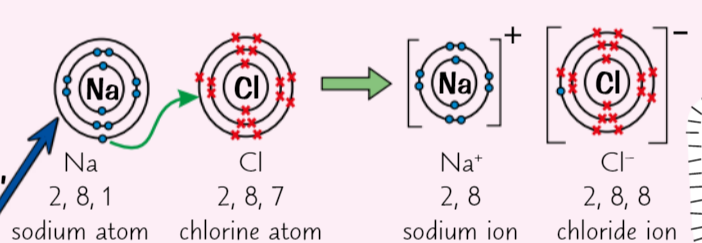
What does Sodium chloride do (NaCl), What does chlorine then do
Sodium chloride (NaCl) → sodium give atom up to its outer electron = Na+ ion
Chlorine atom picks up electron becoming Cl- (chloride) ion
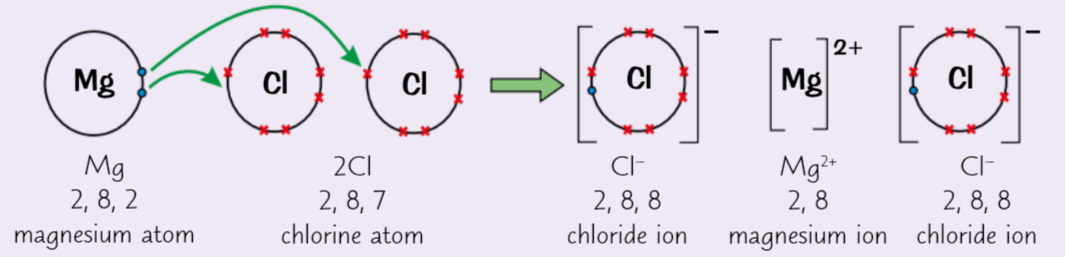
what does Magnesium chloride (MgCl2) do, what does chlorine then do
Magnesium atom gives up 2 outer electrons = Mg2+
2 chlorine atoms pick up 1 electron each = 2 Cl- (chloride) ions
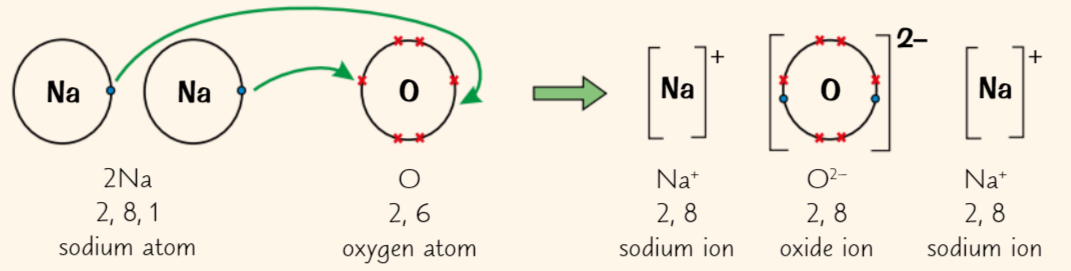
what does Sodium Oxide (Na2O) do, and what does oxygen then do
2 sodium atoms each give up single outer electrons = 2 Na+ ions
Oxygen atom picks up 2 electrons = O2- ion
whats an ionic compounds structure, what do ions form..
Ionic compounds structure = giant ionic lattice
Ions form closely packed regular lattice arrangement & very strong electrostatic forces of attraction between oppositely charged ions (all electrons)
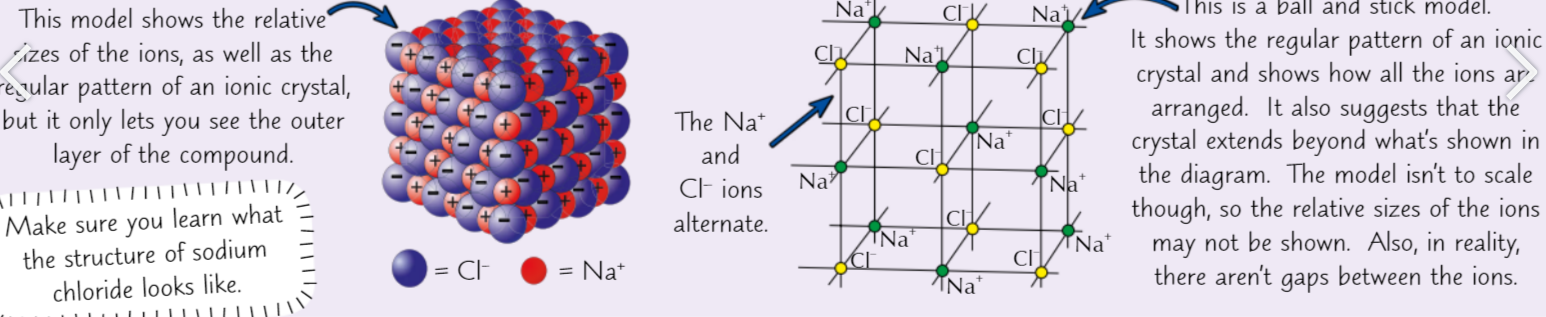
what does a single crystal of sodium chloride make, What r they held tgther in
single crystal of sodium chloride (table salt) = 1 giant ionic lattice. Na+ & Cl- ions held together in regular lattice (can be represented in different ways)
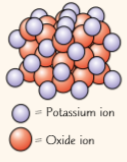
whats K₂O (its ions), what groups r they ions & charges, how do they balance out for empirical formula
compound contains potassium & oxide ions
potassium (group 1) = 1+ ions
oxygen (group 6) = 2- ions
potassium ion only has 1+ charge, need balance out 2- charge of oxide ion, empirical formula = K2O
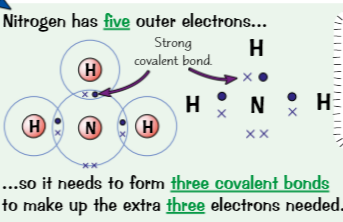
how to draw dot & cross diagrams, its useful because..
Electrons drawn in overlap & the in between has shared atoms
useful → showing which atoms electrons in covalent bond come from, don’t show relative sizes/ how atoms arranged
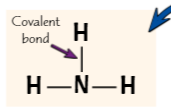
Displayed formula shows covalent bonds as single lines between atoms - Ammonia (pro, 2 cons)
great way showing how atoms connected in large molecules,
dont show 3D structure of molecule/ which atoms electrons in covalent bond come from
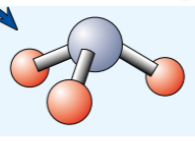
3D model ammonia shows atoms can…, they dont show…, can find molecular formula of…
can quickly get confusing for large molecules where lots atoms to include
dont show where electrons in bonds come from
can find molecular formula of simple molecular compound by counting up how many atoms of each element there are
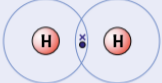
example of simple molecular substances - hydrogen, H2
have 1 electron in outer shell = need 1 more to complete outer shell
often form single covalent bonds with hydrogen atoms or other elements
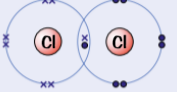
example of simple molecular substances - chlorine, Cl2
needs 1 more electron to complete outers shell
2 chlorine atoms can she pair of electrons & form single covalent bond
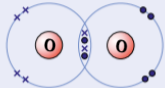
example of simple molecular substances - oxygen, O2
needs 2 more electrons to complete outer shell
in oxygen gas 2 oxygen atoms share 2 pairs electrons with each other making double covalent bond
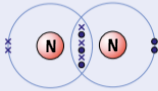
example of simple molecular substances - nitrogen, N2
need 3 more electrons
2 nitrogen atoms share 3 pairs electrons to fill outer shell creating triple bond
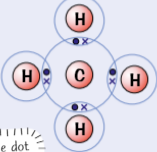
example of simple molecular substances - methane, CH4
carbon has 4 outer electrons (half full shell)
form 4 covalent bonds with hydrogen to fill outer shell
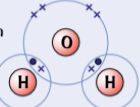
example of simple molecular substances - water, H2O
in water molecules, oxygen shares 2 pairs electrons with 2 H to form 2 single covalent bonds
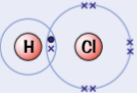
example of simple molecular substances - Hydrogen chloride, HCl
similar to H2 & Cl2
both atoms need 1 more electron to complete their outer shells
Substances containing covalent vonds usually have? Atoms within the molecules are held together by? To melt/boil a simple molecular compound? Most are? Molecules get bigger when? Molecular compounds dont conduct?
substances containing covalent bonds usually have simple molecular structures
atoms within molecules held together by very strong covalent bonds, forces of attraction between molecules are very weak
to melt/ boil simple molecular compound → only need feeble intermolecular forces & not covalent bonds, melting & boiling points are very low (molecules are easily parted from each other)
most are gases/ liquids at room temperature
molecules get bigger = strength of intermolecular forces increases, more energy needed to break them, mp & bp increase
molecular compounds don’t conduct electricity → aren’t charged, so no free electrons/ ions
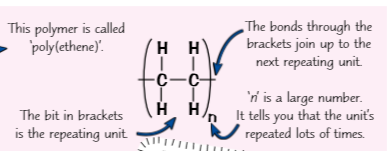
Polymer are made of? Atoms are joined together by? Whats a repeating unit? How to find molecular formula of polymer? Intermolecular forces between polymer molecules r larger than? Intermolecular forces are weaker than?
lots of small units linked together forming long molecule has repeating sections
atoms joined together by covalent bonds
draw shortest repeating section (repeating unit)
find molecular formula of polymer → write molecular formula of repeating unit in brackets & put ‘n’ outside (C2H4)n
intermolecular forces between polymer molecules' are larger than simple covalent molecules → more energy needed to break them, most solid at room temperature
intermolecular forces weaker than ionic covalent bonds = lower boiling points
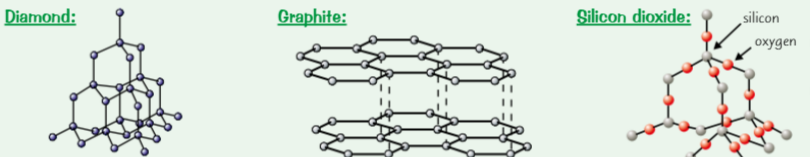
Properties of main macromolecules (diamond, graphite, silicon dioxide)
diamond → each carbon atom forms 4 covalent bonds in very rigid giant covalent structure
Graphite → each carbon atom forms 3 covalent bonds to create layers of hexagons, each carbon atom has 1 delocalised (free) electron
Silicon dioxide → ‘silica’, what sand is made of, each grain is 1 giant structure of silicon & oxygen
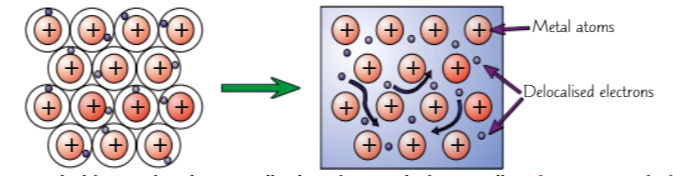
Metallic bonding involves delocalised electrons (consist, outer & attraction, hold, substances, properties)
Metals consist of giant structures
electrons in outer shell of metal atoms are delocalised (free to move around), strong forces of electrostatic attraction between the positive metal ions & shared negative electrons
forces of attraction hold the atoms together in regular structure → metallic bonding (very strong)
substances held by metallic bonding include metallic elements & alloys
delocalised electrons in metallic bonds produce all properties of metals
Most metals are solid at room temperature (strong, points)
Electrostatic forces between metal atoms & delocalised sea of electrons = very strong, need lots energy to be broken
means most compounds with metallic bonds have very high melting & boiling points = generally solid at room temperature
Metals are good conductors of electricity & heat (charge)
delocalised electrons carry electric charge & thermal (heat) energy through whole structure = metals are good conductors of electricity & heat
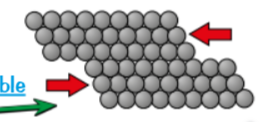
most metals are malleable (shaped)
layers of atoms in metal can slide over each other = making metals malleable → can be bent/ hammered/ rolled into flat sheets
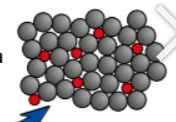
Alloys are harder than pure metals (pure metals & fix, alloys & what, slide)
pure metals → arent right for certain jobs, often too soft when pure so are mixed with other metals to make them harder.
Most metals we use are alloys (mixture 2/more metals or metals & another element) are much harder & more useful
different elements = different sized atoms, when another element is mixed with pure metals the new metals atoms will distort layers of metal atoms = more difficult for them to slide over each other = makes alloys harder than pure metals
3 states of matter - solid, liquid gas (state depends, how strong forces, particle theory)xa\q
state of something is at a certain temperature (solid, gas, liquid) depends on how strong the forces of attraction between particles of material
how strong forces are depends on:
Material (structure of substance & type bonds holding particles together)
Temperature
Pressure
Use particle theory to explain how particles in a material behave in each of 3 states of matter by considering each particle as a small, solid, inelastic, sphere

Solids (state of matter) (forces, don’t move, vibrate)
strong forces of attraction between particles, holds them close together in fixed positions to form very regular lattice arrangement
particles don’t move from positions, all solids keep definite shape & volume, don’t flow like liquids
particles vibrate about positions → hotter solid becomes the more they vibrate (causing solids expand slightly when heated)
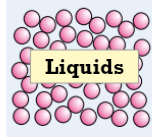
Liquids (state of matter) (force, definite, random motion)
weak force of attraction between particles, randomly arranged & free to move around but tend to stick closely together
definite volume but don’t keep a definite shape & flow to fill the bottom of container
particles constantly moving with random motion, the hotter liquid gets the faster they move (causes liquids expand slightly when heated)
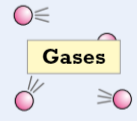
Gases (state of matter) (force, definite, random motion)
force of attraction between particles = very weak, free to move & are far apart, particles in gases travel in straight lines
don’t keep definite shape or volume & always fill any container
particles move constantly with random motion, hotter gas gets the faster they move (either expand when heated or pressure increases)
Particle theory model (state of matter) (great, reality, doesn’t show)
great model for explaining 3 states of matter but isn’t perfect
in reality particles aren’t solid/ inelastic & aren’t spheres (they’re atoms, ions, molecules)
model doesn’t show forces between particles, no way of knowing how strong they are
state symbols tell you the state of substance in equation (s, l, g, aq, example: aqueous hydrochloric acid + solid calcium carbonate → aqueous calcium chloride + liquid water + carbon dioxide gas)
(s) = solid
(l) = liquid
(g) = gas
(aq) = aqueous → dissolved in water
example → 2HCl(aq) + CaCO3(s) → CaCl2(aq) +H2O(l) + CO2(g),
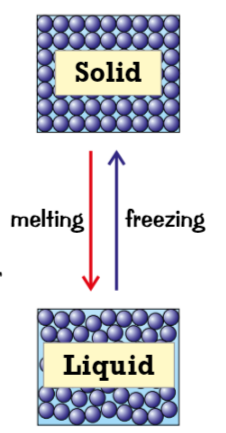
Melting (changes of state) (heated, vibrate, melting point)
when solid is heated, particles gain more energy
makes particles vibrate more, weakens the forces that hold solid together
at certain point (melting point) the particles have enough energy to break free from positions → melting & solid turns into a liquid

Boiling (change of state) (heated, faster, boiling point)
when liquid is heated, particles get even more energy
energy makes particles move faster, weakens & breaks bonds holding liquid together
At certain point (boiling point) particles have enough energy to break their bonds → boiling/ evaporation, liquid becomes gas
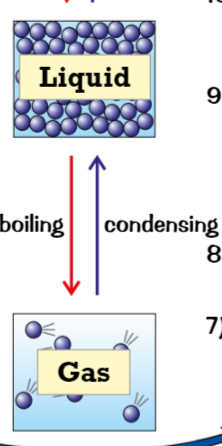
condensing (change of state) (cools,, bonds form, boiling point)
gas cools, particles no longer have enough energy to overcome forces of attraction between them
bonds form between particles
at boiling point many bonds have formed between gas particles, gas becomes liquid → condensing
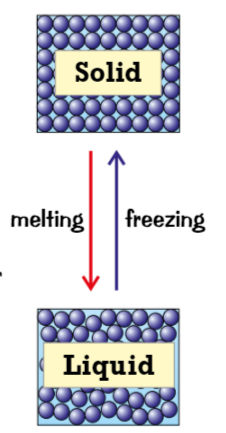
freezing (change of state) (cools, not enough, melting point)
when liquid cools, particles have less energy so move around less
not enough energy to overcome attraction between particles, more bonds form between them
at melting point, so many bonds have formed between particles that they’re held in place, liquid becomes sloid → freezing
how to predict state of a substance (below, above, in between)
if temperatures below melting point of substance it’ll be a solid
if its above the boiling point it’ll be a gas
if its in between 2 points its a liquid
examples of nanoparticles used (sun cream, better, not clear, washed away)
used in sun cream → shown to be better than materials in traditional sun creams at protecting skin from harmful UV ray
give better skin coverage than traditional sun creams
not clear whether nanoparticles can get into body & if they do whether might damage cells
possible when they are washed away they might damage environment