10 - Cytoskeleton and Motors: Actin and Myosin; Microtubules
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Microtubules
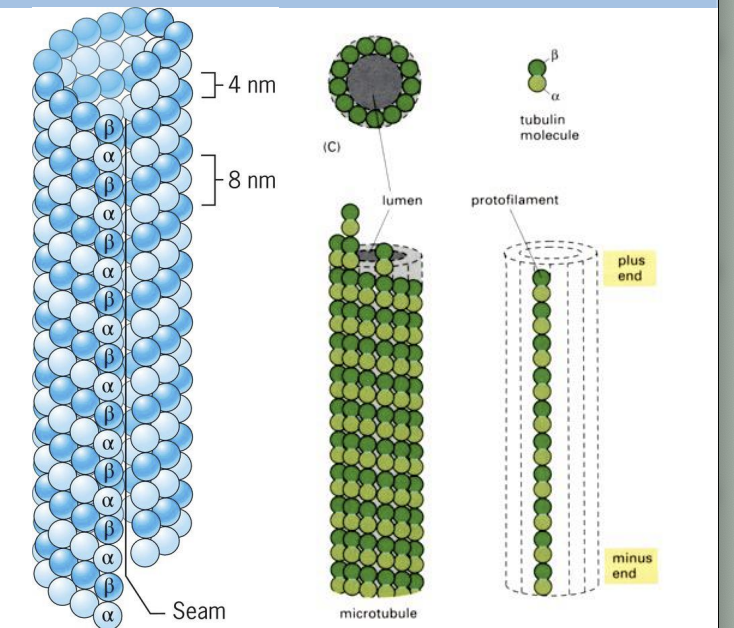
hollow, tubular structures found in almost all eukaryotic cells.
Microtubules (MTs) are built from tubulin proteins: alpha and beta tubulin subunits.
Both types of subunits are very similar in structure and form dimers (heterodimers)
Beta-tubulin subunit on (+) end and alpha-tubulin subunit on (-) end.
protofilament
One polymer of tubulin subunits
Protofilaments are asymmetric, has polarity.
Alpha-tubulin
has a bound GTP that does NOT get hydrolyzed or exchanged!
Beta tubulin
has a bound GTP that is slowly hydrolyze to GDP after polymerization occurs
Heterodimers of alpha and beta tubulin subunits are added to
(+) end.
strcuture of microtuble
Each microtubule is made up of 13 protofilaments together.
Aligned side-by-side with a slight stagger, subunits of same type interact by non-covalent interactions.
Microtubules have polarity as protofilaments are aligned with same polarity.
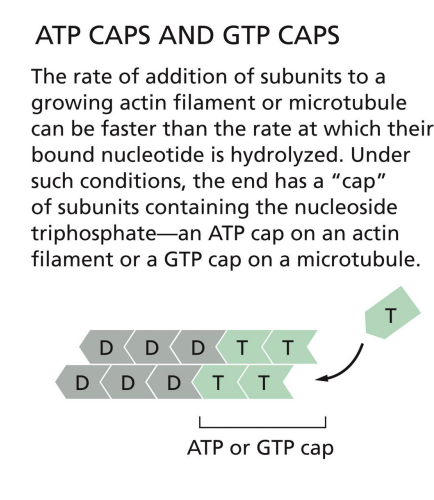
why do Microtubules Have GTP Caps
Polymerization requires energy = GTP
Beta-tubulin carries GTP that is hydrolyzed to GDP soon after assembly into polymer.
Rapid polymerization = GTP cap,
while slow addition = hydrolysis catches up.
= dynamic instability.
MTs assemble and disassemble rapidly, growing and shrinking MTs coexist in cell. Rapid conversion between growth and shrinkage = dynamic instability
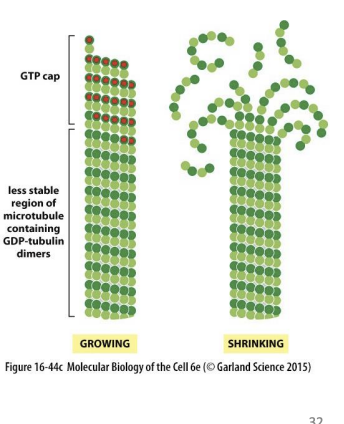
If GTP hydrolysis is slower than addition of GTP dimers =
GTP cap formed.
GDP tubulin is very unstable at (+) end. If hydrolysis catches up to tip of MT, =
GTP cap is lost.
catastrophe
Switch from growth to shrinking
rescue
Switch from shrinking to growth
Microtubules: Depolymerization
One hypothesis around microtubule depolymerization is:
GTP-bound Beta-Tubulin subunits create straight MTs.
While GDP-bound Beta-Tubulin subunits lead to relaxation and curving of the MT = disruption.
How are microtubules nucleated in cells?
Concentration of tubulin subunits needed for nucleation is very high!
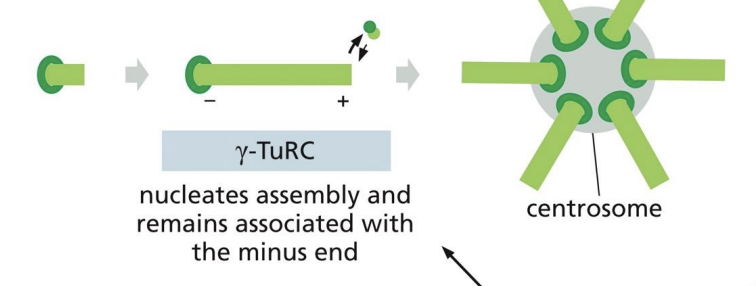
Gamma-tubulin, is involved in nucleation and forms a ring (gamma-tubulin ring complex, gamma-TuRC).
Polymerization begins from this gammaTuRC along with two accessory proteins.
Where is the (-) end of a microtubule anchored, and what is the role of the centrosome?
The (-) end of a microtubule is anchored in the centrosome (perinuclear region of cell with pair of centrioles and is enriched in gamma-tubulin) = microtubule organizing centre (MTOC).
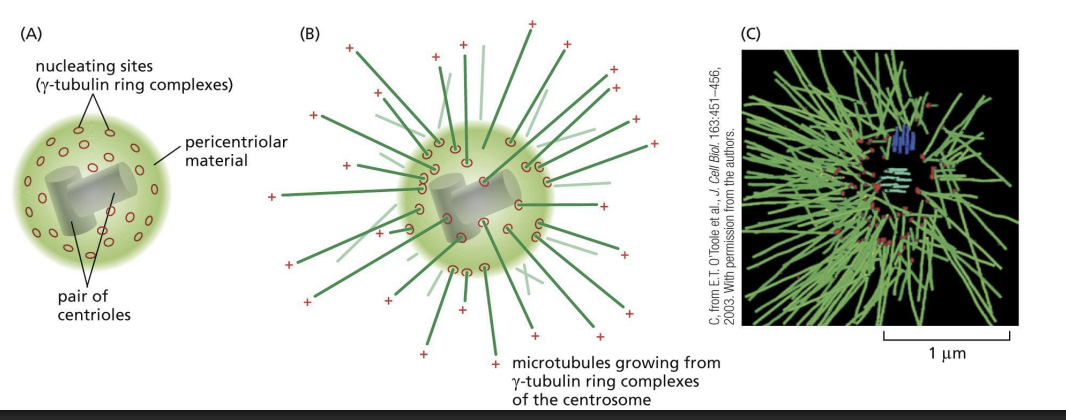
Are centrosomes the only site of microtubule nucleation?
Centrosomes are not the only site of microtubule nucleation, as plant cells and fungi lack centrosomes.
MTs And GammaTuRC
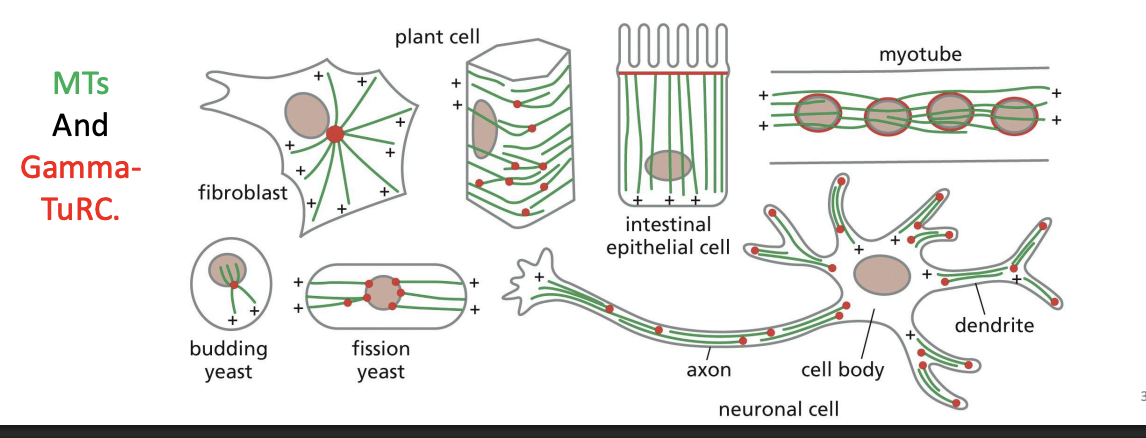
Microtubules in axons and dendrites are all oriented in the same direction, with
their plus ends pointed away from the cell body.
A) True
B) False
b
Microtubules: Accessory Proteins
Microtubules also have interacting proteins that help with:
• Nucleation (initiation and branching)
• Capping
• Subunit sequestration
• Stabilization (through side-binding)
• Severing
• Bundling and crosslinking
y- TuRC
Microtubules: Stabilizing Proteins
Microtubule associated proteins (MAPS)
Microtubule associated proteins (MAPS)
typically bind to MTs and increase stability.
MAPs have 2 domains = one that binds MT and one that acts as filament.
MAPs can determine how closely MTs pack together (e.g. Tau causes tighter packing than MAP2).
Microtubules: Nucleating Proteins
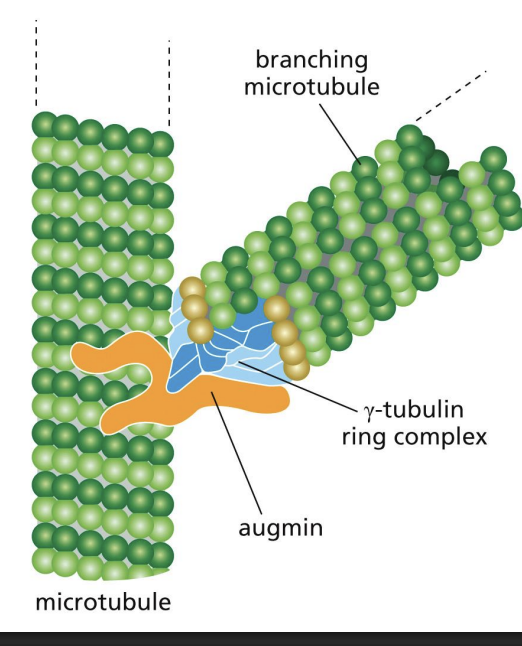
MAPs can also recruit proteins that organize MTs.
and Augmin
Augmin
protein complex that binds MT and recruits gamma-TuRC to nucleate a branch.
Microtubules: Plus-End Tracking Proteins (+TIPs)
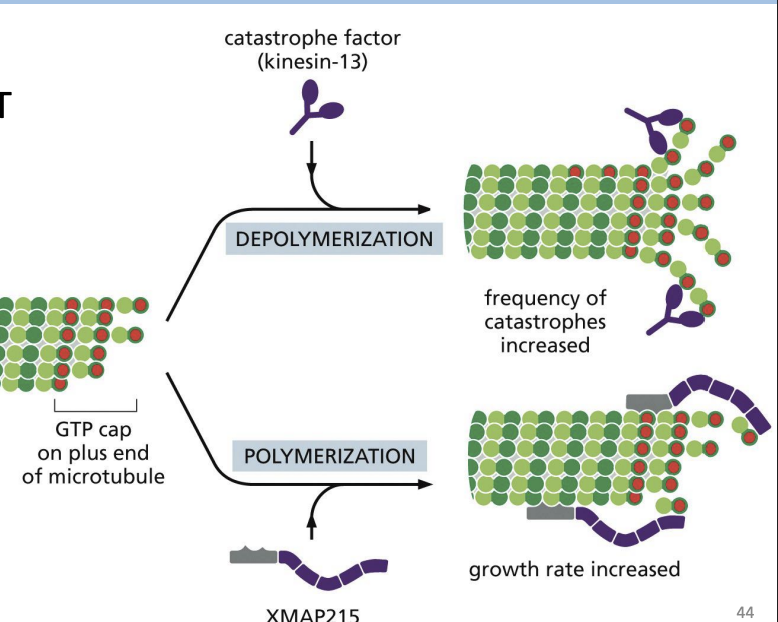
Catastrophe factors and MAPs (e.g. XMAP215)
Catastrophe factors
proteins that bind (+) end of MTs and promote MT curving to “pry” protofilaments apart (e.g. Kinesin-13)
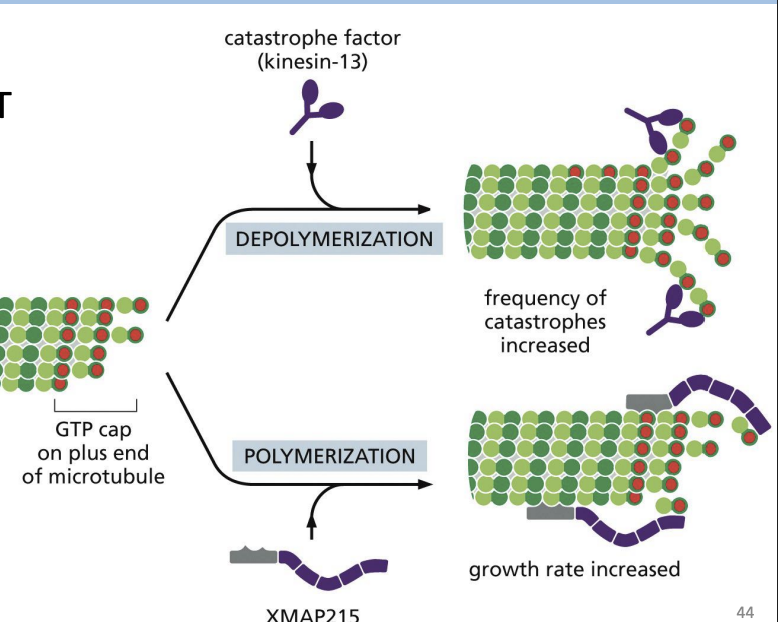
MAPs (e.g. XMAP215)
proteins that bind (+) ends of MTs and delivers free tubulin subunits to increase polymerization
Microtubules: Sequestering (isolate oneself) Proteins
Tubulin sequestering proteins and Stathmin
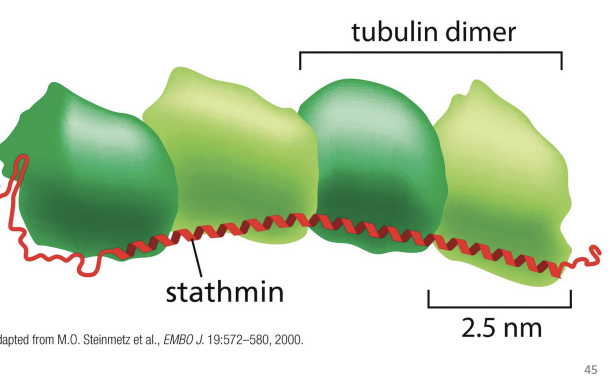
Tubulin sequestering proteins
proteins that sequester unpolymerized
Stathmin
protein that binds Tubulin heterodimers and prevents their addition to MTs
Phosphorylation of Stathmin prevents its binding to MTs.
Microtubules: Severing Proteins
MT severing proteins and Katanin (named after Japanese word for sword)
MT severing protein
proteins that break bonds in all 13 protofilaments to sever the MT.
Katanin (named after Japanese word for sword)
protein that extracts Tubulin subunits out of wall from MT, weakening it to promote breakage
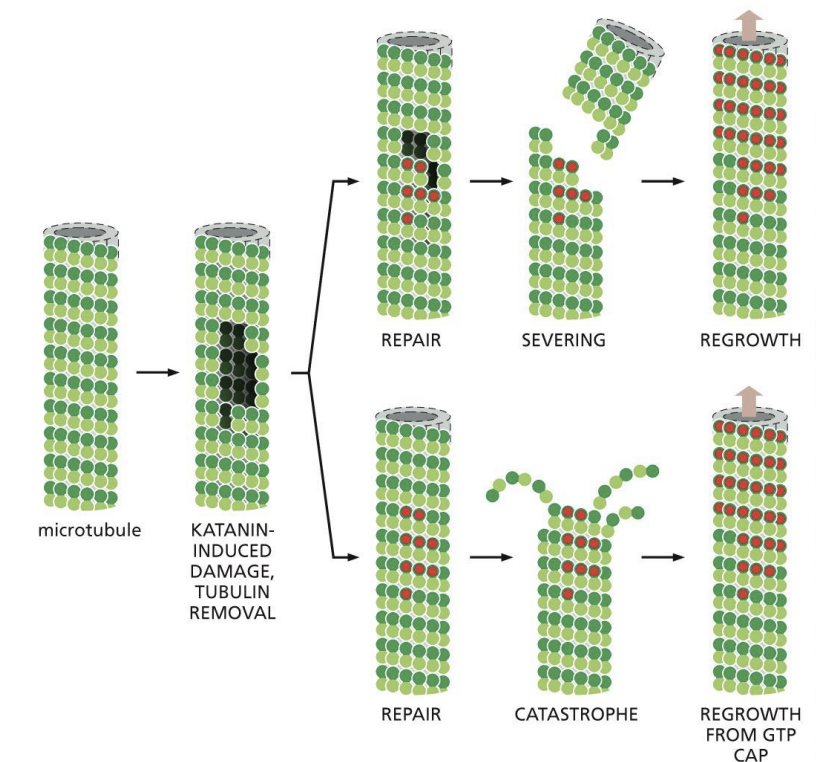
When cells enter mitosis, their existing array of cytoplasmic microtubules must be rapidly broken down and replaced with the mitotic spindle, which pulls the chromosomes into the daughter cells. The enzyme Katanin is activated during the onset of mitosis. Katanin extracts tubulin subunits from the wall of cellular microtubules, weakening its structure and promoting breakage. What do you suppose is the usual fate of the microtubule fragments created by katanin?
A) The fragments serve as seeds for growth from their plus ends.
B) The fragments are rapidly stabilized by end-binding proteins.
C) The fragments depolymerize because they contain GDP-tubulin.
D) The fragments are joined to rapidly form mitotic microtubules.
c
Dynamic MTs vs stable MTs
Dynamic MTs = cell motility.
Whereas stable MTs = positioning of organelles
Two types of MT motors:
1) Dynein = moves to (-) of MT = retrograde transport.
2) Kinesin = moves to (+) end of MT = anterograde transport.
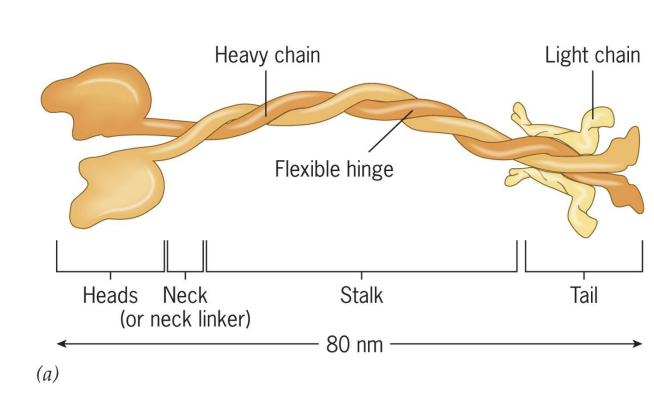
Kinesins
= Tetramer of 2 heavy chains and 2 light chains.
moves to (+) end of MT = anterograde transport.
Part of superfamily (45 different types!).
4 features of Kinesins
1) Head (ATPase and binds MTs)
2) Neck – direction of movement
3) Stalk = flexibility/movement of heads and tail
4) Tail = binds cargo
Kinesin 1 is most similar to
Myosin II.

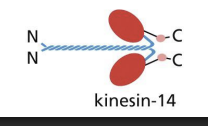
Kinesin 14 has
motor domains at C-terminal and moves to minus end.
Kinesins: Movement
Each motor head binds ATP, which undergo cycles of hydrolysis to dock and undock from MT.
1) Rear head is bound to ATP and tightly bound to MT, front head is bound to ADP and loosely bound to MT.
2) Hydrolysis of ATP in rear head and front head binding ATP = forward displacement of rear head (neck linker shifts conformation).
3) Forward displacement of rear head allows for ADP-bound head to be in front and ATP-bound head to be at the back
Dynein
moves to (-) of MT = retrograde transport.
Large head domain = ATP binding Stalk connects MT binding domain to head domain
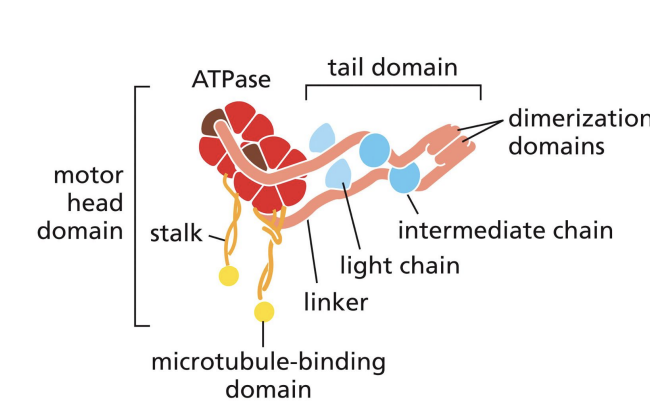
Cytoplasmic Dyneins are
composed of 2 heavy chains, several intermediate and light chains
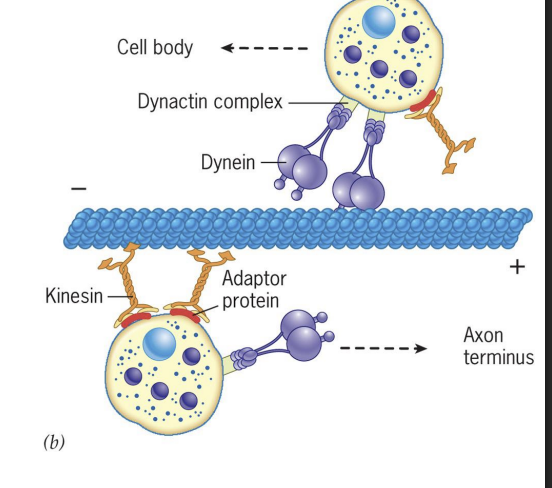
Adaptor Proteins for Dynein and Kinesin
Both Dynein and Kinesin have adaptor proteins that mediate binding to cargo.
Dynein = important for mitosis (spindle positioning and chromosome separation), moving organelles (Golgi, lysosomes), moving endosomes and vesicles in cytosol.
Kinesin = moving organelles (peroxisomes and mitochondria), moving secretory vesicles
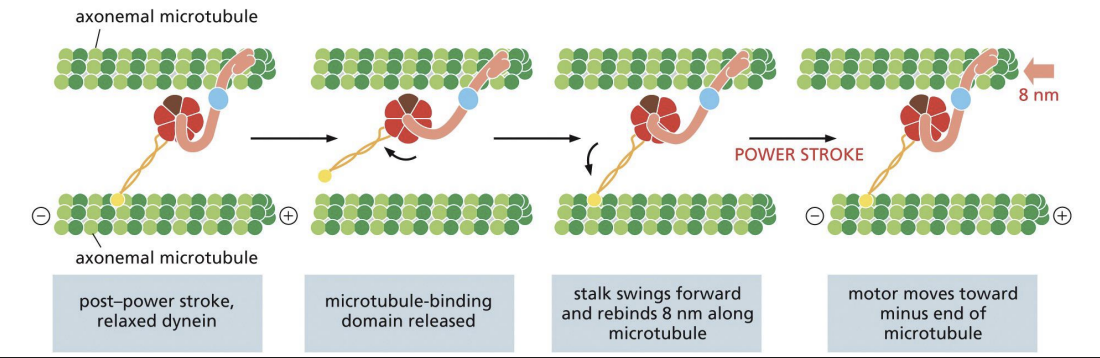
Dyneins: Movement
ATP binding = “linker-swing, dynein-winch” mechanism where head domain is thrown like a fishing hook before reattaching to MT.
Hydrolysis of ATP and release of ADP + Pi = power stroke that pulls tail and attached MT or cargo towards (-) end by 8nm.
how does Dyneins Use Adaptor Proteins to Bind Cargo
Dynein uses an adaptor protein to bind cargo = Dynactin.
Dynactin can
1) Bind weakly to MTs,
2) Bind to Dynein
3) Include actin-like filaments associated with cargo,
4) Associate with two cytoplasmic Dynein proteins along with adaptor protein to be connected to cargo.
Cilia and Flagella
Motility structures built from MTs and Dynein that are “hair-like” organelles in Eukaryotes
Cilia beat with whiplike motion.
Flagella create undulating motion.
Axoneme
Inner core of cilium or flagellum that bends to produces movement
9 doublets of MTs (one complete and one partial MT) on outer ring and 1 central pair of MTs.
Microtubulues in Cilia and Flagella
Motion requires MTs and Axonemal Dynein proteins
Dyneins are attached to one MT doublet and move along adjacent doublet.
Moves MTs relative to each other to cause bending.
Intact Axonemes have flexible protein links that prevent sliding and cause bending motions instead.
Taxol
drug that binds MTs and prevents disassembly; also binds tubulin dimers to prevent assembly.
Taxol used as chemotherapy agent = reduced division of cancer cells = death.
Anticancer drugs like Taxol are used to kill certain kinds of tumour cells because
these drugs are not toxic to rapidly dividing normal cells.
A) True
B) False
b
Alzheimer’s Disease relation to microtubles
Defects in MAPs (e.g. Tau protein) = loss of stabilized MTs.
Destabilized MTs = tangled MTs = neurofibrillary tangles = neuronal death.
Lissencephaly
cells fail to migrate to cerebral cortex of developing brain.
Caused by defective Lis1 (Dynein binding protein) that is required for nuclear migration.
Leads to developmental delays and neurological effects.
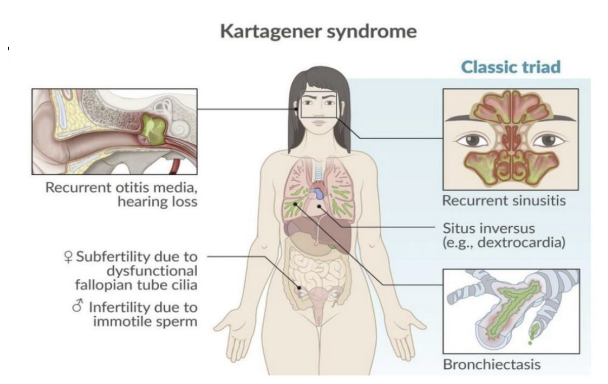
Kartagener’s syndrome (
Defects in Axonemal Dynein = Kartagener’s syndrome (type of Primary Ciliary Dyskinesia).
Situs Inversus
Defective cilia = body organ symmetry is reversed
Embryonic cilia move fluid in embryo to the left of the midline = development of specific organs on left, which causes our body organ symmetry
Primary Ciliary Dyskinesia or Kartagener’s syndrome.
Defects in Axonemal Dynein = Primary Ciliary Dyskinesia or Kartagener’s syndrome.
Situs Inversus = asymmetry of internal organs.
Patients are prone to lung infections and lack of sperm motility = sterile.
Bardet-Biedl syndrome
disease caused by defects in proteins that play important role in cilia structure and function.
Patients cannot smell and develop retinal degeneration.
Also leas to hearing loss, polycystic kidney disease, Diabetes, obesity and polydactyly.
GTP hydrolysis and whether GTP or GDP is bound to tubulin is an important
mechanism to control the dynamic instability of microtubules. EB1 is a capping
protein that binds to the GTP-cap on microtubules. Dynamic instability can be
viewed using a GFP-EB1 fusion protein, which process(es) is it useful for visualizing
and why?
A) Growing and shrinking microtubules, because EB1 binds to the GTP-tubulin cap
on microtubules
B) Growing microtubules, because EB1 binds to the GTP-tubulin cap on
microtubules
C) Shrinking microtubules, because EB1 binds to the GTP-tubulin cap on
microtubules
D) Growing and shrinking microtubules, because EB1 binds to the GDP-tubulin
cap on microtubules
b
How would the microtubule dynamics change after adding a non-hydrolyzable
analog of GTP to the cells?
A) Microtubules would shrink.
B) Microtubules would grow longer.
C) Microtubule dynamics would not change.
D) Dynamic instability would increase as microtubules rapidly switch between
growing and shrinking
b
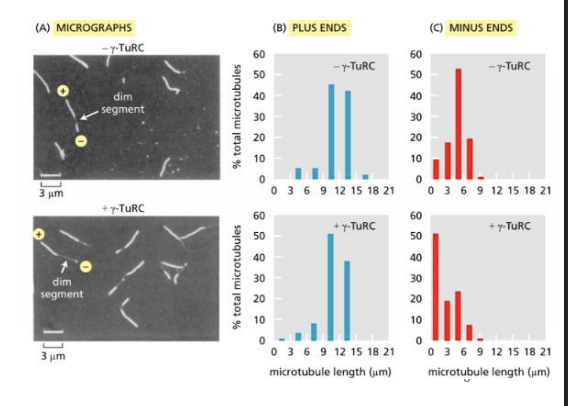
To determine whether γ-TuRC nucleates microtubules at plus ends or at minus ends, microtubules were polymerized in two steps. In the first step, microtubules nucleated with or without γ-TuRC were allowed to form in the presence of a low proportion of rhodamine-labeled αβ-tubulin, which makes the microtubules fluoresce dimly.
In the second step, these microtubules were allowed to extend at both ends in the presence of a higher proportion of rhodamine-tagged tubulin to label the ends brightly. The longer bright segment extending from a dim segment identifies the plus end, and the shorter segment, the minus end (see the figure Part A). Measurements of the lengths of numerous bright segments in individual microtubules that also contained a dim segment yielded the data in Parts B and C.
To determine whether γ-TuRC nucleates microtubules at plus ends or at minus ends, microtubules were polymerized in two steps. What do these data tell you about how γ-TuRC nucleates microtubules?
A) Gamma-TuRC nucleates at the minus end; plusend lengths do not change, minus-end lengths are shorter.
B) Gamma-TuRC nucleates at the minus end; plus-end lengths increase, minus-end lengths decrease.
C) Gamma-TuRC nucleates at the minus end; plus-end lengths do not change, minus-end lengths are longer.
D) Gamma-TuRC nucleates at the minus end; plus-end lengths increase, minus-end lengths remain the same.
a
Mice that are homozygous for a knockout of the gene for the kinesin motor protein KIF1B die at
birth. Heterozygous knockouts (one copy deleted) survive but suffer from a progressive muscle
weakness similar to human neuropathies. Humans with Charcot–Marie–Tooth disease type 2A
have a mutation in one copy of the gene for KIF1B that prevents the protein from binding to ATP.
The heterozygous mice and the human patients have very similar progressive neuropathies.
How do you suppose that the loss of one copy of a gene for a kinesin motor can have such
profound effects on nerve function?
A) The human gene with a point mutation makes slow moving kinesin motors.
B) One normal gene makes enough kinesin.
C) The normal gene produces twice as much kinesin but can’t keep doing so.
D) Half the usual amount of kinesin cannot keep up with the needs of nerves.
d