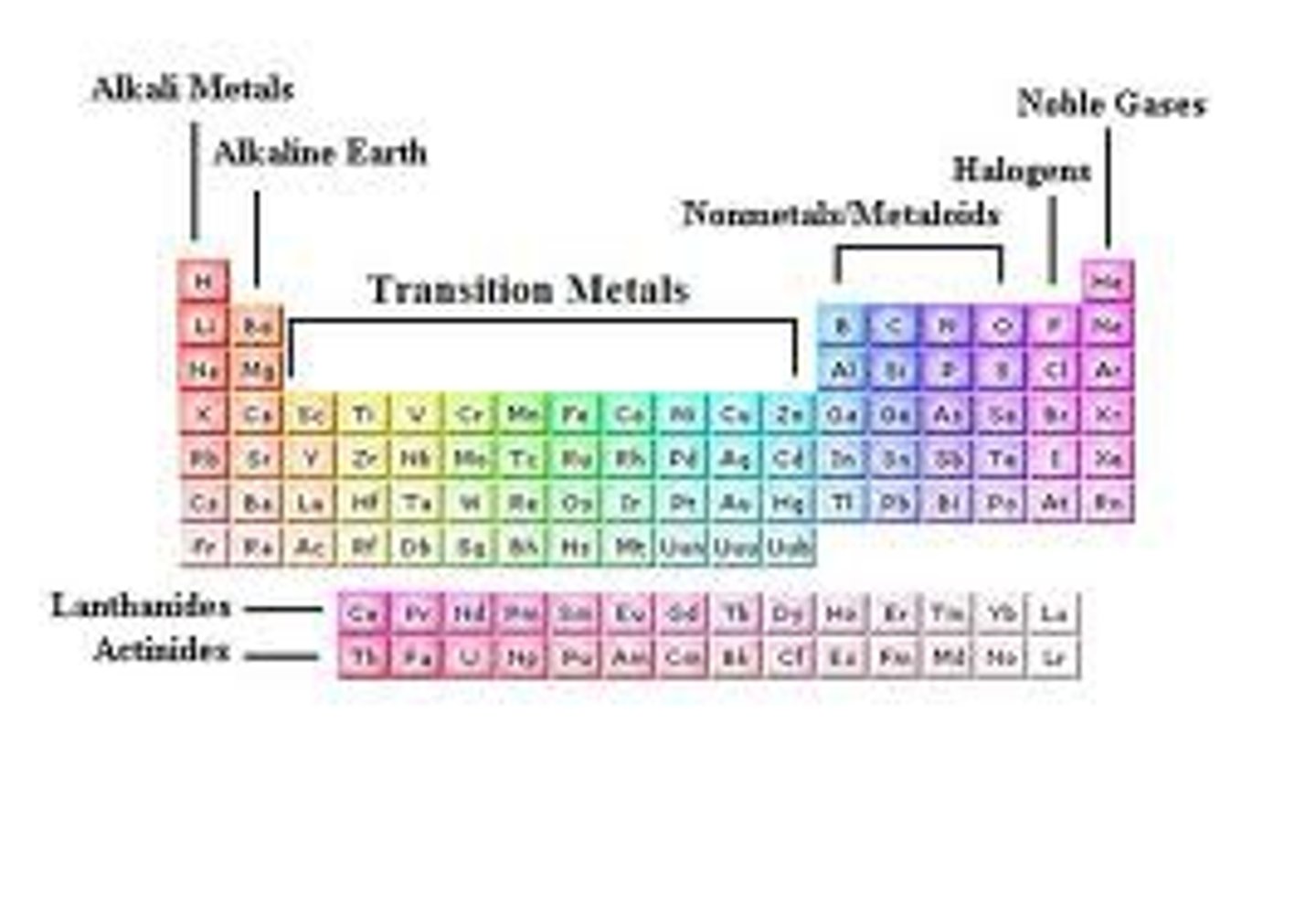
Calculating Protons, Neutrons, and Electrons
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
Neutrons
Which of the parts of an atom have no charge and a mass of 1 amu?
electron cloud or energy level
Where are the electrons located in the atom?
The atomic number
How can you tell the number of protons in an element?
Atomic Mass - # of Protons
How do you find the number of neutrons in a neutral atom?
2
How many neutrons does Helium have?
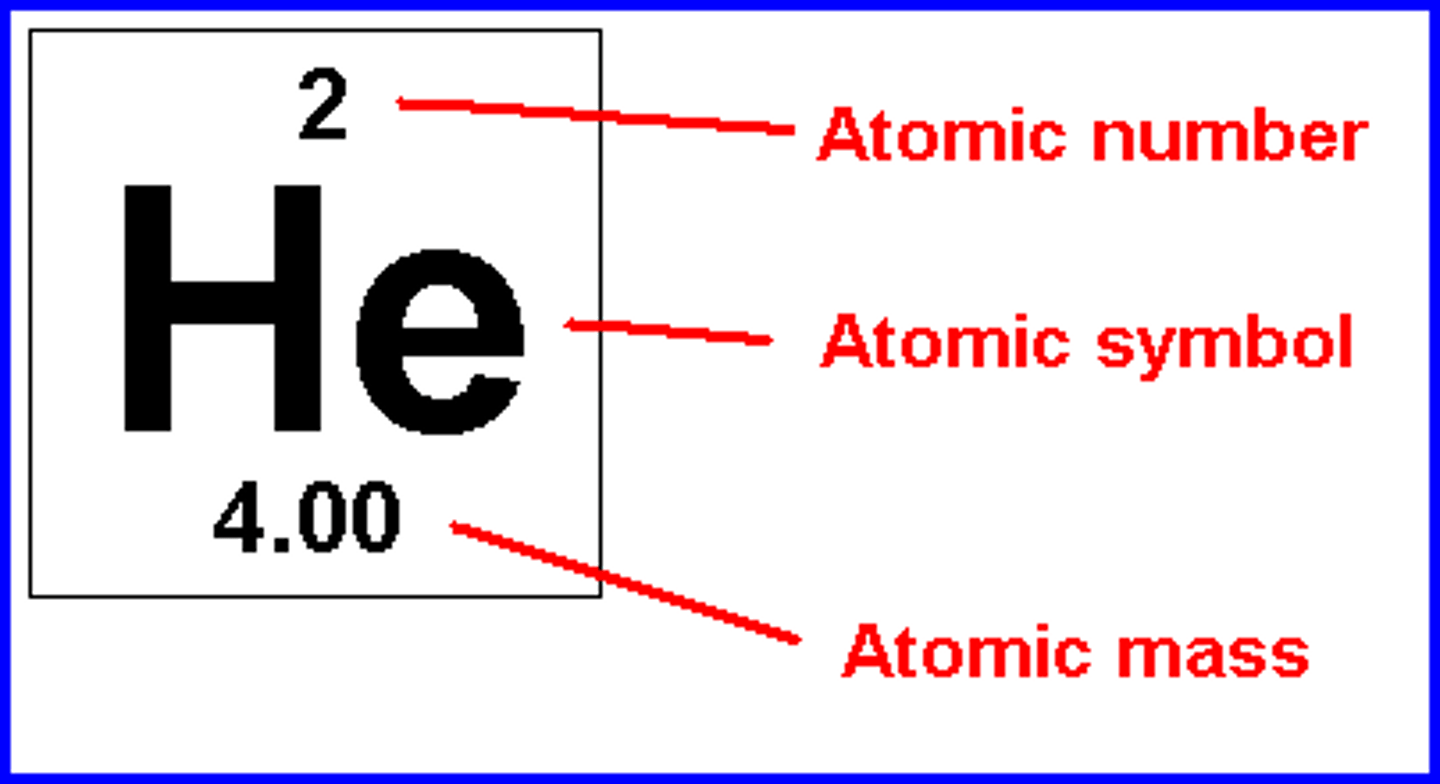
26
What is the number of protons in this atom?

Atomic Mass
What does the letter Z represent in this graphic? The atomic number or atomic mass?
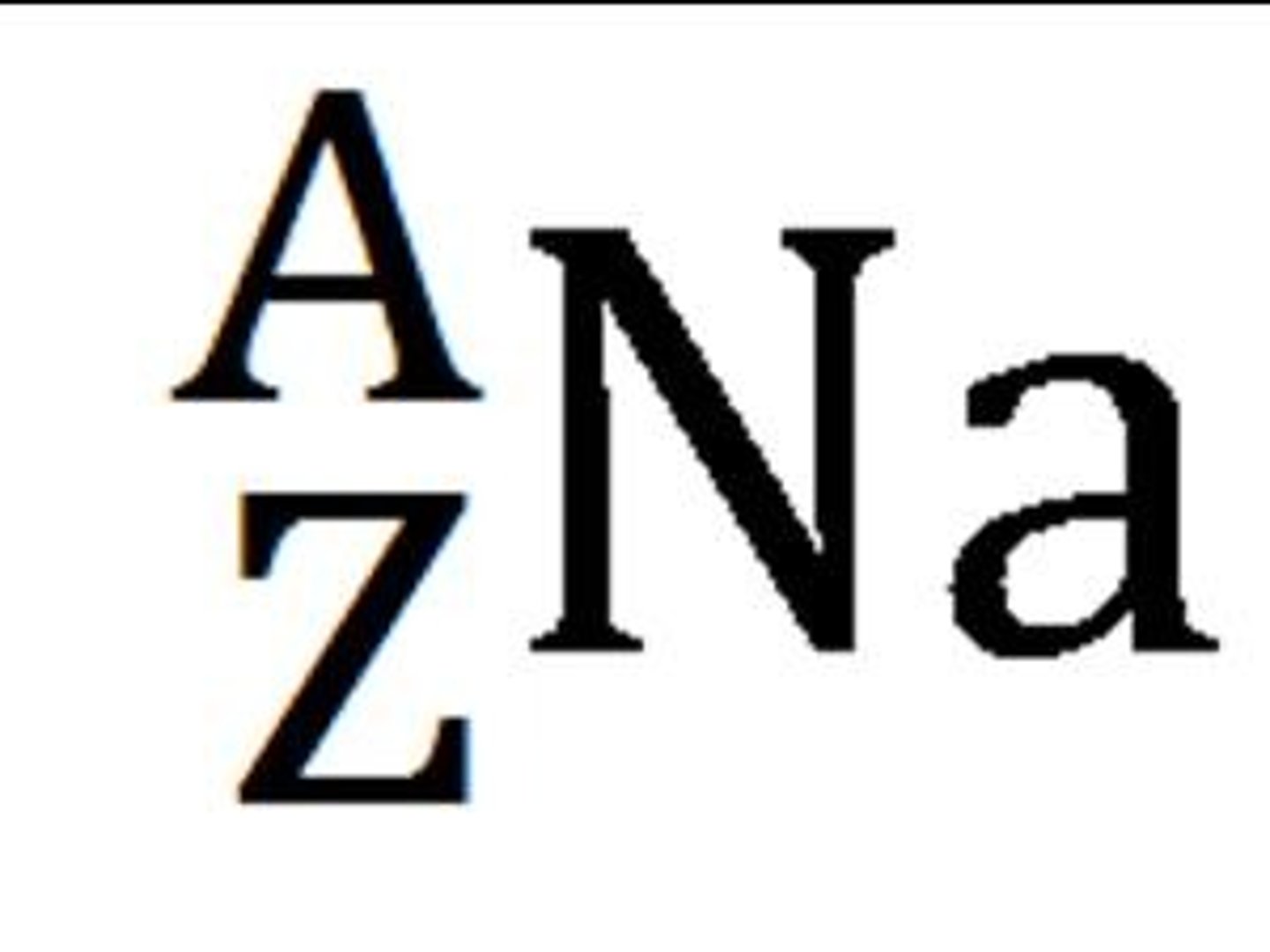
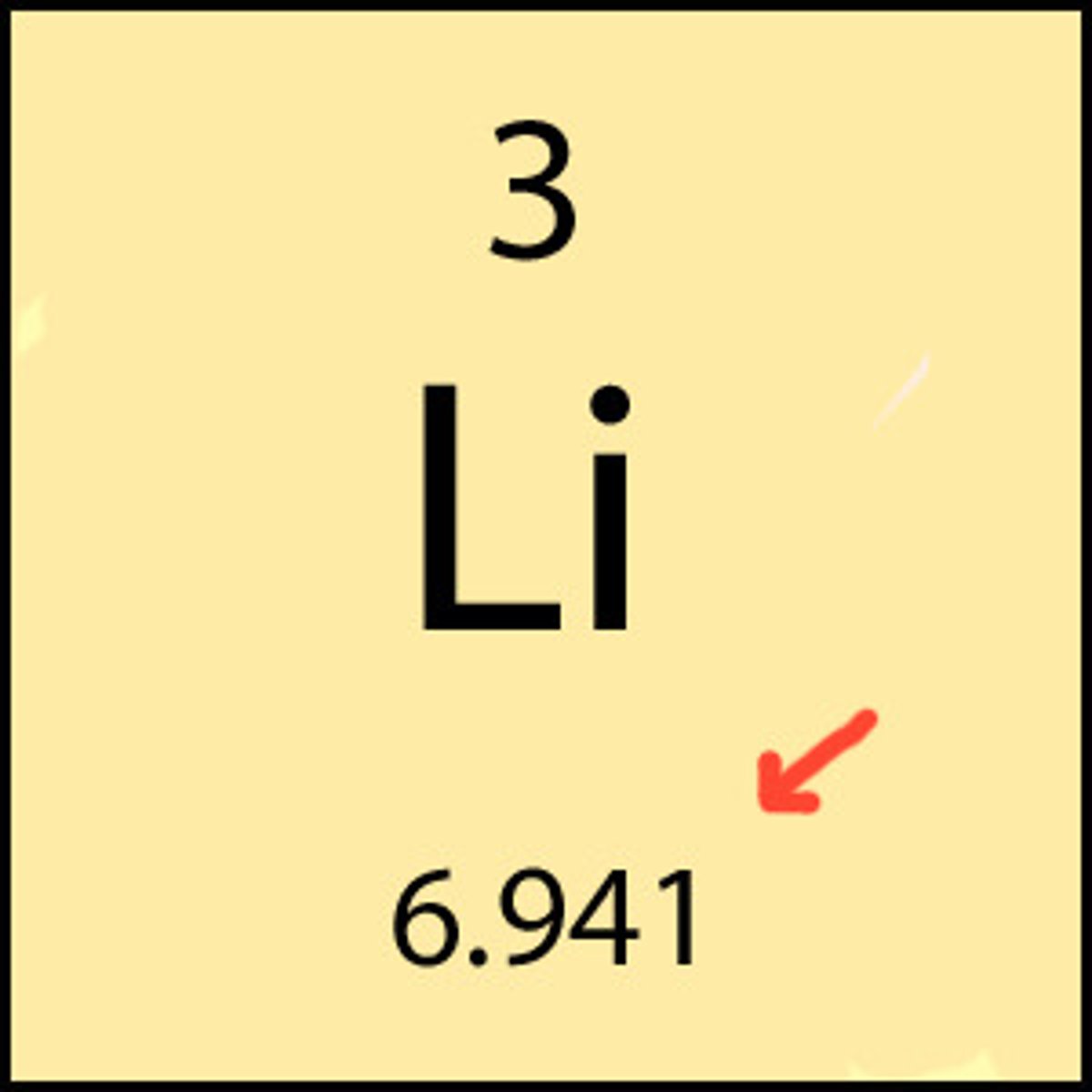
29
How many protons does copper (Cu) have?

4
How many neutrons does this element have?
Protons + Neutrons
The sum of what subatomic particles make up the mass of an atom?
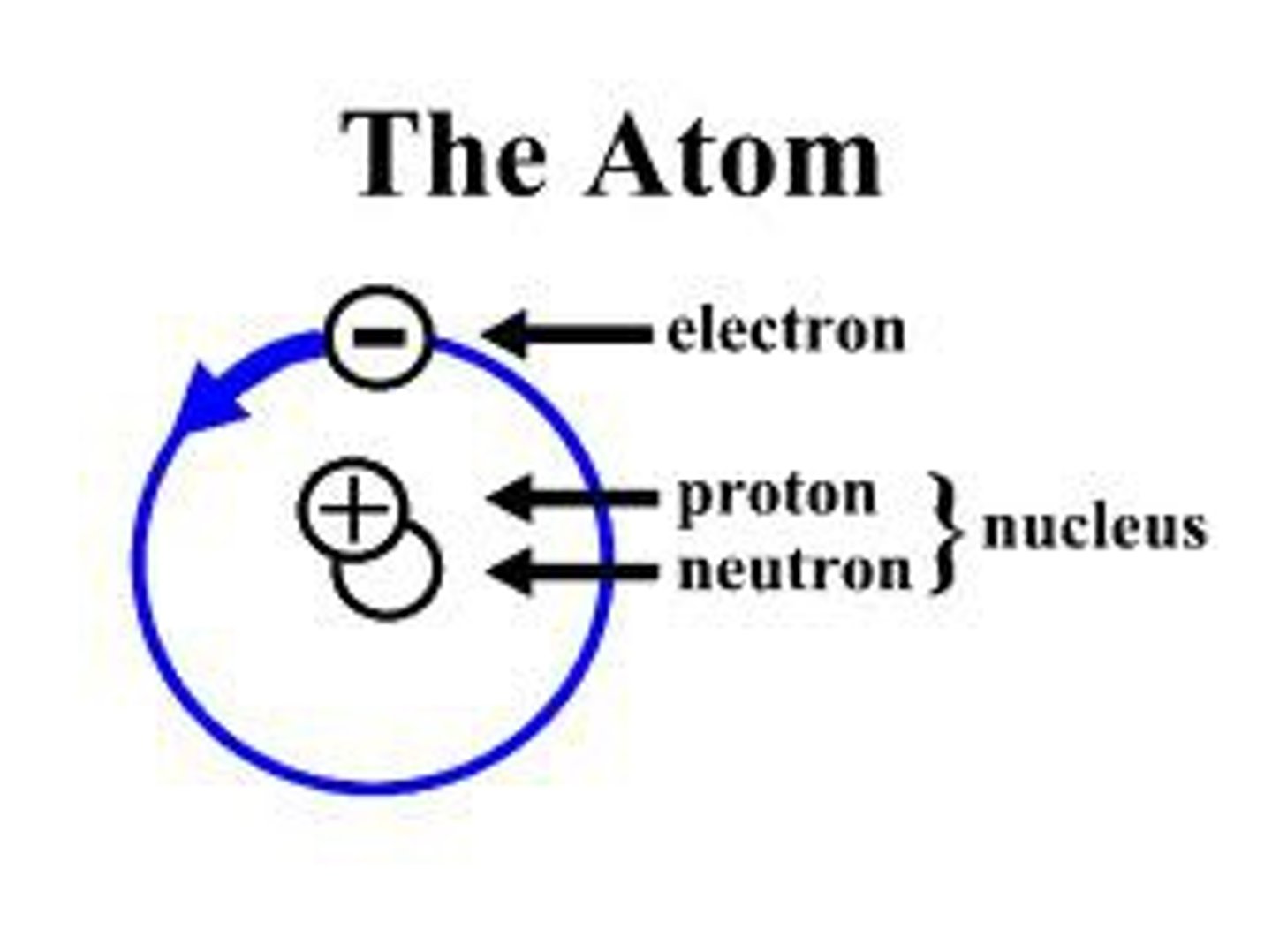
Helium
What element does this model represent?
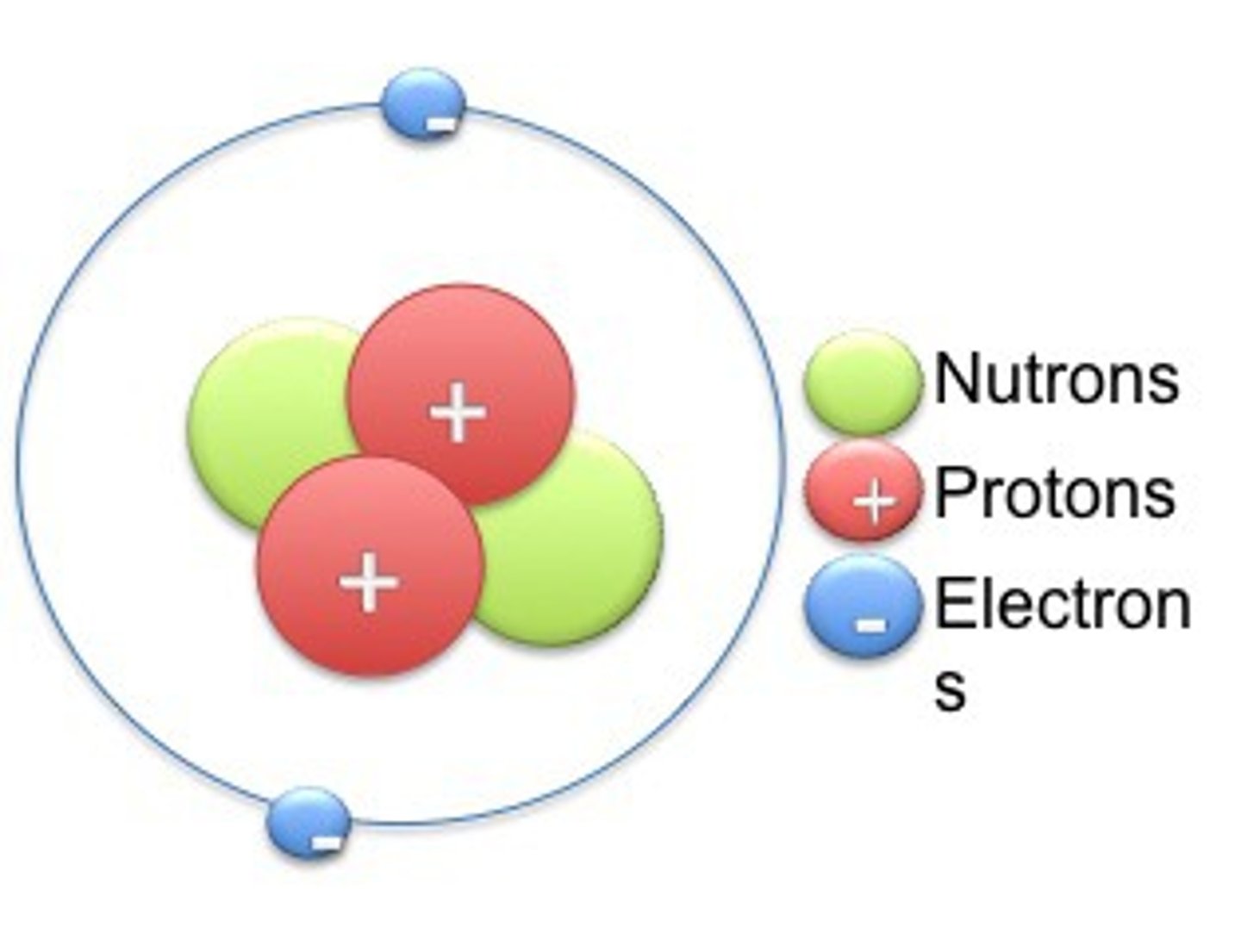
20
How many protons does Calcium (Ca) have?

11
How many electrons does Sodium (Na) have?

Proton
Part of the atom with a positive charge; a mass of 1 amu; found in the nucleus
Electron
Part of the atom with a negative charge; a mass of 0 amu; located outside the nucleus in the energy levels
Atom
Makes up all matter, made up of protons, neutrons, and electrons
10
How many neutrons does Neon have?

24
How many neutrons does Scandium have?

Gallium
What element has 39 protons?
Radon
What element has 86 Electrons?