Quiz 6: Medicinal Chemistry
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
What is the mechanism of action of aspirin as an antiplatelet?
irreversibly acetylates COX-1 and COX-2 at Ser530, blocking formation of thromboxane A₂ (TXA₂) and preventing platelet aggregation
Why does aspirin have a short half-life but long duration?
plasma half-life ≈ 16-20 min, but effect lasts 5-7 days due to irreversible COX inhibition in platelets that cannot synthesize new enzyme
What is the recommended aspirin dose for acute MI vs. prevention?
162-325 mg (chewable) for acute MI; 81 mg daily for secondary prevention
Why is routine aspirin not advised in adults > 60 years?
risk of GI bleeding outweighs preventive benefit when no prior CV disease
What do P2Y₁₂ inhibitors block and why?
inhibits ADP-induced platelet activation by blocking P2Y₁₂ purinergic receptors, preventing fibrinogen binding to GPIIb/IIIa
How do clopidogrel and prasugrel differ in activation?
Both are prodrugs, but...
Clopidogrel: needs 2 CYP2C19 steps → active thiol metabolite
Prasugrel: activated by esterases (CES2 > CES1) and CYP3A4/2B6, not CYP2C19
S-Clopidogrel Structure

What polymorphisms reduce clopidogrel activation?
**CYP2C192 and 3 cause poor metabolism
- 10-15 % in Asians are PMs, 50 % IMs
What are FDA recommendations for CYP2C19 PMs or IMs on clopidogrel?
- perform genotype testing
- switch to prasugrel/ticagrelor (PM)
- increase dose 75 → 150 mg (IM) based on platelet function
What type of inhibitor is ticagrelor vs. cangrelor?
both are reversible P2Y₁₂ inhibitors
Compare ticagrelor vs. cangrelor (route, duration, recovery).
Ticagrelor: oral, t½ 6-12 h, recovery 3-5 days
Cangrelor: IV only, t½ 3-6 min, recovery ~60 min
What are GPIIb/IIIa inhibitors and when are they used?
IV agents blocking fibrinogen/vWF binding to platelet integrin αIIbβ₃; used during PCI or for unstable angina/MI
What are examples of GPIIb/IIIa inhibitors?
Abciximab (mAb), Eptifibatide (snake-derived cyclic peptide), Tirofiban (small molecule)
What is the MOA of heparin and LMWHs?
bind antithrombin III, enhance its inhibition of factor Xa and thrombin (IIa)
***LMWHs are more selective for Xa
How are heparin and LMWH administered?
heparin - IV
LMWH - SubQ q12h dosing
What is a clinical use for LMWHs?
used to bridge patients prior to
surgery for patients on warfarin or direct oral anticoagulants (DOACs)
What vitamin is essential for synthesis of factors II, VII, IX, X?
Vitamin K, via γ-carboxylation enabling Ca²⁺ binding and activation
What enzyme does warfarin inhibit?
Vitamin K epoxide reductase (VKORC1) → prevents recycling of reduced vitamin K
Which warfarin enantiomer is more potent, and how is it metabolized?
S-warfarin (~80 % activity) metabolized mainly by CYP2C9 to 7-OH-warfarin
What are key warfarin pharmacogenes?
CYP2C9 (*2, *3 reduce metabolism) and VKORC1 variants; genotype explains ~50 % of dose variability
What is the therapeutic INR range for warfarin?
2-3 for most patients
What is the antidote for warfarin toxicity?
Vitamin K (oral or IV)
Compare heparin, LMWH, and warfarin by onset and route.
Heparin: rapid (IV)
LMWH: 1-2 h (SC)
Warfarin: slow (12-24 h oral)
What distinguishes DOACs from warfarin?
directly inhibit Xa or thrombin; less intracranial bleeding; no INR monitoring; fewer DDIs; high cost
Which DOACs are Factor Xa inhibitors?
Apixaban (Eliquis®), Rivaroxaban (Xarelto®)
Apixaban (Eliquis) Structure

What is the MOA of Dabigatran (Pradaxa®)?
direct thrombin inhibitor, activated from prodrug dabigatran etexilate via CES1 & CES2
Dabigatran (Pradaxa) Pro Drug Structure
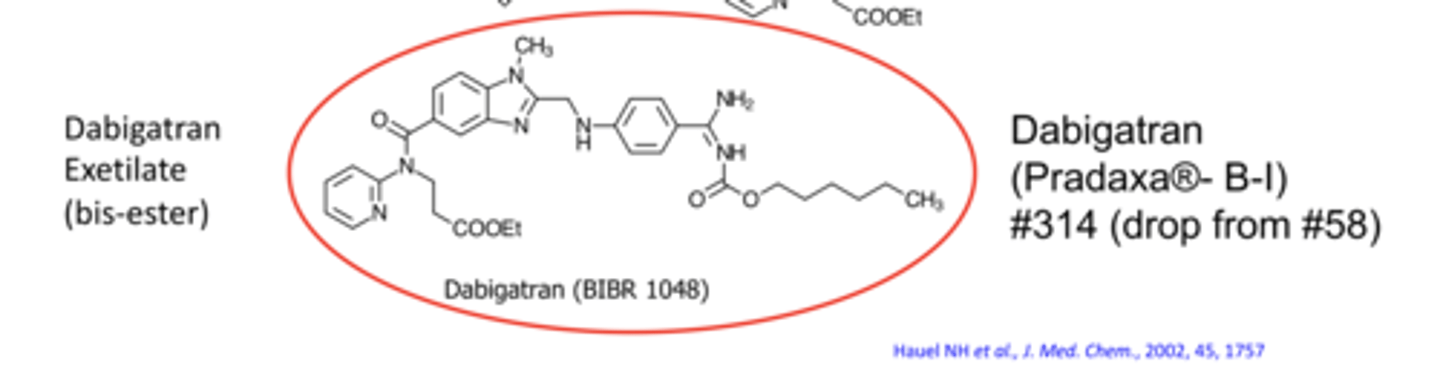
Why is dabigatran rarely used now?
increased mortality in patients with artificial heart valves; requires renal dosing adjustments
What is Bivalirudin (Angiomax®) and when is it used?
- synthetic 20-aa peptide (hirudin analog)
- IV direct thrombin inhibitor used in patients with HIT
Which anticoagulant class has reduced intracranial bleeding vs. warfarin?
DOACs – Factor Xa and thrombin inhibitors (e.g., Apixaban, Rivaroxaban, Dabigatran)
Which genetic or drug interactions most affect warfarin dose?
CYP2C9 *2/*3 variants and co-administration with amiodarone (CYP inhibitor) → dose reduction required
What is the overall mechanism of KATP-channel activators?
they open ATP-sensitive K⁺ channels → K⁺ efflux → membrane hyperpolarization → closure of voltage-gated Ca²⁺ channels → ↓ intracellular Ca²⁺ → less MLCK activation → smooth-muscle relaxation and arterial vasodilation
Why are these agents most effective in small arteries/arterioles?
have high baseline tone; their relaxation markedly lowers systemic vascular resistance and arterial pressure
What compensatory effect can occur with KATP activators?
reflex (baroreceptor-mediated) tachycardia due to sudden fall in blood pressure
Which drugs act as KATP-channel activators used in CHF or HTN?
hydralazine and minoxidil
What are the three complementary mechanisms of hydralazine?
- opens K⁺ channels
- inhibits IP₃-induced Ca²⁺ release from SR
- stimulates NO formation → cGMP-mediated vasodilation
What type of smooth muscle does hydralazine act on?
arteriolar smooth muscle > venous; direct arterial relaxant
What enzyme metabolizes hydralazine?
N-acetyltransferase-2 (NAT2) acetylates hydralazine
What causes variability in hydralazine metabolism?
polymorphisms produce slow and rapid acetylator phenotypes
In which patient groups is hydralazine most effective?
slow acetylators (≈60 % whites, 30 % Black Americans, 7-17 % East Asians)
What is hydralazine's oral bioavailability in rapid acetylators?
roughly 10 % because of extensive first-pass acetylation
What are hydralazine's primary clinical uses?
- HFrEF with isosorbide dinitrate (esp. African Americans)
- hypertension in pregnancy (second/third line)
What severe idiosyncratic reaction can hydralazine cause?
drug-induced systemic lupus erythematosus (SLE) due to its hydrazine moiety forming reactive metabolites
How is lupus risk related to dose and acetylator status?
0 % at 50 mg, ≈5 % at 100 mg, ≈10 % at 200 mg
- 11 of 14 cases occurred in slow acetylators
Is minoxidil active as administered?
No, it is a prodrug requiring hepatic SULT1A1-mediated conversion to minoxidil N-O-sulfate, the active metabolite
What is the inactive major metabolite of minoxidil?
N-O-glucuronide conjugate
What percentage of oral minoxidil becomes active?
only ~10-20 % converted to the active sulfate; ~20 % excreted unchanged
What are the main therapeutic uses of oral minoxidil?
severe or refractory hypertension and topically for androgenic alopecia
What concurrent therapy is recommended with oral minoxidil?
combine with a loop diuretic to prevent Na⁺ retention and plasma volume expansion
What is the mechanism of SGLT2 inhibitors?
block renal SGLT2 in proximal tubules → ↓ glucose reuptake → glycosuria + osmotic diuresis + mild Na⁺ loss
Why is selectivity for SGLT2 over SGLT1 important?
SGLT1 is expressed in gut and brain; blocking it would interfere with nutrient and glucose uptake in critical tissues
Which SGLT2 inhibitor has the highest SGLT2:SGLT1 selectivity?
Empagliflozin (Jardiance®)
What are key structural/chemical features of this drug class?
C-glucoside linkage (stable ether bond) instead of hydrolysis-prone O-glucoside found in phlorizin (the apple natural product)
What are the main therapeutic effects of SGLT2s beyond glucose lowering?
cardioprotective and renoprotective benefits in HF and CKD; also cause modest weight loss (~3-5 kg)
When are SGLT2 inhibitors not recommended?
when eGFR < 45 mL/min due to reduced renal efficacy
What are the three structural units of cardiac glycosides?
sugar unit (at C-3), a steroidal core, and an α,β-unsaturated lactone ring
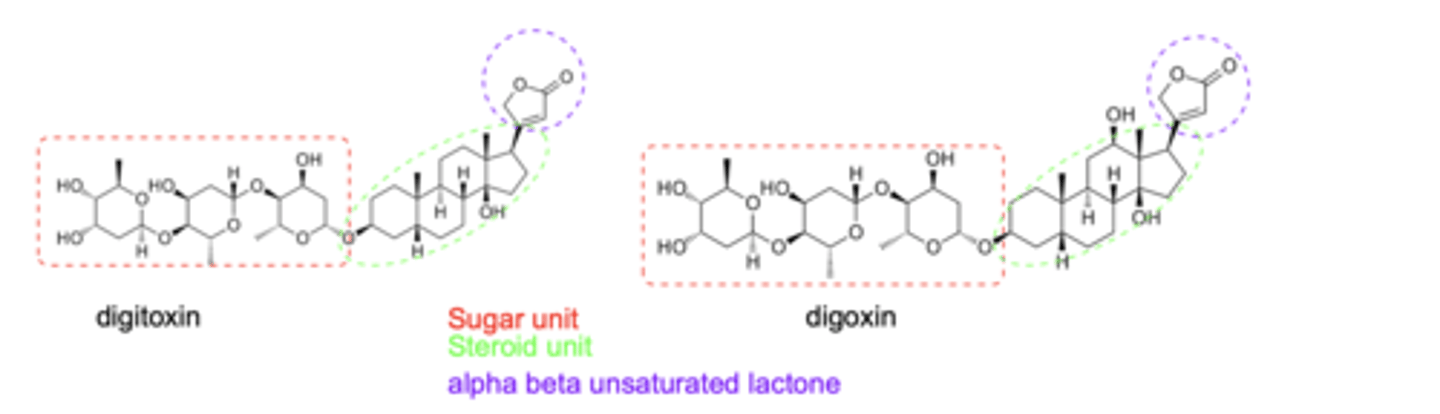
What is significant about the lactone unit of digoxin/digitoxin?
makes covalent bonds and irreversible inhibition
What is the pharmacologic target of digoxin?
Na⁺/K⁺-ATPase inhibition → ↑ intracellular Na⁺ → ↓ Na⁺/Ca²⁺ exchange → ↑ Ca²⁺ in myocytes → positive inotropy
What additional cardiac effect does digoxin have?
negative chronotropic effect via ↑ vagal tone (parasympathomimetic action)
How do the pharmacokinetic properties of digoxin and digitoxin differ?
GI absorption: Digitoxin > 90 %; Digoxin 70–85 %
Protein binding: Digitoxin 90–95 %; Digoxin 25–30 %
Half-life (t½): Digitoxin 5–7 days; Digoxin 1–2 days
Elimination: Digitoxin — hepatic; Digoxin — renal (largely unchanged)
Which agent is clinically preferred between digoxin and digitoxin and why?
Digoxin - shorter half-life, better dose control, less accumulation
What are mild to moderate manifestations of digoxin toxicity?
nausea, vomiting, anorexia, weakness, bradycardia
What are severe manifestations of digoxin toxicity?
blurred vision (yellow/green tint), disorientation, diarrhea, ventricular arrhythmias
How is digoxin toxicity treated?
K⁺ supplementation, atropine, phenytoin, activated charcoal (within 2-3 h), and Digibind® (ovine Fab antibody)