M1 - Course overview RBC and platelet preservation
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What are the main requirments for a successful transfusion?
a non-toxic anticoagulant
appropriate preservative solutions
appropriate devices to perform the transfusion
avoiding circulatory overload
appropriate storage
component therapy
patient testing
donor testing
compatibility tests
Describe three key aspects of red blood cell (RBC) biology that are essential for normal RBC survival and function, and state the normal lifespan of an RBC in circulation.
The three key aspects are:
RBC membrane – maintains cell shape, flexibility, and integrity.
RBC metabolism – provides energy (ATP) to preserve membrane function and ionic balance.
Haemoglobin structure and function – enables efficient oxygen transport.
Abnormalities in any of these can reduce RBC function or survival.
Normal RBC lifespan: approximately 120 days in circulation.
Describe the structure and composition of the red blood cell (RBC) membrane and explain how its organization contributes to cell function.
The RBC membrane is a semi-permeable lipid bilayer supported by a protein cytoskeleton that maintains the cell’s shape and flexibility.
It contains phospholipids, integral proteins, and peripheral proteins that regulate membrane deformability and permeability.
The membrane has an asymmetrical organization:
External layer: glycolipids and choline phospholipids
Internal (cytoplasmic) layer: amino phospholipids
This organization preserves RBC flexibility, stability, and the ability to pass through narrow capillaries.
Explain the biochemical composition and permeability properties of the red blood cell (RBC) membrane, and describe how these features help maintain RBC stability.
The RBC membrane is composed of 52% protein, 40% lipid, and 8% carbohydrate.
It is permeable to water and anions (Cl⁻, HCO₃⁻).
It is impermeable to cations (Na⁺, K⁺), helping to maintain osmotic balance.
Calcium (Ca²⁺) is actively pumped out of the RBC using ATP to prevent intracellular accumulation.
These properties, along with active cation transport, prevent colloid hemolysis, control cell volume, and maintain RBC structural integrity.
Why are red blood cell (RBC) metabolic pathways primarily anaerobic, and what is the significance of these pathways?
RBCs rely mainly on anaerobic glycolysis for ATP production because they are anucleate and lack mitochondria, preventing aerobic respiration.
The anaerobic glycolytic pathway provides ATP needed to maintain membrane integrity and ion transport.
Additionally, three ancillary pathways support haemoglobin function and RBC survival by protecting against oxidative damage and maintaining red cell flexibility.
Explain the factors that affect red blood cell (RBC) membrane deformability and describe what happens when deformability is lost.
RBCs must remain flexible, deformable, and permeable to pass through narrow capillaries.
Loss of ATP reduces phosphorylation of spectrin, a cytoskeletal protein essential for membrane flexibility.
Calcium accumulation in the membrane increases rigidity and decreases pliability.
As a result, old or damaged RBCs become less deformable and are sequestered and removed by the spleen, leading to the formation of spherocytes and bite cells.
Describe the haemoglobin oxygen dissociation curve and explain the factors that cause it to shift to the right or left.
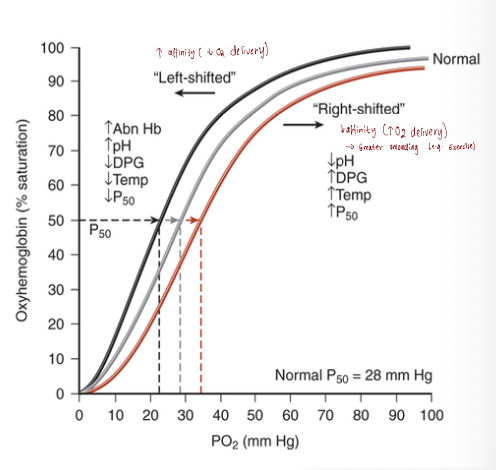
The haemoglobin oxygen dissociation curve is sigmoidal (S-shaped), reflecting allosteric changes in haemoglobin as it loads and unloads oxygen.
2,3-DPG (diphosphoglycerate), an organic phosphate in RBCs, plays a key role in regulating oxygen release.
A shift to the right indicates reduced haemoglobin affinity for oxygen, allowing more O₂ to be released to tissues (e.g., during high metabolism, acidosis, or increased 2,3-DPG).
A shift to the left indicates increased haemoglobin affinity, resulting in less O₂ released to tissues (e.g., in alkalosis or decreased 2,3-DPG levels).
What is the main goal of blood preservation, and what are the key requirements for maintaining red blood cell (RBC) viability during storage according to the FDA?
The main goal of blood preservation is to ensure that viable and functional RBCs are available for transfusion.
To maintain viability during storage, RBCs must meet FDA requirements, which include:
An average 24-hour post-transfusion survival of more than 75%, and
Free haemoglobin levels of less than 1% of total haemoglobin.
These criteria ensure stored RBCs remain functional and safe for clinical use.
Explain how the viability of red blood cells (RBCs) is affected during storage and describe the role of 2,3-DPG in post-transfusion oxygen delivery.
RBC viability post-transfusion is assessed by measuring survival of transfused cells.
Loss of RBC viability is associated with the “storage lesion,” which includes various biochemical changes during storage.
2,3-DPG levels in RBCs decrease significantly after ~2 weeks of storage.
Reduced 2,3-DPG lowers the ability of RBCs to release oxygen to tissues compared to fresh RBCs.
Discuss the purpose of anticoagulant-preservative solutions in blood collection and explain the physiological consequences of transfusing RBCs with low 2,3-DPG levels.
Purpose of anticoagulant-preservative solutions:
Prevent coagulation during collection (e.g., CPD, ACD-A, 4% sodium citrate).
Preserve RBC viability by maintaining ATP levels through added adenine, supporting glycolysis.
Physiological consequences of low 2,3-DPG in transfused RBCs:
Increased haemoglobin affinity for oxygen → reduced O₂ delivery to tissues.
Compensatory increase in cardiac output.
Decreased mixed venous pO₂ in the pulmonary artery.
Effects may occur in combination depending on patient status.
Additional note: Plastic bags use DEHP as a plasticizer, which can influence RBC preservation.
Explain the role of additive solutions in red blood cell (RBC) preservation and describe the composition and benefits of SAGM and SAGM2.
Role of additive solutions (AS):
Preserve RBCs after plasma removal.
Maintain RBC viability and affect the viscosity of RBC concentrates.
Approved for up to 42 days of storage for packed RBCs.
Examples of additive solutions:
Australia: SAGM and SAGM2.
Composition and benefits:
SAGM: NaCl (isotonic), Adenine (maintains ATP), Dextrose monohydrate (supports metabolism), Mannitol (reduces RBC lysis).
SAGM2: NaCl, Adenine, Dextrose anhydrous, Mannitol.
Both solutions help maintain RBC metabolism and reduce hemolysis during storage.
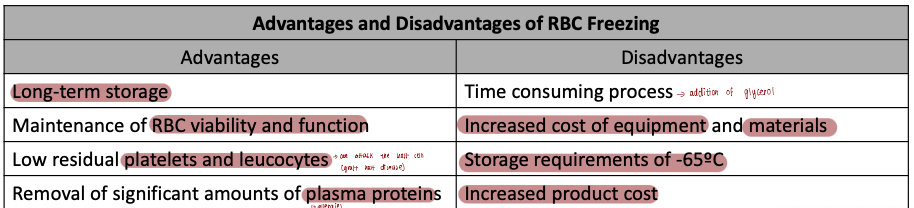
Describe the process and purpose of red blood cell (RBC) freezing, including the role of cryoprotective agents.
Purpose of RBC freezing:
Primarily for autologous transfusions and storage of rare blood types.
Process:
RBCs less than 6 days old are used.
A cryoprotective agent, usually glycerol (20–40% w/v), is added slowly with vigorous shaking to allow permeation into RBCs.
RBCs are then rapidly frozen and stored at temperatures below −65°C.
Storage duration:
FDA licenses frozen RBCs for up to 10 years from the date of freezing.
What are the common advantages and disadvantages of RBC freezing
Explain the purpose and process of red blood cell (RBC) rejuvenation, including the types of solutions used and its clinical significance.
Purpose:
Restore or enhance ATP and 2,3-DPG levels in stored RBCs to improve oxygen delivery.
Process:
RBCs are incubated with rejuvenating solutions (pyruvate, inosine, phosphate, adenine) for ~1 hour.
RBCs are then washed and transfused within 24 hours.
Clinical use:
Can prepare RBCs for transfusion up to 3 days after expiration if stored in solutions like CPD or CPDA-1.
Mainly used for rare or selected autologous units.
Limitations:
Expensive and time-consuming process.
Discuss the challenges of platelet preservation and the key quality control measures used to ensure platelet concentrate viability.
Challenges of platelet storage:
Short shelf life (7 days in Australia; previously 5 days).
Risk of bacterial contamination at 22°C.
Platelet activation and aggregation during storage.
Release of intracellular granules.
Decline in ATP and ADP levels, affecting platelet function.
Quality control measurements:
Platelet concentrate volume.
Platelet count.
pH of the unit.
Residual leukocyte count (if leuko-reduction claims are made).
Platelet swirl assessment (visual indicator of platelet viability).
List the clinical indications for platelet transfusion and explain how the efficacy of transfused platelets is assessed.
Clinical indications:
Treatment of bleeding associated with thrombocytopenia.
Prophylactic treatment for haematology-oncology patients with thrombocytopenia.
Assessment of efficacy:
Measured using Corrected Count Increment (CCI):
CCI = (platelet increment per µL) × (body surface area in m²) / number of platelets transfused (× 10¹¹).
Pre- and post-transfusion platelet counts at 1 hour and/or 24 hours used to calculate CCI.
Describe the current conditions for storage of platelet concentrates, including temperature, agitation, and pH requirements.
Stored at 20°–24°C with continuous agitation.
Shelf life: up to 7 days (expiration midnight of day 7).
Platelets may be prepared from whole blood (pooled from buffy coats) or apheresis (single donor).
pH maintenance is key; standard for satisfactory viability is pH ≥ 6.2.
Second-generation storage containers improve gas transport properties.
Additive solutions and irradiation are used to maintain viability and safety.
Explain why bacterial contamination is a major concern in platelet transfusion and describe the strategies used to detect and prevent it.
Stored at room temperature (20°–24°C) in oxygen-rich conditions → bacterial growth.
Sepsis from contaminated platelets is the most common infectious complication.
10–40% of patients transfused with contaminated units may develop life-threatening sepsis.
Prevention includes pathogen reduction/inactivation methods and maintaining appropriate storage conditions.
Summarize the current trends and innovations in platelet preservation.
Extended storage: Methods now allow platelets to be stored up to 7 days.
Additive solutions/synthetic media support platelet viability.
Pathogen reduction/inactivation procedures for safer transfusions.
Platelet substitutes:
Frozen platelets with 5% albumin and lyophilization.
Platelet-like nanoparticles for haemostatic function.
Cold storage (1°–6°C): Reduces bacterial contamination risk.
Cryopreservation:
Dimethyl sulfoxide added to apheresis platelets, stored at −80°C up to 2 years.
Recovery after transfusion ~33%.