Potassium
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
what are ranges of potassium that are life-threatening?
2.0-3.0: dangerous! take pt to ER
3.0-3.5: replete potassium levels
3.5-5.0: normal
5.0-5.5: investigate...
5.5-6.0: fix the high levels
6.0-7.0: dangerous! take pt to ER
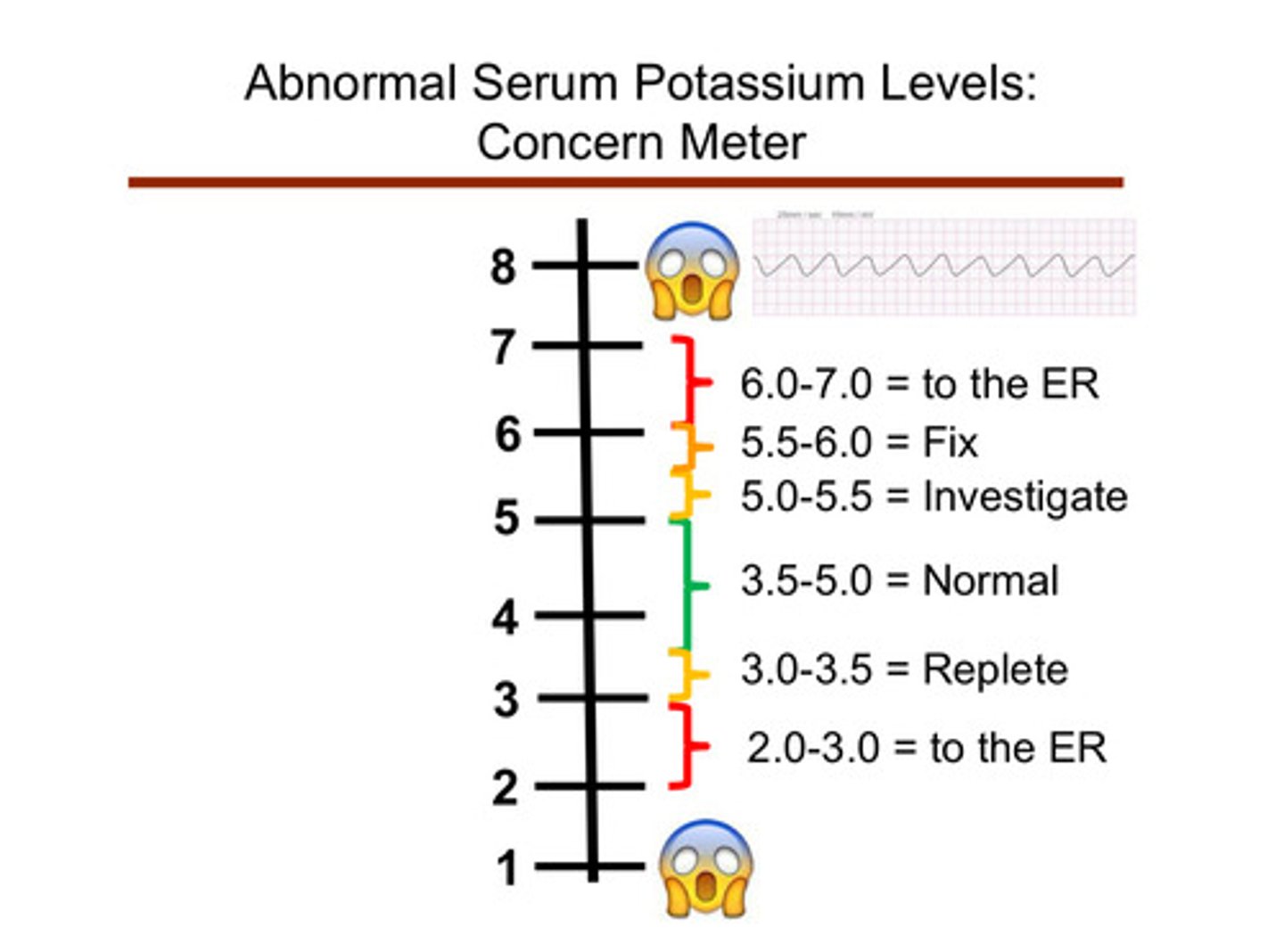
what can happen to the patient in dangerously low or high levels of potassium?
muscle weakness and bradyarrythmias
can patients "feel" that their potassium is "off"
NO!
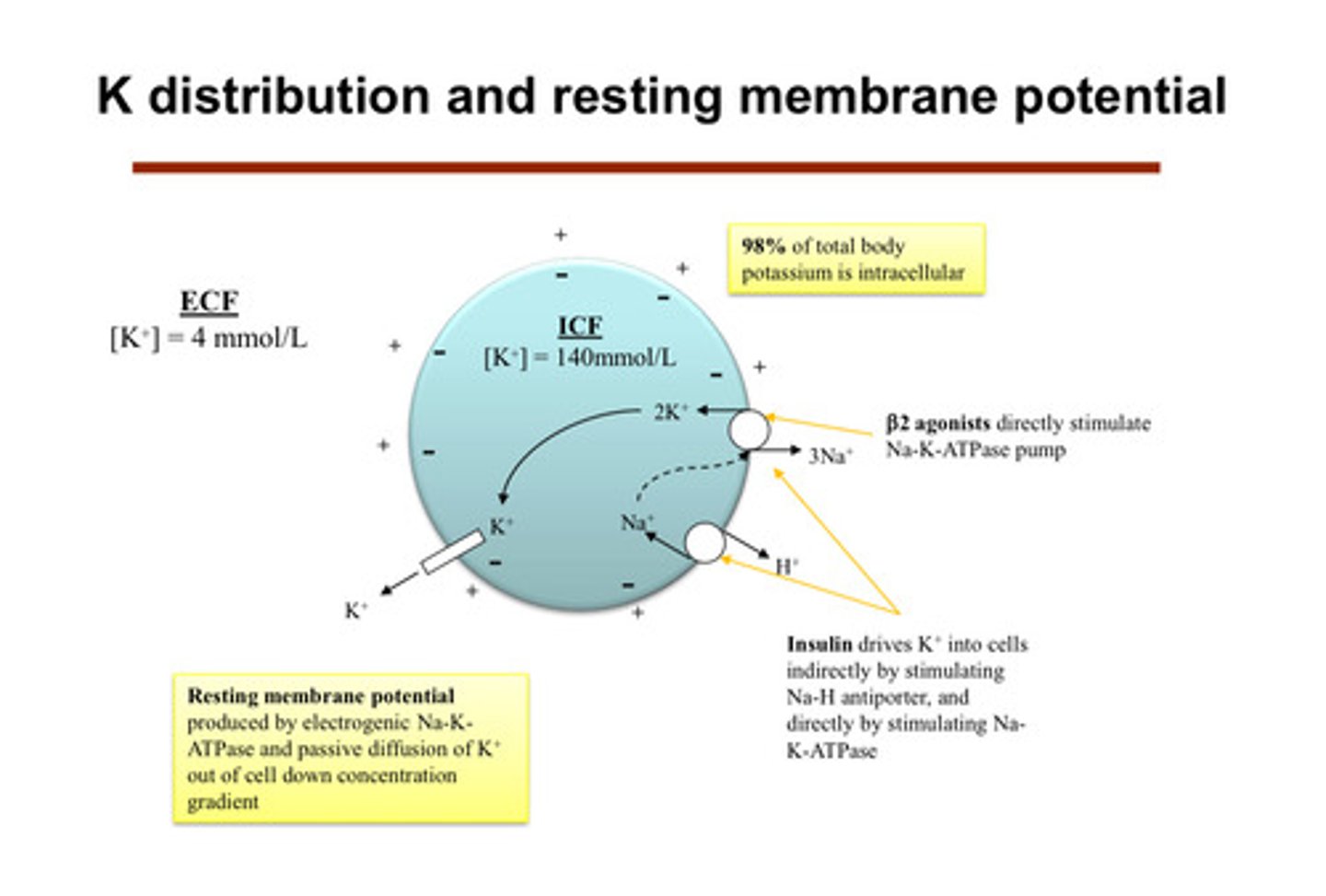
what common hormone/medication can affect potassium? does it push potassium into or out of cells?
insulin! it drives K+ into the cells (gets it out of the extracellular space into the body) indirectly by stimulating Na-H antiporter, and directly by stimulating Na-K-ATPase
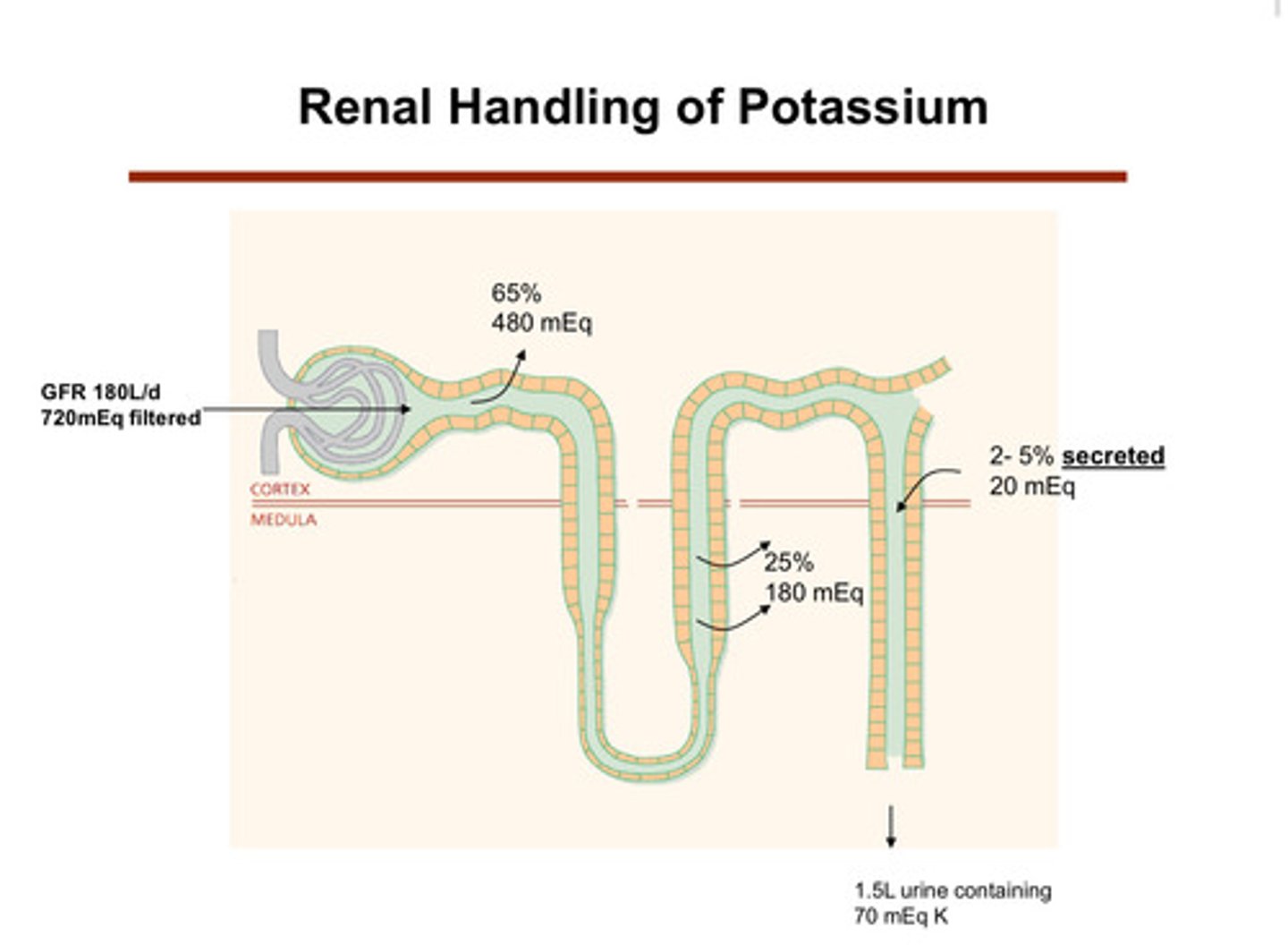
what happens to potassium in the nephron tubules?
1) potassium is filtered at the glomerulus
2) in the proximal tubule (65%) and the thick ascending limb of the loop of henle (25%), most of the potassium is reabsorbed (tubule to blood/body)
3) later, potassium is secreted (blood/body into tubule) into the tubular lumen (urine side) in the distal tubule and collecting duct (2-5%)
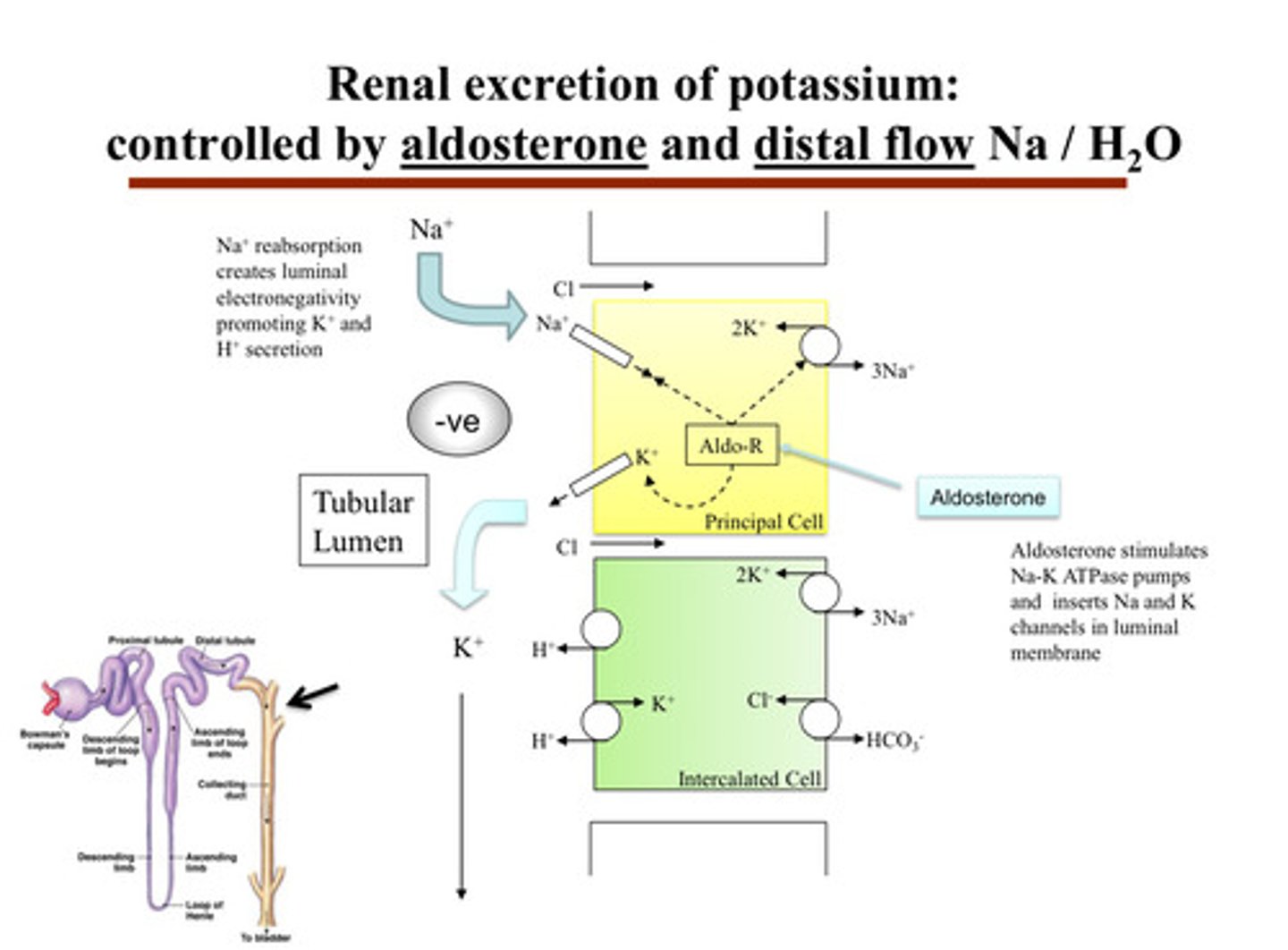
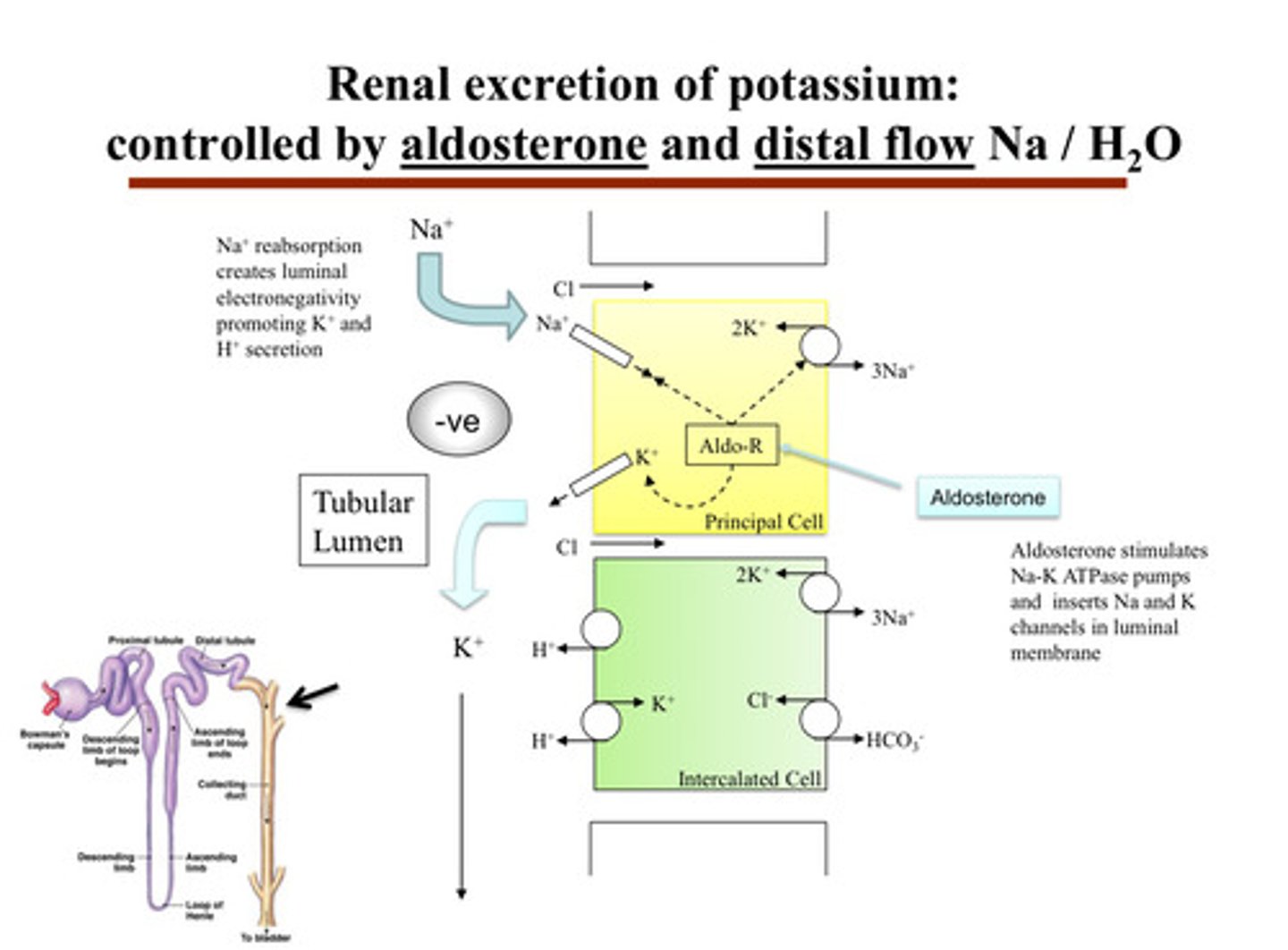
describe renal excretion of potassium
- inside the principal cells of the CD, potassium is brought in from the blood by the Na/K ATPase pump on the basolateral side (side facing the capillaries)
- then, potassium exits the principal cell on the apical side (facing the urine tubule) through potassium channels (ROMK) into the urine
where does aldosterone come from and what stimulates its release?
aldosterone is under control of the RAAS system; it is a systemic hormone, and it is released in response to elevations of the renin angiotensin system BUT can also be released in response to hyperkalemia (states that would lead to high renin and high angiotensin (hypovolemia), as well as hyperkalemia will stimulate aldosterone)
image of aldosterone acting on CCD to increase Na reabsorption, and therefore, K secretion
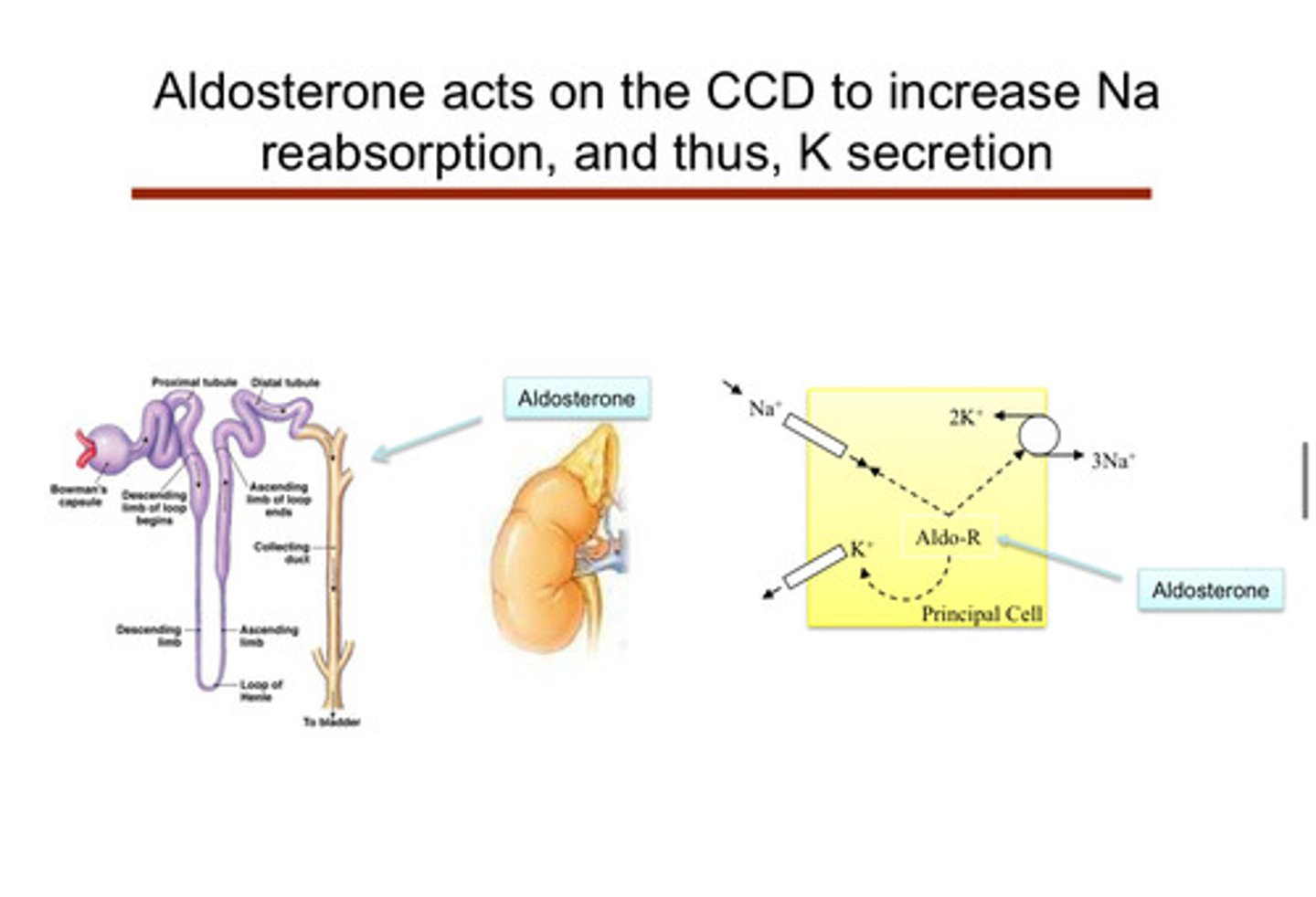
since the kidneys are in charge of getting rid of potassium, any kidney disease can lead to?
hyperkalemia
what hormone controls the fine tuning of potassium levels and where is this happening in the tubule (what segment)?
aldosterone is the main hormone —> it increases potassium secretion and sodium reabsorption in the principal cells of the distal convoluted tubule and collecting duct:
1) aldosterone acts on the cortical collecting duct in the principal cells to increase Na reabsorption, and therefore K secretion (pushing potassium out of body cells into the tubule in order for it to go into urine)
2) aldosterone stimulates Na-K ATPase pumps and inserts Na and K channels in the luminal membrane
3) Na+ reabsorption creates luminal electronegativity, promoting K+ and H+ secretion
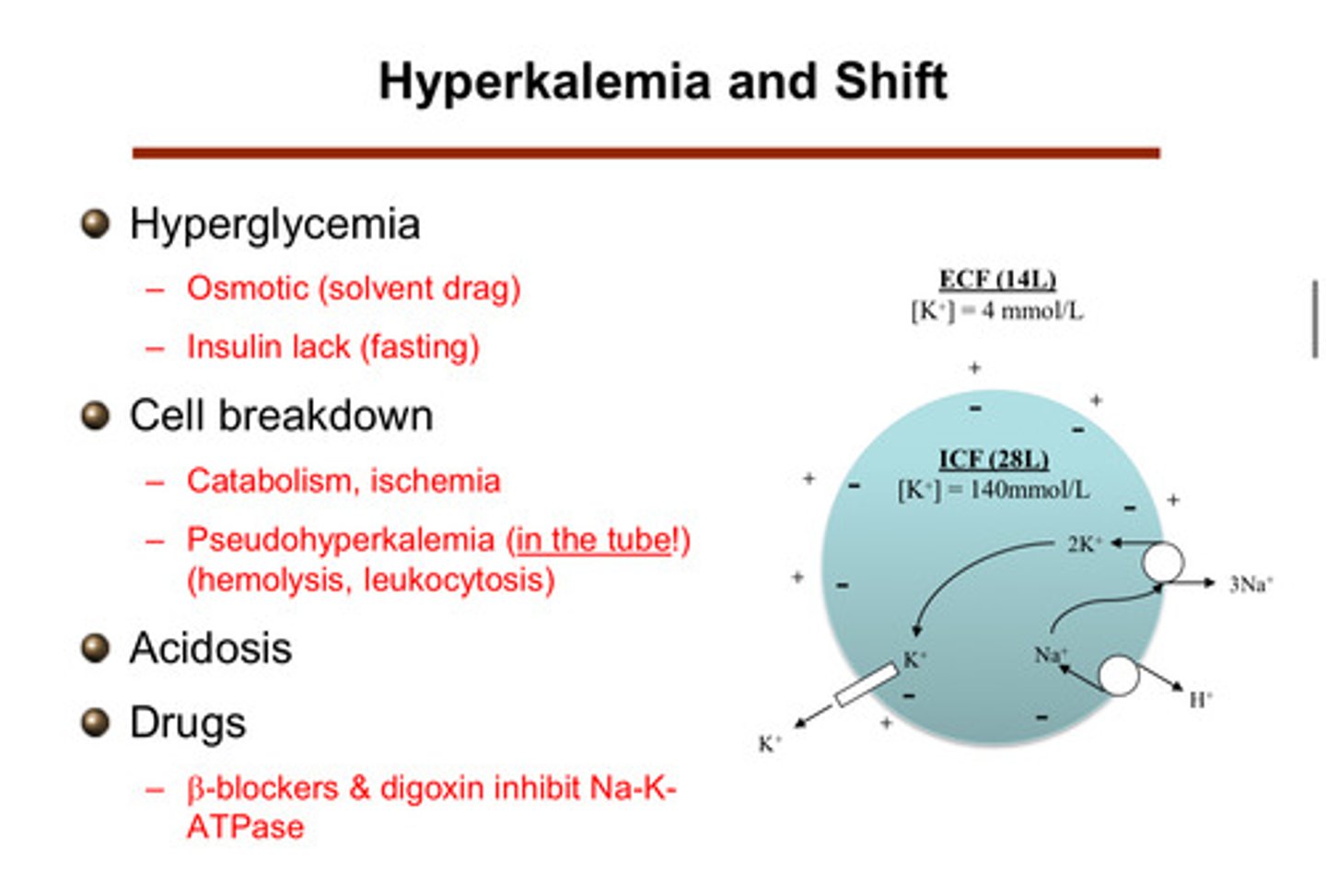
what are some "shift" situations that lead to hyperkalemia (where the potassium that is normally in the cells genes shifted to the extracellular fluid and blood)?
1) hyperglycemia
2) cell breakdown
3) acidosis
4) drugs
since aldosterone is the hormone that controls the excretion of potassium, what are some ways that aldosterone levels can be altered abnormally?
things that can lead to low levels of aldosterone or resistance to action are anything that interferes with the RAAS process —> DRUGS (can lead to lower levels of aldosterone and can lead to high potassium)
describe how cell breakdown can cause a shift to hyperkalemia
cells that die and break down and can no longer maintain that barrier —> release all of the potassium they had inside of them. this can come from massive hemolysis or muscle break down or even tumor lysis (leukocytosis). catabolism and ischemia are also things that can cause cell breakdown
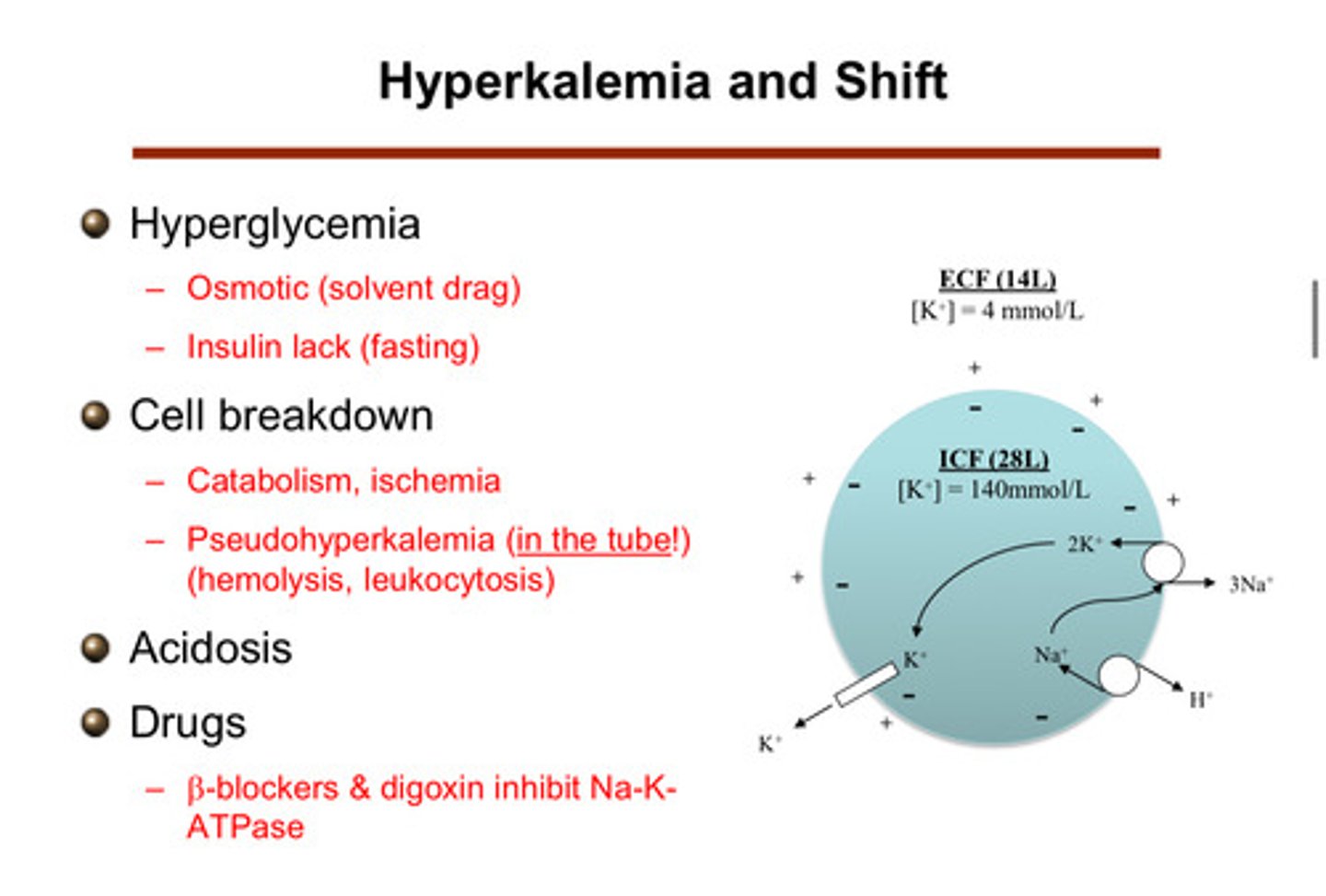
describe how drugs can cause a shift to hyperkalemia
beta blockers & digoxin are drugs that inhibit the Na-K ATPase! (note that beta agonists lead to a shift of potassium into the cells)
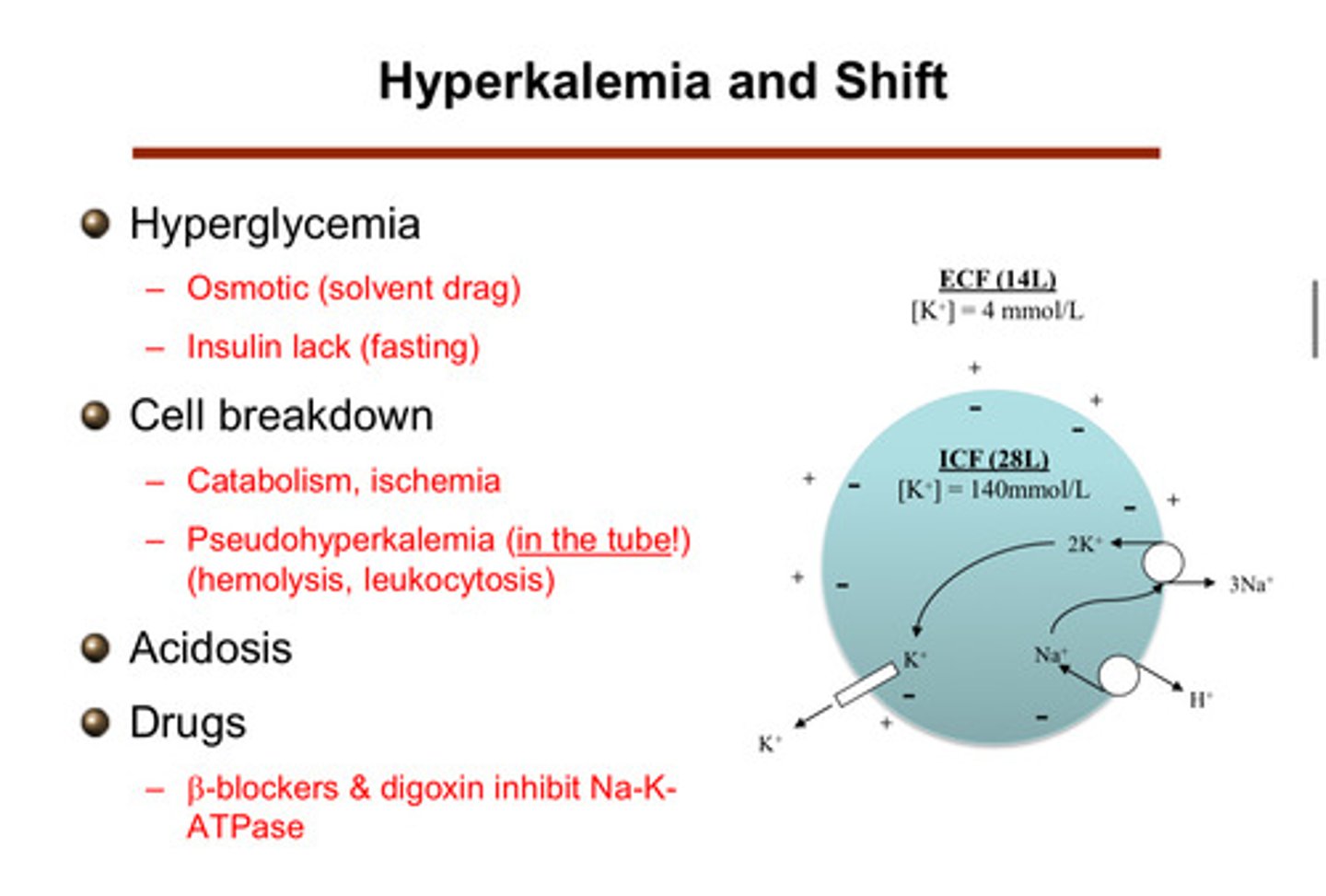
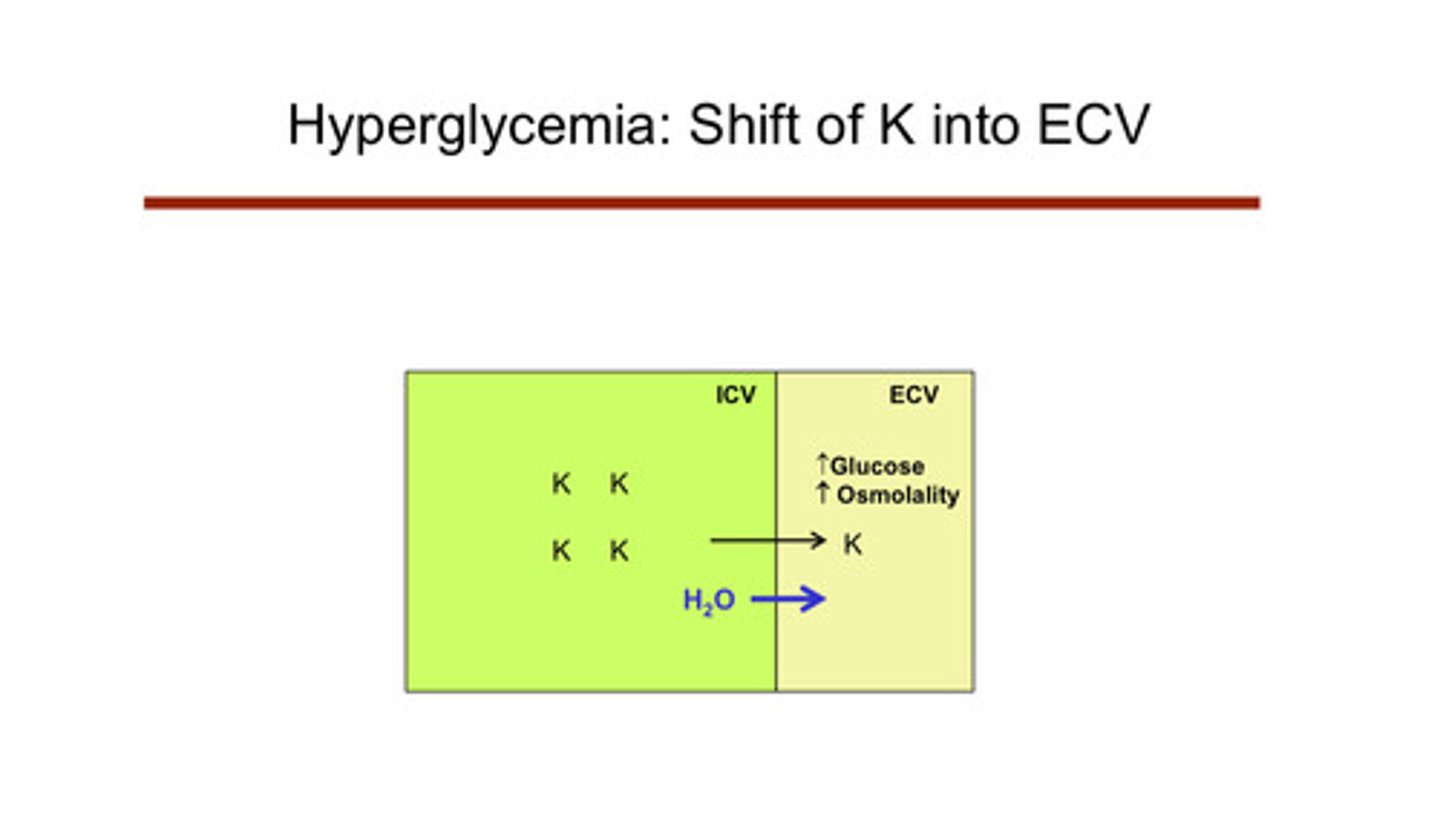
describe how hyperglycemia can cause a shift to hyperkalemia
if you have high glucose in blood, you get hyperosmolarity. as a result, water comes across from the ICV into the ECV bc it is attracted to the high osmolarity. this expands the size of the ECV relative to the ICV. this expansion creates a gradient for more potassium to come across, therefore causing potassium to shift into the ECF and blood. insulin does something similar in that it shifts glucose into the cells (does the opposite, but this is why insulin is related to hyperkalemia; lack of insulin (fasting) —> hyperkalemia)
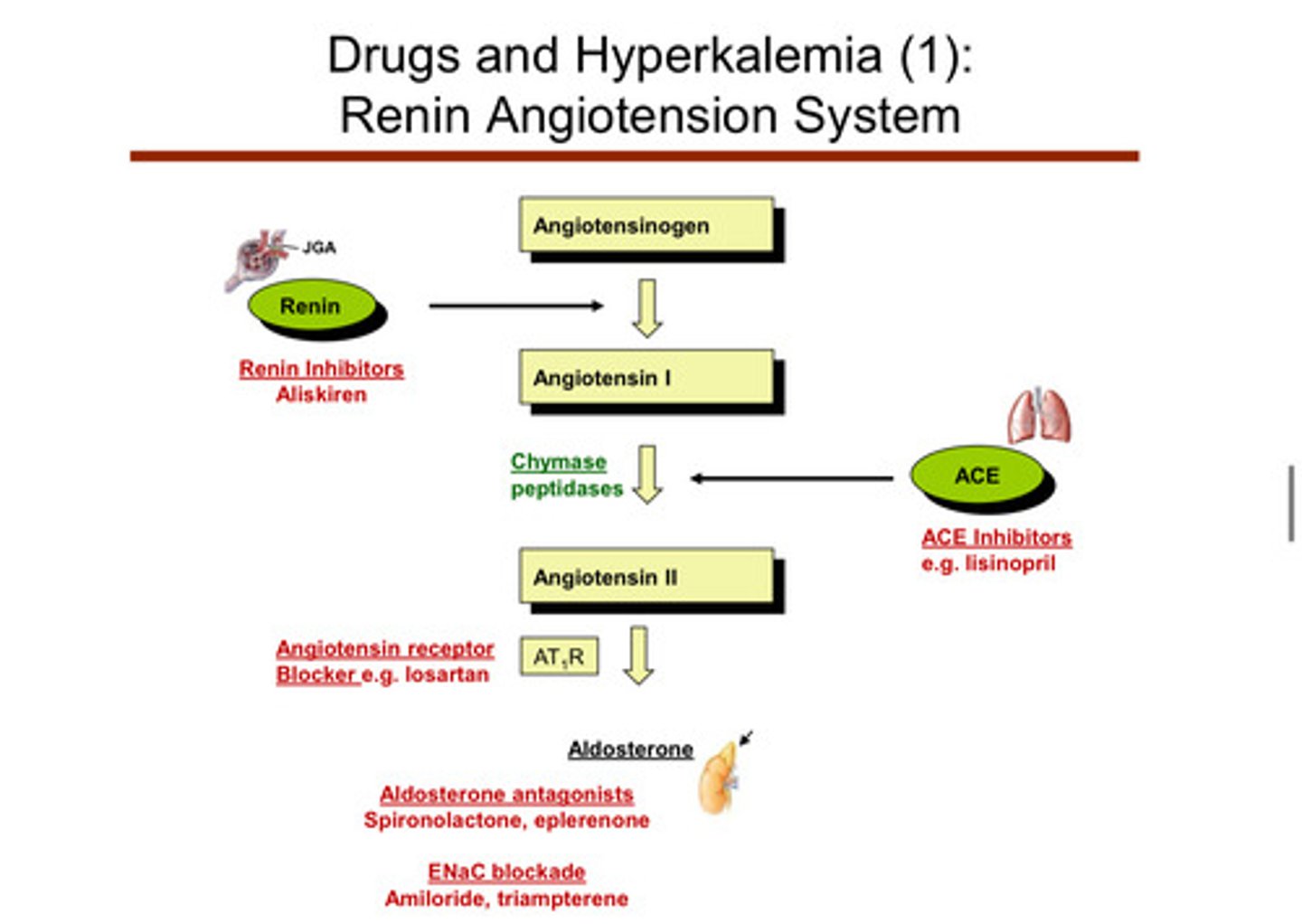
recall that things like heart failure are a state of excess RAAS activation
yup
note that some drugs that act on the RAAS system (like ACE inhibitors) can decrease protein in the urine and protect the kidney from diabetic kidney disease. however, giving these medications to somebody with already bad kidneys may cause more harm than good. there is also a more harmful affect associated with the coupling of multiple RAAS medications!
yup
which medications that act on the RAAS system may lead to hyperkalemia as a side effect?
1) ACE inhibitors: lisinopril —> blocks the converting enzyme —> hyperkalemia
2) angiotensin receptor blockers: iosartan
3) aldosterone antagonists: spironolactone, eplerenone
4) ENaC blockade: amiloride, triampterene (ENaC, epithelium sodium channels, are sodium channels that help reabsorb sodium into the cell and therefore, enhances potassium secretion via ROMK channels)
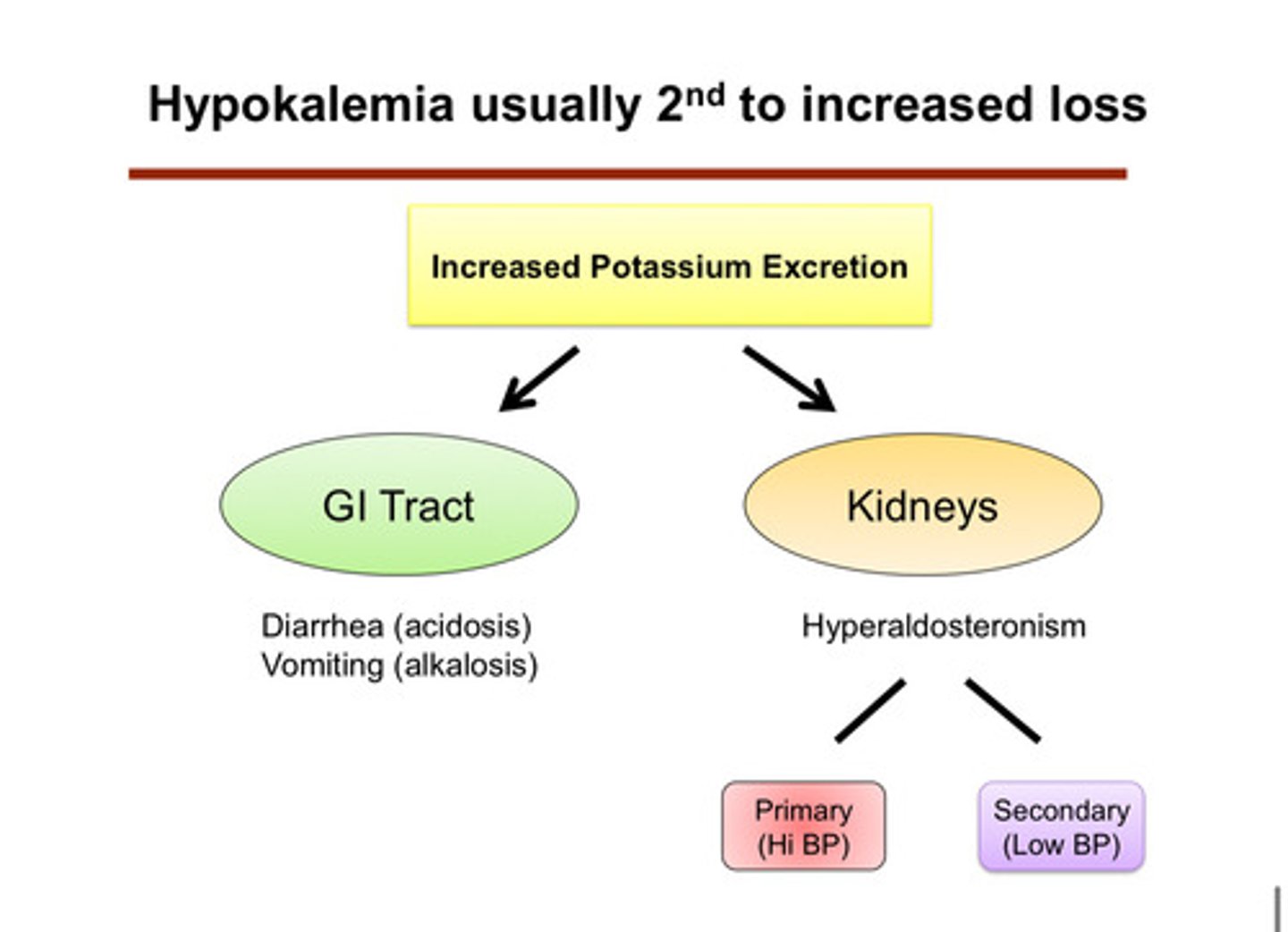
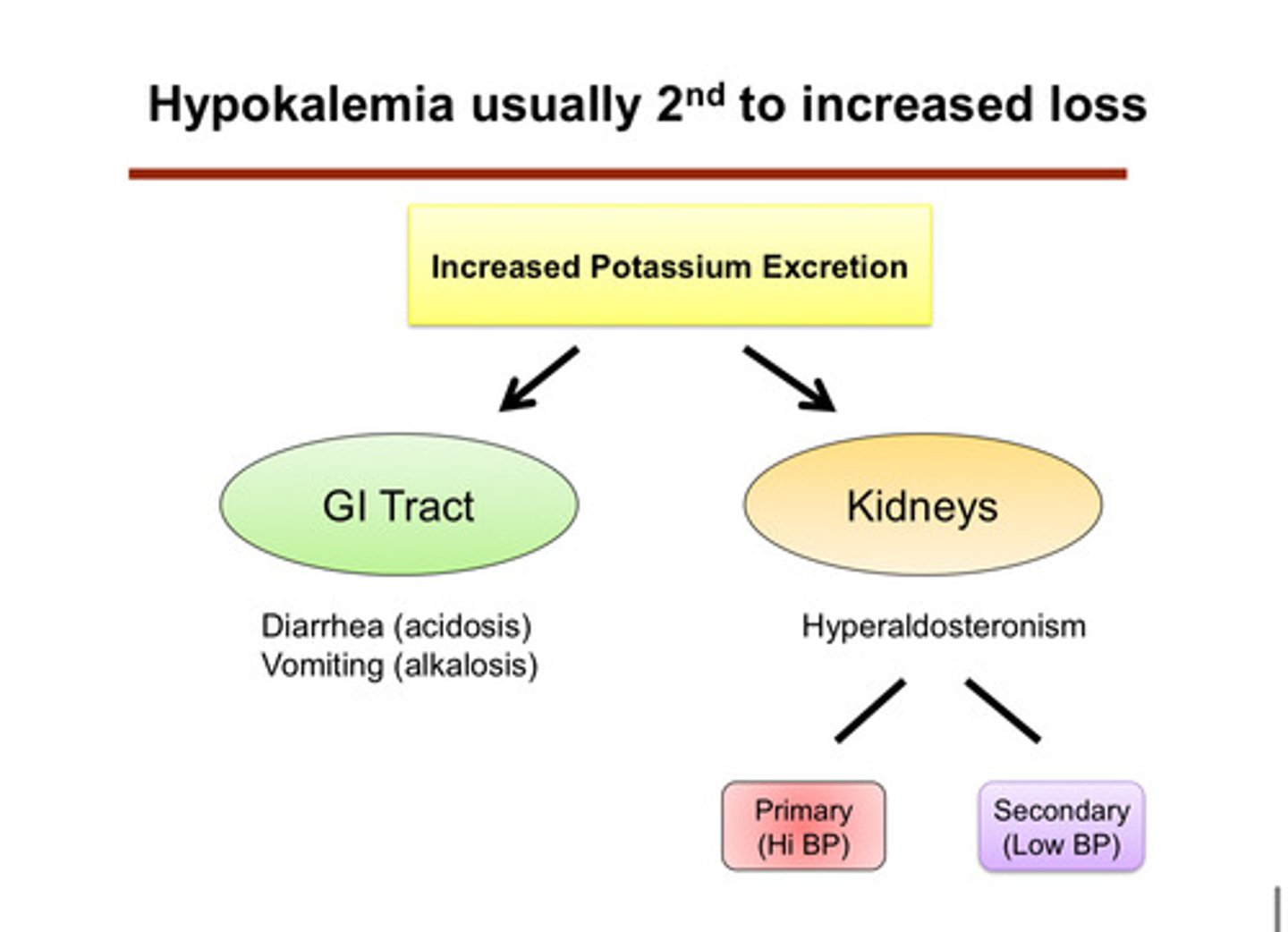
hypokalemia is usually second to what?
increased loss of potassium (waste of potassium): we see that the cell is continuing to kick out and excrete potassium even though it should not
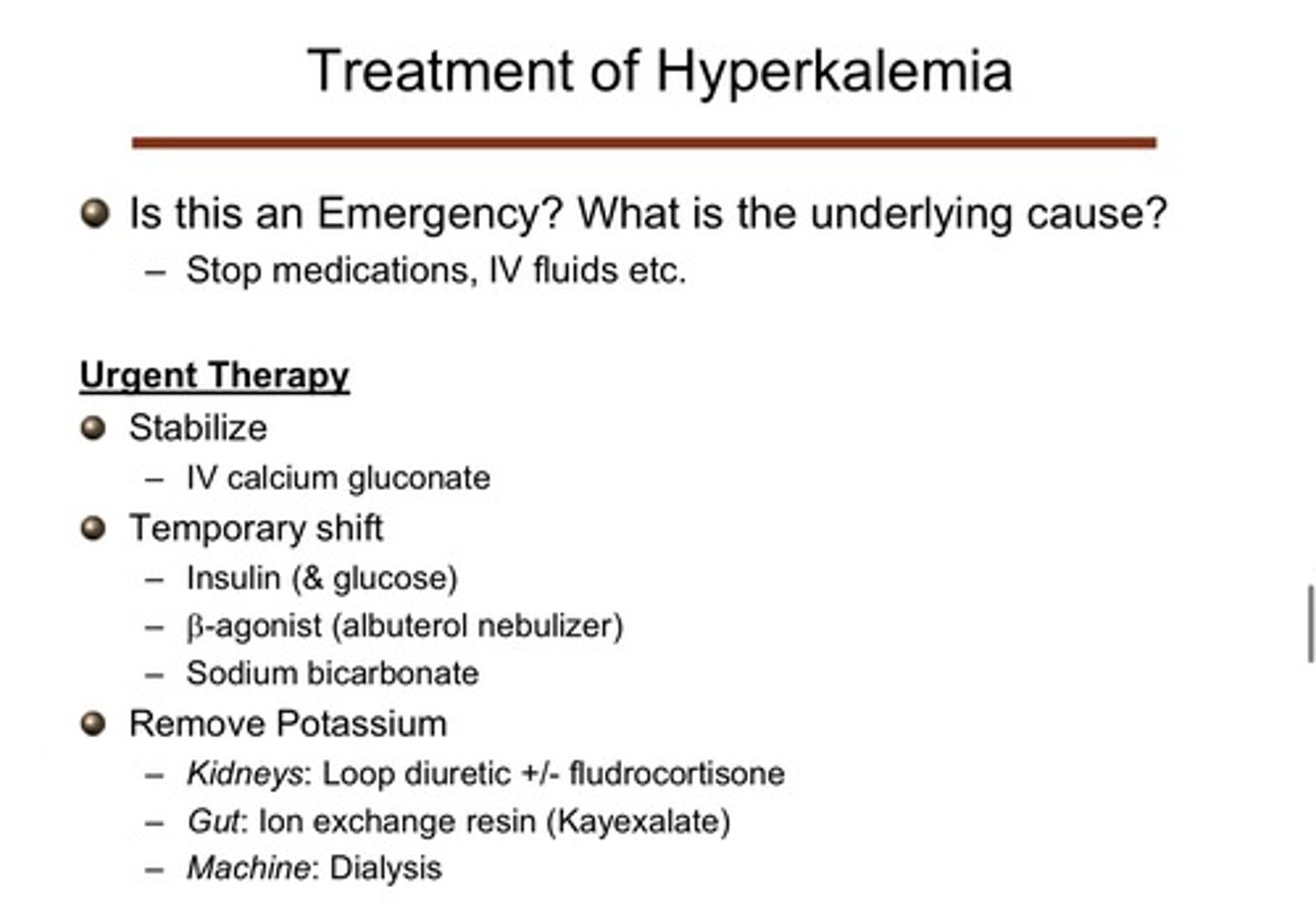
what are the general treatments for hyperkalemia that they use in the ER?
1) stabilize!: provide IV calcium gluconate; this does not help the potassium issue, but helps stabilize the problem by stabilizing the cardiac membrane (should no longer have any problems w possible arrhythmias)
2) temporary shifts: give insulin (& glucose), beta agonists, and sodium bicarbonate)
3) remove potassium: give loop diuretics +/- fludrocortisone for the kidneys, give ion exchange resin (kayexalate) for the gut, or use machine dialysis
what are the 2 most common causes (categories) of hypokalemia
1) out through urine (kidneys)
2) out through GI tract
describe how potassium can be lost through the GI tract
1) vomiting: leads to alkalosis which increases distal delivery of NaHCO3, and leads to volume depletion which increases aldosterone levels
2) diarrhea: leads to acidosis and has a pretty high potassium content sometimes
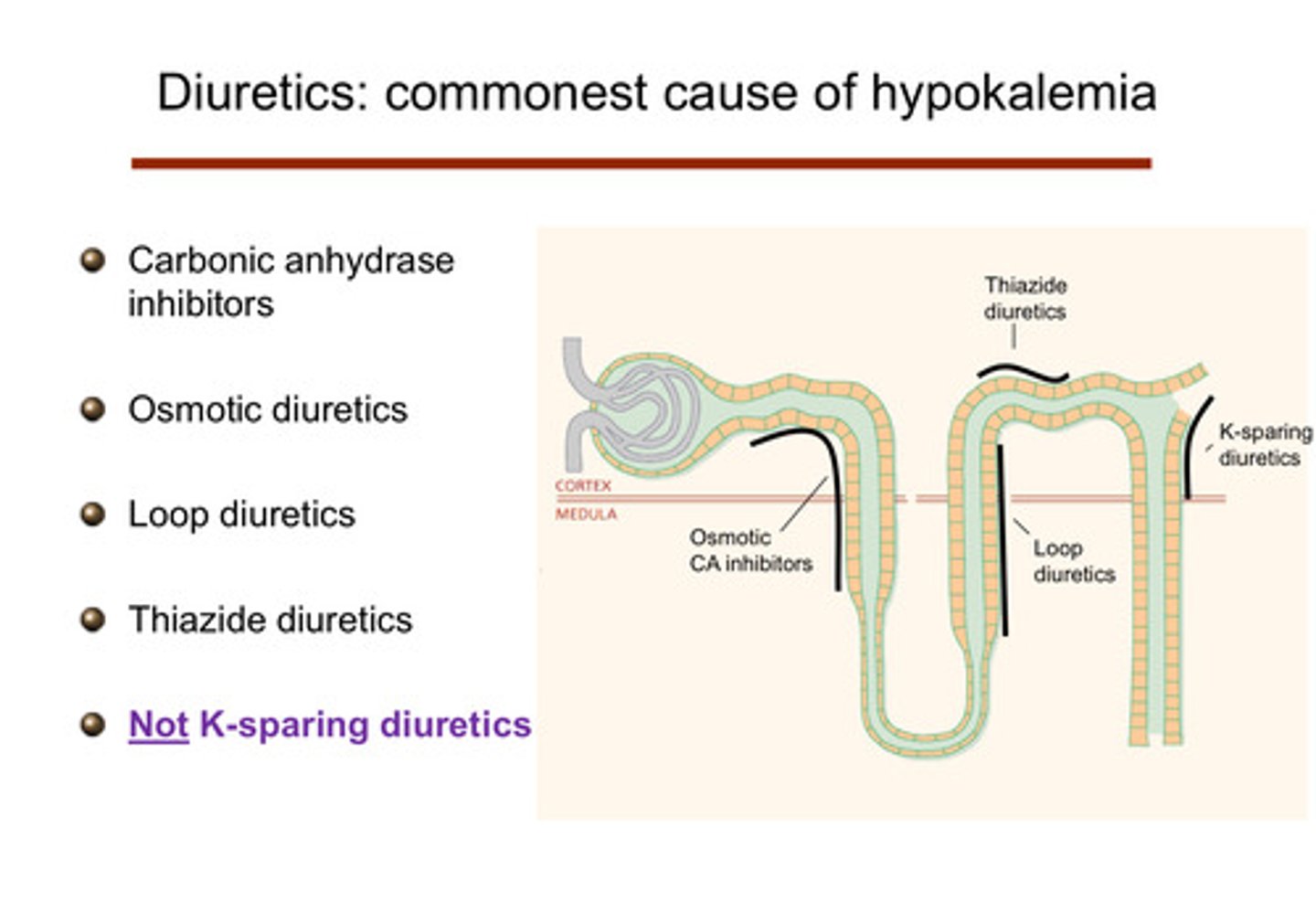
what are the renal causes of hypokalemia in terms of medications?
diuretics such as carbonic anhydrase inhibitors, osmotic diuretics, loop diuretics, thiazide diuretics, not-K sparing diuretcs can cause:
1) sodium waste through increased distal delivery of NaCl
2) elevation of aldosterone simultaneously through volume depletion
describe how diuretics cause sodium waste
they block sodium channels so the kidney does not reabsorb sodium, which means there is a lot of sodium available for exchange with potassium (when you waste sodium in the urine, the body tries to reabsorbs some of that sodium later down the nephron in the distal tubule and collecting duct; it will swap sodium for potassium, meaning the more sodium lost and reabsorbed later, the more potassium is lost too)
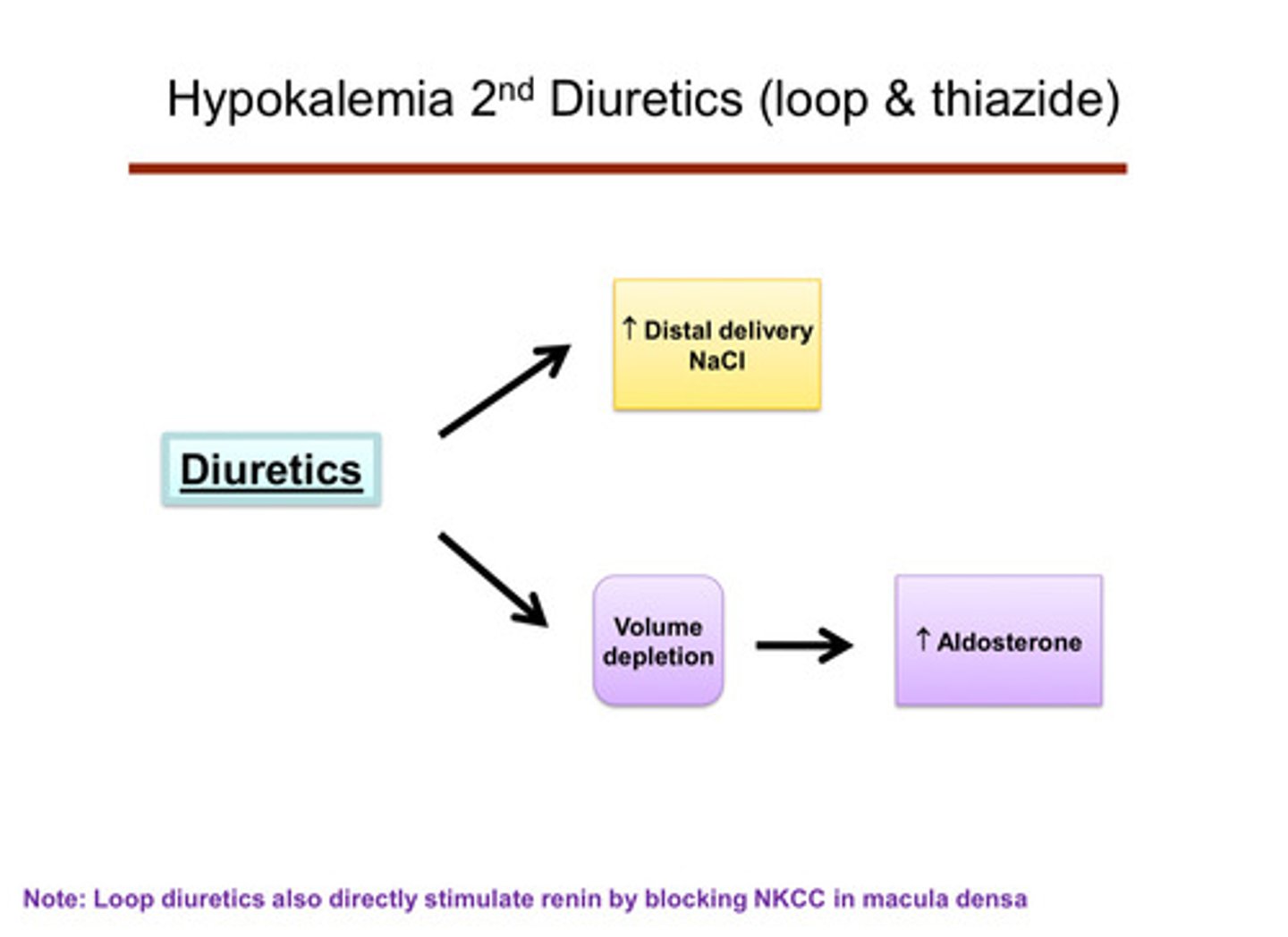
describe how diuretics cause an elevation of aldosterone
diuretics cause you to be volume depleted —> activates RAAS system —> extra aldosterone (loop diuretics also directly stimulate renin by blocked NKCC in macula densa)
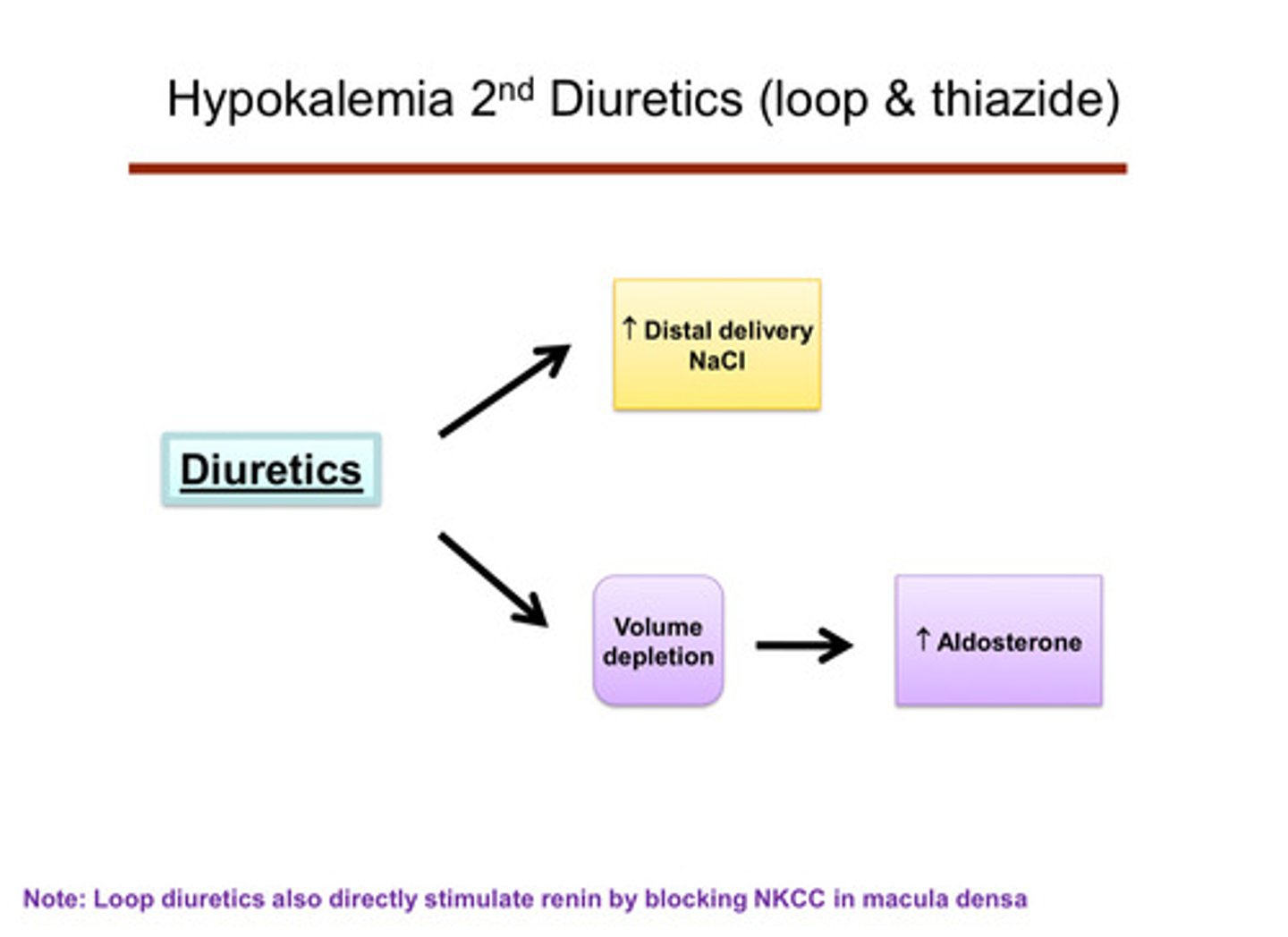
describe how potassium can be lost through the kidneys
hyperaldosteronism —> causes further potassium excretion/wasting in the cortical collecting duct
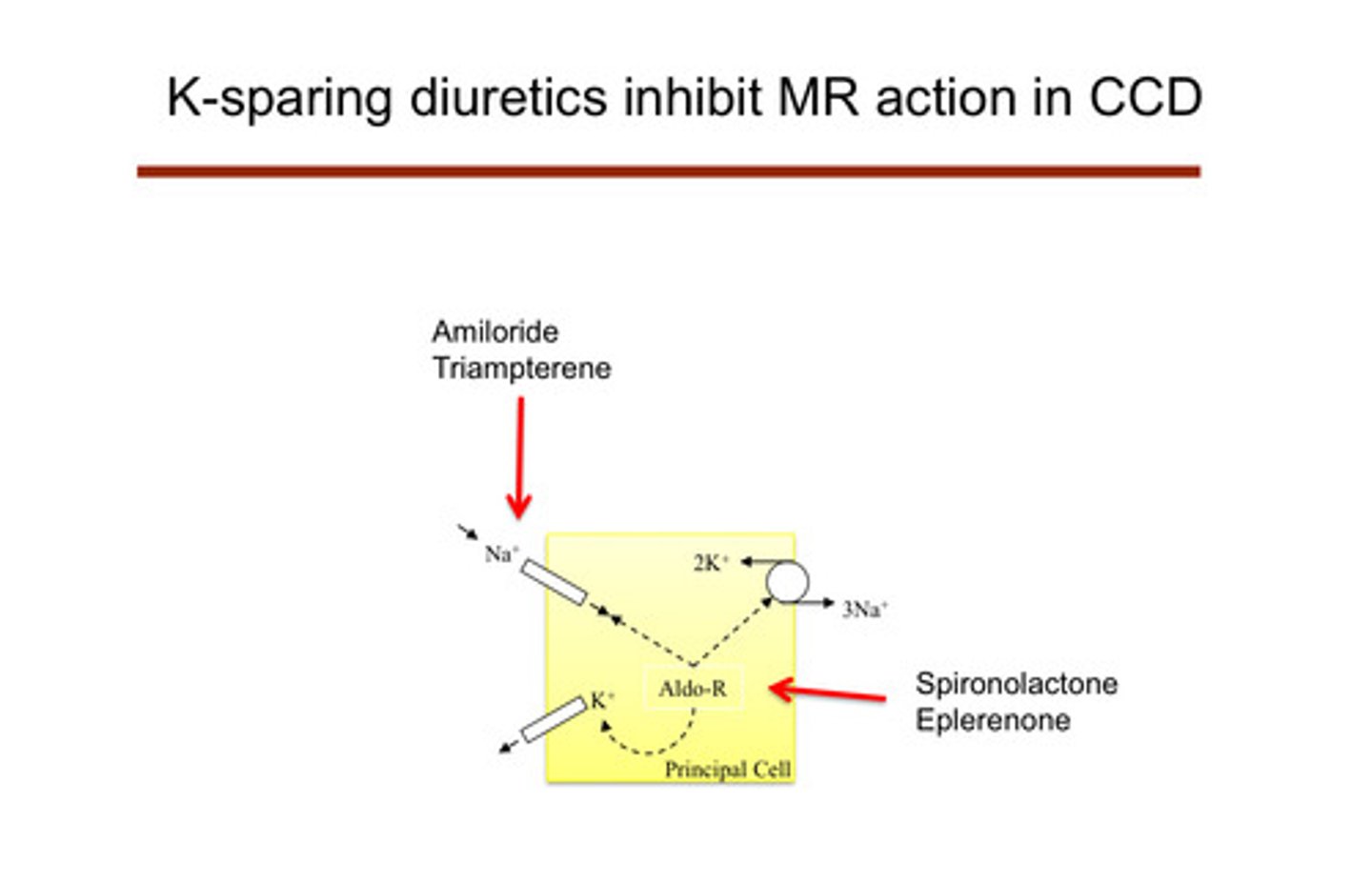
what are the "potassium-sparing" diuretics? (remember that all the other diuretics cause hypokalemia)
amiloride and triampterene —> act on the ENaC channel and cause sodium wasting that way (ENaC blockade); spironolactone and eplerenone are also potassium sparing (aldosterone antagonists) —> block aldosterone
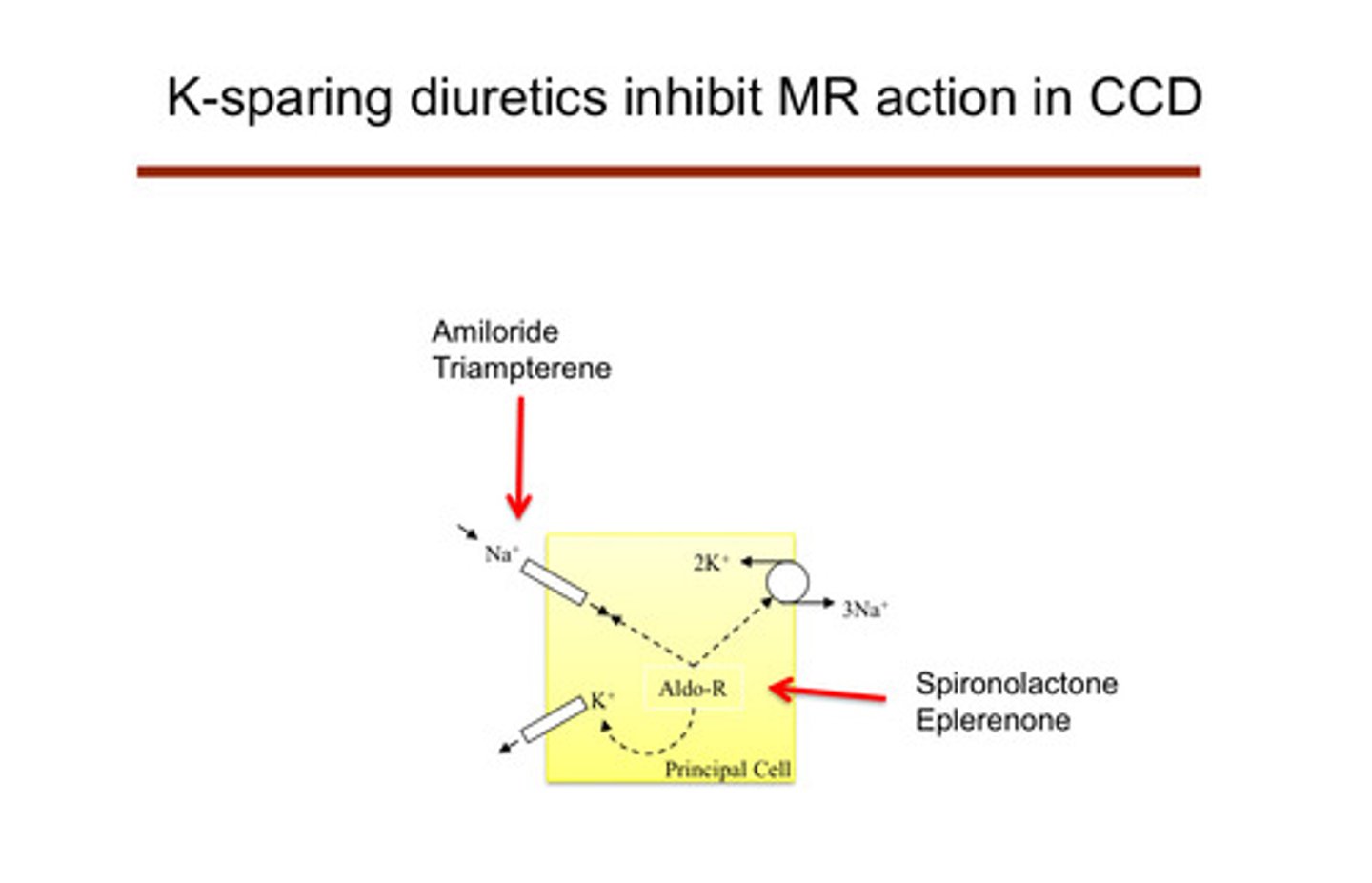
describe how the K-sparing diuretics work
they affect the principal cells of the cortical collecting duct themselves: do not lead to low potassium because they are shutting down the cell that causes the potassium excretion
1) amiloride and triampterene: block the ENaC channels of the principal cells, preventing sodium reabsorption —> no electrical drive to secrete potassium
2) spironolactone and eplerenone: aldosterone normally binds to mineralocorticoid receptors in the principal cells —> this usually increases activity of ENaC channels and Na-K ATPase pumps, promoting potassium secretion and sodium reabsorption; these drugs block the mineralocorticoid receptor actions
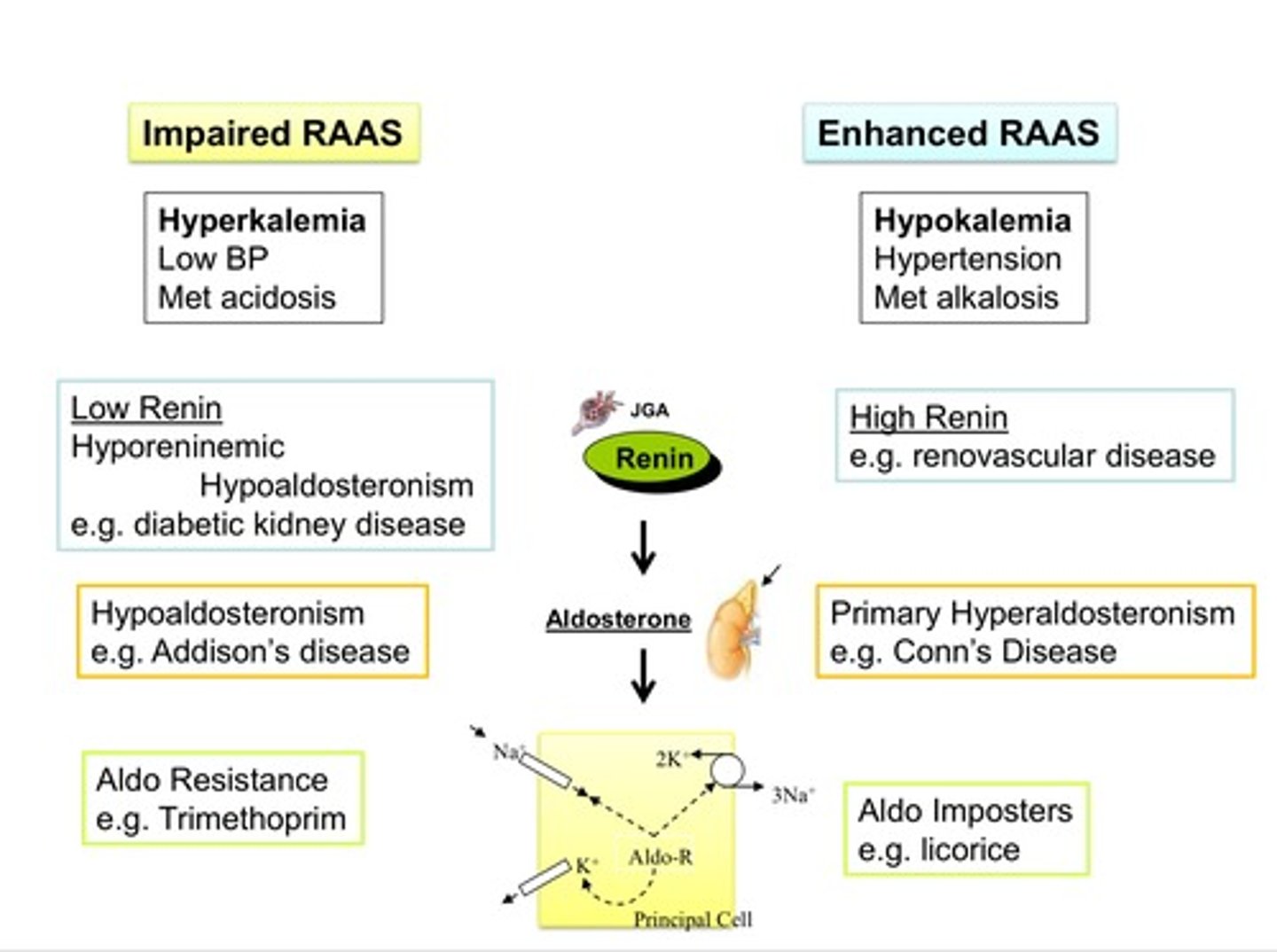
note that addison's disease (an autoimmune disease that cripples the adrenal glands) causes _____ aldosterone, which in turn causes ________
low aldosterone (hypoaldosteronism), which in turn causes hyperkalemia
loop and thiazide diuretics routinely cause hypokalemia how?
by providing more sodium for the principal cell in the ENaC channel