Uncertainty
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
The uncertainty of a measurement which requires two readings is…
double the uncertainty of each individual reading
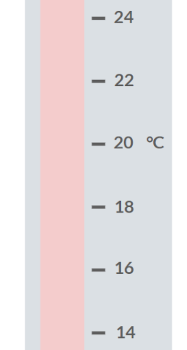
When you make a measurement of the change in temperature using the thermometer pictured above, what will the uncertainty in your answer be?
+- 2 degrees celsius
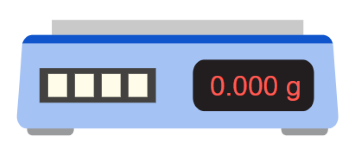
When you make a measurement of mass using the balance pictured above, what will the uncertainty in your answer be?
The resolution of the balance is 0.001g.
The uncertainty of a single=
reading=±resolution/2=±0.0012=± 0.0005g.
Because we’re measuring the mass, we need two readings, so the uncertainty is doubled:
2×±0.0005=±0.001g
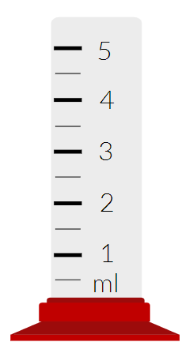
When you make a measurement of the change in volume using the measuring cylinder pictured above, what will the uncertainty in your answer be?
±0.5 ml
How do you calculate percentage uncertainty?
uncertainty of measurement/measurement *100
Each reading from a thermometer has an uncertainty of ±2°C
In an experiment, this thermometer is used to measure a temperature change of 16°C
Using the equation above, calculate the percentage uncertainty in the temperature change.
Give your answer to 2 significant figures
To record temperature change, two readings must be taken. This means the uncertainty of measurement is double the uncertainty in each reading.
Uncertainty of measurement=2×±2°C=±4°C
Measurement=16 °C
% uncertainty=4/16×100=25 %
A temperature sensor is used to measure the change in the lab’s temperature while a reaction runs overnight.
The minimum and maximum readings are shown below.
Calculate the percentage uncertainty in the change in temperature.
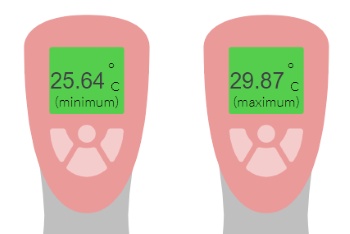
uncertainty of each reading= ±0.005°C
percentage reading = 0.24%
How can you reduce the percentage uncertainty in a measurement? (2)
Change the instrument you measure with to one with a smaller resolution.
Alter the experiment to increase the quantity of whatever you’re measuring.