10. Amines and Amides
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is an Amine?
An ammonia molecule that has one (or more)
of its hydrogens replaced by an alkyl group
General Formula: CₙH₂ₙ₊₃N
Functional Group: NH₂
How do you determine if an Amine is Primary (1°), Secondary (2°), or Teritiary (3°)?
one alkyl group attached to the N
two alkyl groups attached
three alkyl groups attached
What are an Amine’s Properties?
strong odours
amines found in urine, decaying flesh
small ones soluble in water
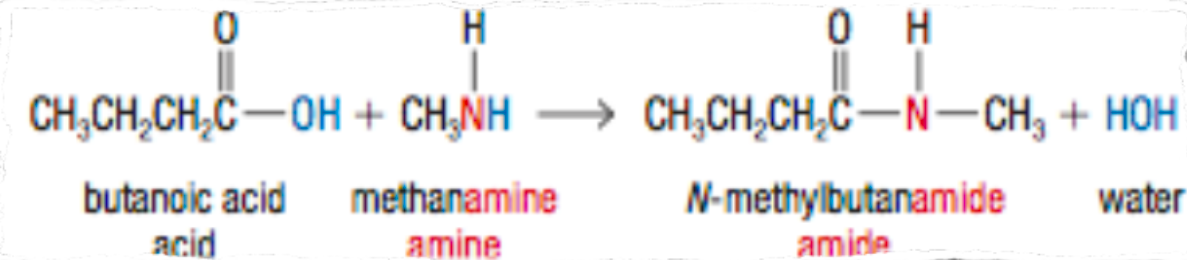
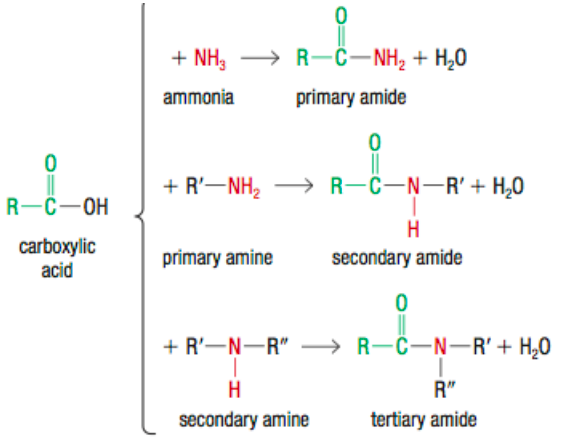
What are Amides?
carbonyl group bonded with an amine
carboxyamide functional group
carboxylic acid + ammonia, primary/secondary amines to produce amides
What is the General Formula for Amides?
CₙH₂ₙ₊₁NO
How do you form Amines?
alkyl halide (carbon chain with a halogen) + ammonia
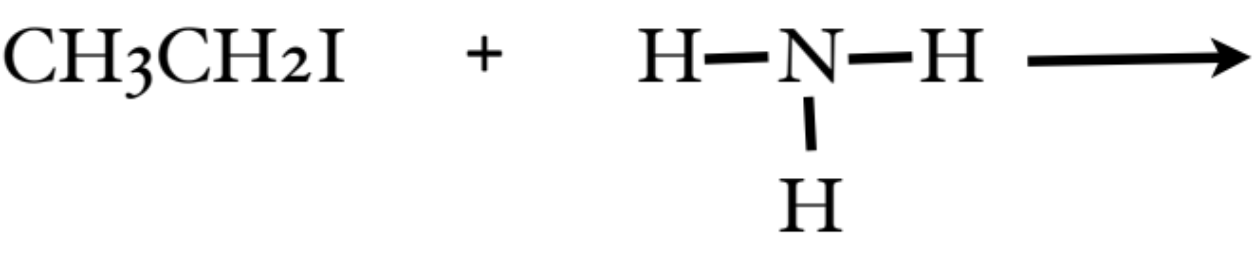
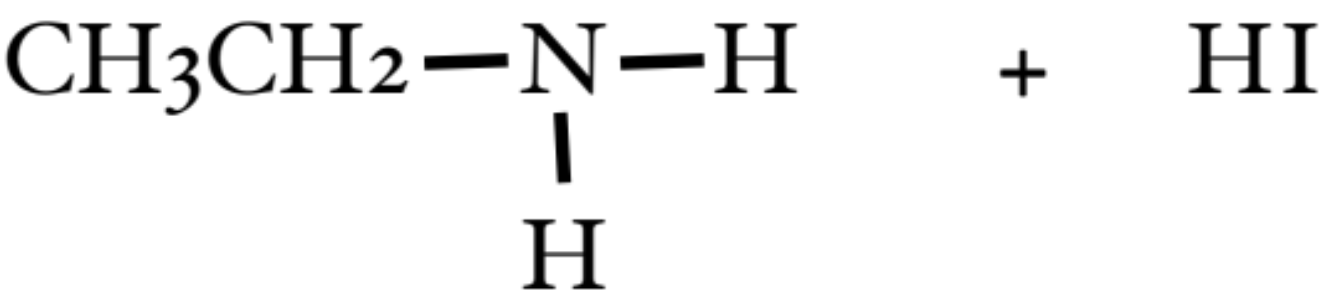
What is the classification of Amine presented in the photo? What can further do with it?
it is a primary amine
by adding another alkyl halide, we can create a secondary amine
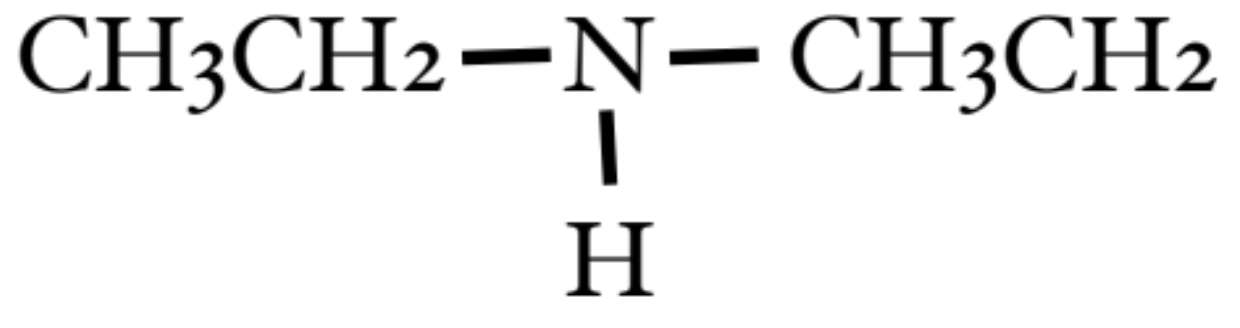
What classification is presented here?
a secondary amine
How can you create Amides?
carboxylic acid + ammonia/primary or secondary amine
a condensation reaction
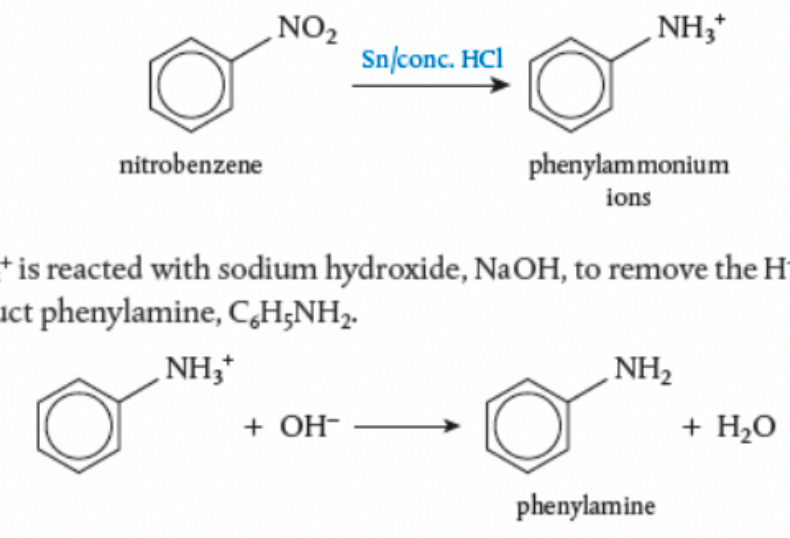
Reduction of Nitrobenzene
First Step
Nitrobenzene, C₆H₅NO₂, is dissolved in a mixture of tin and concentrated HCl
the mixture is heated under reflux in a water bath
phenylammonium ions, C₆H₅NH₃⁺
Second Step
phenylammonium + NaOH removes the H⁺ (creates water) and creates phenylamine, C₆H₅NH₂