Carbonate Diagenesis
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
What trend arises from mixing diagenetic end members
A linear trend between Sr and Mn concentrations
What would be the trace metal content of a primary, unaltered carbonate mineral
High Sr concentration and low Mn concentration
What would be the trace metal content of a fully altered carbonate mineral
Low Sr and high Mn concentrations
What does an open system refer to in diagenesis
Successive iterations of a one step reaction which mimic continuous water-rock interactions
What does the single element distribution coefficient D mean
Ratio of concentrations of an element between mineral and fluid phases at equilibrium
What is the exchange reaction distribution coefficient (Kd)
Ratio of two elements, equilibrated between solid and fluid phase (Kd = Di/Dj)
What does Coi represent
Concentration of element i in the entire fluid-rock system before any interaction occurs
Recipe for closed system diagenesis
Put the fluid and rock into the box. Calculate Coi in whole F-R system
React fluid with rock. Calculate concentration of element i in the rock after equilibration with the fluid by using Coi and Di
Is a carbonate rock homogeneously reflective of its diagenetic history
No! Different minerals/regions may be more susceptible to diagenesis than other regions.
What is the bulk rock composition of a carbonate rock represent
Mixing of any diagenetic end members within the structure
Why would the concentration of Mn and Sr behave differently during diagenesis
Mn increases in concentration due to the very high compatibility to the solid phase while Sr decreases due to its lesser solid phase compatibility
How do you find equilibrium concentrations of an element
Multiply the fluid concentration by the distribution coefficient
Why does it take Mn longer to reach equilibrium with the fluid during open system diagenesis
Mn has a higher distribution coefficient which means that it needs more passes of fluid to change composition. Increased D = increased rock buffer
Why is it that C and O concentrations do not change during diagenesis
Masses are conserved and concentrations wont change as long as the same mineral/polymorph are dissolved and reprecipitated (not true for trace elements like Sr and Mn whose concentrations do change)
Are d13c values in pore fluids similar to seawater
NOPE, water-rock equilibration can result in very different isotope values
What is the isotopic concentration of rock with no fractionation between solid-liquid phases (alpha = 1)
D13c of the rock will equal d13c of the whole system
Recipe for isotopic diagenesis model
put fluid and rock in box. Calculate concentration of element i in entire system (Coi)
Calculate isotopic composition of the rock after equilibration (d13co)
React the fluid with the rock. Calculate the iso composition of the rock after equilibration (d13Cs)
What is the fractionation factor alpha
Distribution coefficient for isotopes (alpha = d18os + 1000 / d18o + 1000)
Is C or O more sensitive to alteration
Oxygen is extremely sensitive to alteration
Why is O extremely sensitive to diagenetic alteration
Pore fluids contain much more O than C so less interaction is needed to change the isotopes
What is closed system Rayleigh distillation
Exponential relationship that describes the partitioning of isotopes between two reservoirs as one of them decreases in size
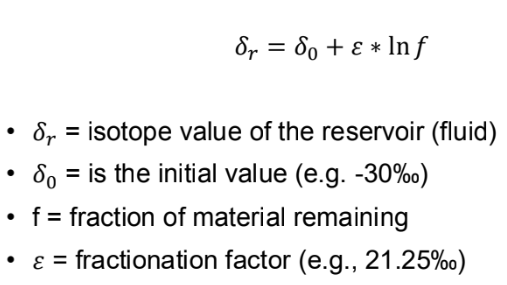
Why does burial diagenesis mainly impact oxygen isotopes
D18O fractionation factor between the fluid and carbonate phase is large and temperature dependent. Higher temperatures lower fractionation (decrease isotope values)
Under water scenarios is covariation during diagenesis possible
mixing of different mineral end-members (linear trend)
Mixing of diagenetic fluids (linear or not linear relationship (arced))
Which trace element is more susceptible to diagenetic alteration
Sr (smaller distribution coefficient)