Week 7 Material Bacteriology II
1/200
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
201 Terms
what is a nosocomial infection
an infection that occurs during a hospital-based stay that was neither present nor in the prodromal (incubation) state when the patient entered the hospital
nosocomial UTI infections
33% of all nosocomial infections
E. coli is the most common organism
nosocomial lung infections
15% of nosocomial infections
Risk factors: advanced age, chronic lung disease, large volume aspiration, chest surgery, intracranial pressure monitoring devices, ICU hospitalization, intubation
Most common organisms causing nosocomial pneumonia: Gram-negative rods, S. aureus, M. catharralis
nosocomial surgical site infections
15% of all nosocomial infections
Most common agents: Gram positives, S. aureus and CNS, enterococci, Gram-negative rods, Candida
Risk factors: advanced age, obesity, infection at a remote site, malnutrition, diabetes, extended preoperative stay, extended surgery time
Nosocomial bloodstream infections
13% of all nosocomial infections
risk factors: age 1 year or younger or 60 years and older, malnutrition, immunosuppressive chemotherapy, loss of skin integrity, burns or bedsores, severe underlying illness, undwelling device, ICU stay, prolonged hospital say
Nosocomial infection transmission
Direct: contaminated food or intravenous solution
Indirect: from patient to patient via hands
Droplet: inhalation of droplets (>5 um in diameter) that cannot travel more than 3 feet (pertussis)
Airborne: inhalation of droplets (</= 5 um) that travel long distances (M. tuberculosis)
vector borne: insects and rats, rare mode in developed countries
antibiotic resistance in nosocomial infections
Nosocomial infections have changed due to the use and overuse of antibiotics
patients’ normal flora changes after hospitalization, become colonized with resistant organisms, it patients returned to nursing homes, resistant organisms can be transferred to other patients
risk factors for acquiring highly resistant organism include: prolonged hospitalization, prior antibiotic treatments
nosocomial infection prevention
Hand washing between patient contact is more effective
segregation of infected patients
wearing masks, gloves, and gowns
bagging of contaminated articles
cleaning room thoroughly after patient discharged
placing instructions on patients doors
types of bioterrorist evens
announce: overt
unannounced: covert
category A bioterror agents
Highest concern
variola major
bacillus anthracis
yersinia pestis
clostridium botulinum toxin
francisella tularensis
hemorrhagic fever viruses (filoviruses and arenaviruses)
category B bioterror agents
coxiella buretti
brucella spp.
burkholderia mallei
staphylococcal enterotoxin B
food and waterborne agents
ricin toxin
alphaviruses
category C bioterror agents
nipah virus
hantavirus
yellow fever virus
tickborne encephalitis virus
multidrug resistant mycobacterium tuberculosis
BSL-1
organism that do not cause disease in healthy humans and are of minimal potential hazard to lab personnel and the environment (ex. B. subtilis)
standard microbiology safety practices
no special safety equipment required
laboratory clothing recommended
sink for hand washing, open bench top resistant and impervious to water
BSL-2
organisms associated with human disease and pose moderate potential hazard (ex. shigella)
BSL-1 safety practices plus limited access to lab and extreme precautions with contaminated sharps
class I or II biological safety cabinet
appropriate personal protective equipment
BSL-1 facility requirements plus autoclave and eye wash
BSL-3
organism which pose serious or potentially lethal disease when inhaled (ex. M. tuberculosis)
BSL-2 safety practices plus controlled access to lab and all procedures conducted in biological safety cabinet
Class I or II biological safety cabinet
appropriate personal protective equipment
BSL-2 facility requirements plus negative airflow, air exhaust to outside, self-closing double doors
BSL-4
organisms with life threatening potential and transmission by aerosol or of unknown risk of transmission (ex. Ebola)
maximum containment, special clothing shower upon exit, separate building, special engineering design
route of infection- food
potentially significant route of delivery
secondary to either purposeful or accidental exposure to aerosol
route of infection- water
capacity to affect large numbers of people
dilution factor
water treatment may be effective in removal of agents
route of infection- respiratory
inhalation of spores, droplets and aerosols
aerosols are most effective delivery method
advantages of biologic as weapons
infectious via aerosol, organisms fairly stable in environment, susceptible civilian populations, high morbidity and mortality, person to person transmission (smallpox, plague, VHF), difficult to diagnose and/or treat, easy to obtain, inexpensive to produce, potential for dissemination over large geographic area, creates panic, can overwhelm medical services, perpetrators escape easily
level A laboratory
BSL-2 lab with a certified class II biological safety cabinet, BSL-1 microbiology practices, directed by competent scientists, personnel specifically trained in handling pathogenic agents.
role is to rule out critical biological agents and refer to higher level laboratory
if announced: notify FBI, and the PHL, based on consultation, test and refer
if unannounced: rule out, if unable to rule out call the nearest level B lab
laboratory risk for bioterrorism agents
B. anthracis: BSL-2, low risks
Y. pestis: BSL-2, medium risk
brucella spp: BSL-2/3, high risk
F. tularensis: BSL-2/3, high risk
botulinum toxin: BSL-2, medium risk
smallpox: BSL-4, high risk
viral hemorrhagic fever: BSL-4, high risk
plague epidemiology
US averages 13 cases per year
30% of cases are in Native Americans in the southwest.
15% case fatality
most cases occur in summer
bubonic, septicemic, and pneumonic
Yersinia pestis specimen selection
bubonic: bubo, lymph node aspirate
septicemic: blood, obtain three sets 10-30 min apart
pneumonic: sputum, bronchial washings
yersinia pestis specimen inoculation
inoculate routine plating media and make thin smear for DFA
use Wayson only if DFA is unavailable, Wayson stain is not diagnostic must confirm by DFA and mouse inoculation
yersinia pestis characteristics
small, gram negative bipolar coccobacilli
Wayson stain is pink-blue cells with a closed safety pin looks
BHI broth will have little chunks in it
Botulism
Diagnosis of botulism is made clinically
Health care providers suspecting botulism should contact their State Health Department
Infective dose: 0.001 µg/kg
Incubation period: 18 - 36 hours
Dry mouth, double vision, droopy eyelids, dilated pupils n Progressive descending bilateral muscle weakness & paralysis. Respiratory failure and death
Mortality 5-10%, up to 25%
Level A Procedures for Botulism Event
Properly collected specimens are to be referred to designated testing laboratories
Prior to the shipment of any botulism associated specimen, the designated laboratory must be notified and approved by the State Health Department
Clinical specimens to be collected: Serum, Gastric contents or vomitus, Feces or return from sterile water enema, Wound tissue
Botulism toxins are extremely poisonous
Minute quantities acquired by ingestion, inhalation, or by absorption can cause death
All materials suspected of containing toxin must be handled with CAUTION
anthrax Epidemiology
Primarily a disease of herbivorous animals such as sheep, cattle, goats, and horses
Humans acquire the infection accidentally in agricultural or industrial setting, During processing of hides or animal hair, gains access through cuts or inhalation
Anthrax Clinical Manifestations
Cutaneous anthrax begins 2 to 5 days after inoculation of spores (95%), lesion starts as an erythematous papule that progresses into an ulcerative black eschar or “malignant pustule”
Pulmonary anthrax (rare) is acquired by inhalation of spores, malaise, mild fever, nonproductive cough follows
Gastrointestinal (very rare)
Anthrax Laboratory Diagnosis
Large gram-positive bacilli in short chains
Nonhemolytic, white to gray on sheep’s blood agar
"Medusa head” appearance
Lack of motility
Penicillin inhibition zone
Capsule formation
“STICKY” consistency on SBA
catalase-positive
Aerobic spore formation
Inhalational Anthrax
Infective dose = 8,000 - 15,000 spores
Incubation period = 1-6 days
Duration of illness = 3-5 days
Fever, malaise, and fatigue
Short period of improvement = up to 2 days
Abrupt respiratory distress…death <24hrs
No person-to-person transmission
Anthrax Specimen Selection
Inhalation: Sputum and Blood
Cutaneous: Vesicles and Eschar
Gastrointestinal: Stool and Blood
Francisella tularensis
Plague-like disease in rodents (California), Deer-fly fever (Utah), Glandular tick fever (Idaho and Montana) Market men’s disease (Washington, DC), Rabbit fever (Central States), O’Hara’s disease (Japan)
Poorly staining, tiny Gram-negative coccobacilli
Fastidious, requires cysteine for robust growth: Cysteine Heart Agar (CHA) is ideal, BYCE can be used
tularemia
Contagious --- no
Infective dose --- 10-50 organisms
Incubation period --- 1-21 days (average=3-5 days)
Duration of illness --- ~2 weeks
Mortality --- treated : low, untreated: moderate
Persistence of organism ---months in moist soil n Vaccine efficacy --- good, ~80%
Brucellosis
Zoonotic disease caused by any of 4 Brucella spp.: abortus, melitensis, suis, and canis
Systemic infection characterized by an undulant fever pattern
Relatively rare in the U.S. with approximately 100 cases/year
The most commonly reported laboratory-associated bacterial infection
Infective dose = 10 -100 organisms
Incubation period = 5 days - > 6 months
Duration of illness = weeks to months
Fever, profuse sweating, malaise, headache and muscle/back pain
No person-to-person transmission
Mortality: < 5% n Stable organisms
Brucellosis transmission
Ingestion: The most common mode of transmission
Direct skin contact/puncture: Occupational hazard for farmers, butchers and veterinarians
Aerosols: Highly infectious
Brucella spp. Specimen Selection
Serum
Blood or bone marrow
Tissue (spleen, liver)
Brucella spp. Key Level A Lab Tests
Colonial morphology on SBA, Fastidious ¨Visible growth may take 48 - 72 hrs, Small (0.5-1.0mm), convex, glistening, Non-hemolytic and non-pigmented
Oxidase
Urea hydrolysis: B. suis & B. canis ~15 min, B. abortus & B. melitensis ~24hr
What is a zoonosis?
A disease that occurs predominantly in vertebrate animals and is transmitted to humans through direct contact with an infected animal or its excretions, or by an insect vector that feeds on animals.
Name two zoonoses associated with livestock.
Campylobacter (chicken) and Salmonella (chicken/eggs).
What is Bacillus anthracis commonly known as?
Anthrax, also referred to as Woolsorters Disease.
What disease is caused by Brucella?
Brucellosis.
What is Francisella tularensis associated with?
Tularemia.
What zoonotic disease is associated with rodents?
Leptospirosis, caused by Leptospira interrogans.
What are two vector-borne zoonoses?
Rickettsiae and Borrelia burgdorferi (Lyme Disease).
What is Yersinia pestis known for causing?
Plague.
What are the two bacterial zoonoses associated with Streptobacillus and Spirillum?
Streptobacillus moniliformis and Spirillum minor.
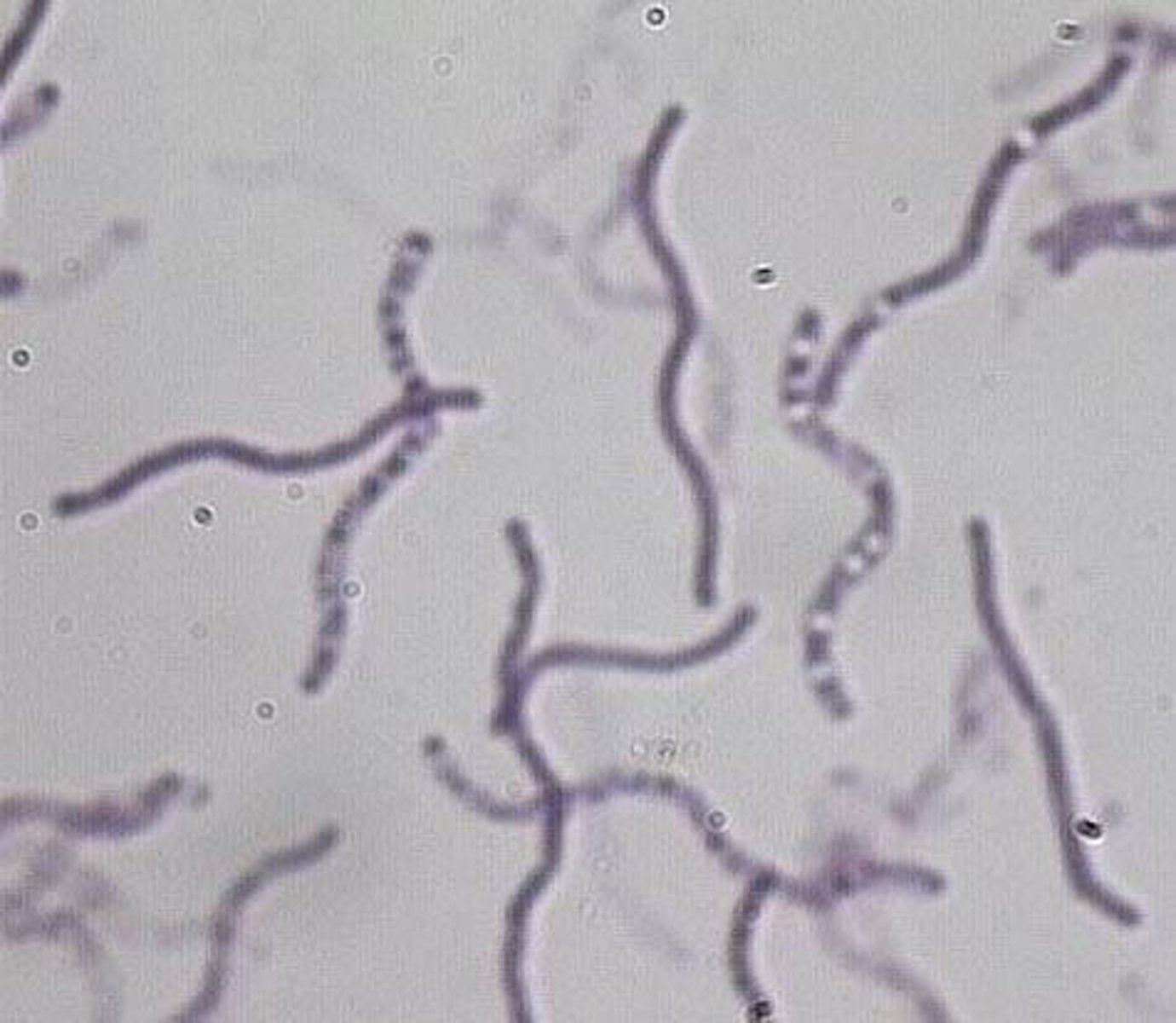
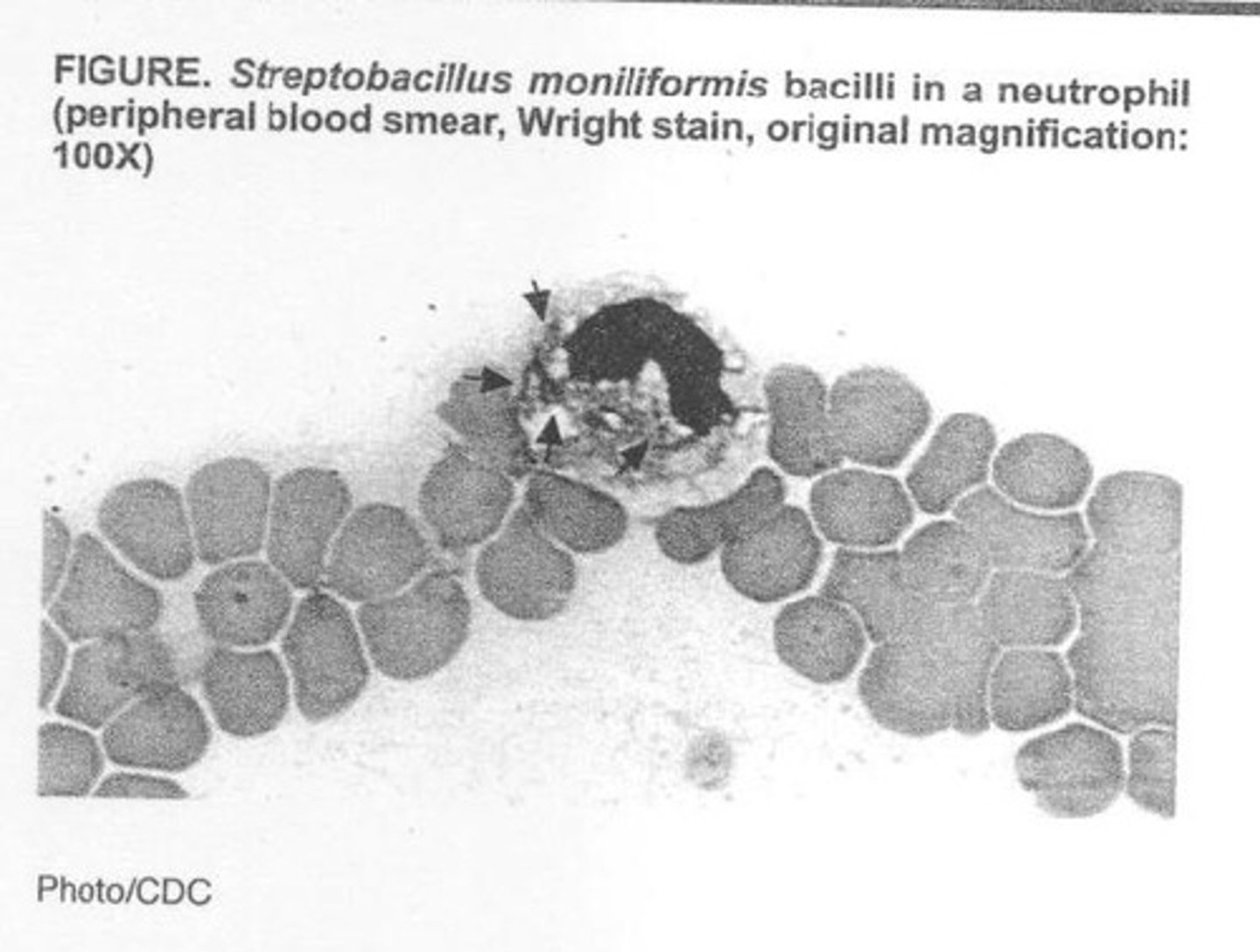
What are the general features of Streptobacillus moniliformis?
Long, thin, pleomorphic, gram-negative, aligns in chains, and forms 'L' forms.
How is Streptobacillus transmitted to humans?
Through a bite from a rat or ingestion of contaminated milk.
What is the incubation period for Streptobacillus moniliformis?
Less than 10 days.
What are common symptoms of zoonotic diseases caused by Streptobacillus?
Abrupt onset, ulceration at the bite site, lymphadenopathy, high fever, headache, rash, and recurrent fevers.
What is the treatment for infections caused by Streptobacillus?
Penicillin.
What is the typical laboratory diagnosis method for Spirillum?
Darkfield microscopy of blood or infected tissue.
What is the habitat of Streptobacillus?
Oropharynx of rats and other rodents.
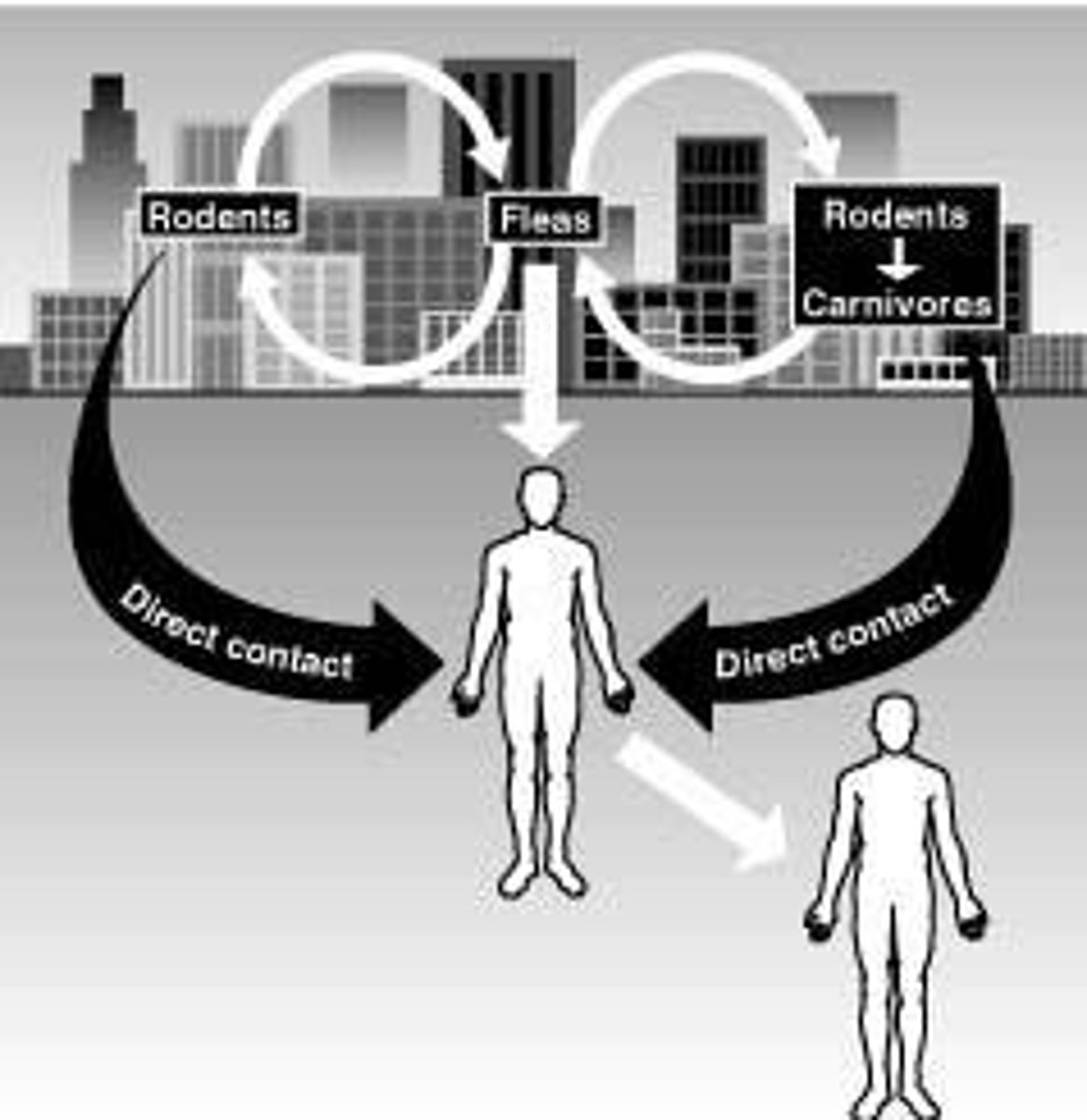
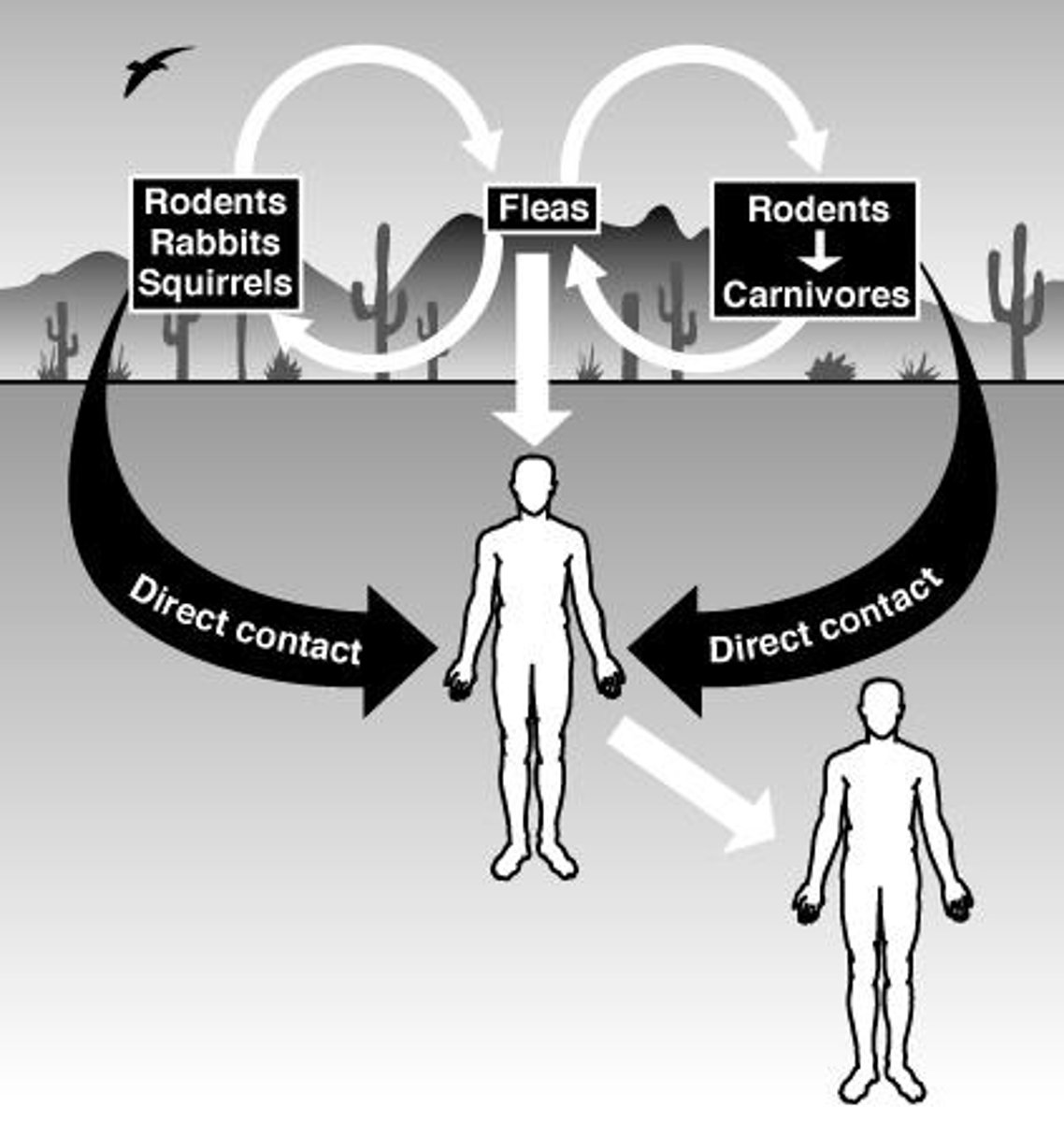
What are the two cycles of plague transmission?
Urban cycle and sylvatic cycle.
What is the primary cause of massive epidemics of plague?
Infected fleas biting domestic rats.
What are the symptoms of bubonic plague?
Buboes, bacteremia, and seeding of the lungs, liver, and spleen.
What is the laboratory diagnosis for Yersinia pestis?
Direct microscopic examination of aspirates from buboes or sputum, showing gram-negative bacilli.
What type of colonies does Yersinia pestis grow on sheep's blood agar?
Nonhemolytic, smooth, and slightly opaque colonies.
What is the incubation period for Spirillum?
Greater than 10 days.
What are the common sources of infection for zoonotic diseases?
Direct contact with infected animals, bites, or ingestion of contaminated food or water.
What are the risk factors associated with zoonotic infections?
Occupational exposure, contact with animals, and environmental factors.
Antibiotics
Substances that inhibit the growth of or destroy microorganisms.
Resistance
The ability of microorganisms to withstand the effects of antibiotics.
Mechanisms
Processes by which antibiotics exert their effects or by which resistance is developed.
Marcia A.
Author or contributor related to the topic of antibiotics and resistance.
Antimicrobial chemotherapy
The use of chemicals to inhibit or kill microorganisms in or on the host
Selective toxicity
The agent used must inhibit or kill the microorganism without harming the host
Antimicrobial chemotherapeutic chemicals
Chemicals synthesized in the lab which can be used therapeutically on microorganisms
Bacteriostatic
Inhibit bacterial growth
Bacteriocidal
Kill the targeted organisms
Broad spectrum
Effective against BOTH gram positive and gram negative bacteria
Narrow spectrum
Effective against one type of organism
Opportunistic infections
Destroy normal flora
Yeast infections
Infections caused by yeast
Ulcerative colitis
A chronic inflammatory bowel disease
Drug toxicity
Adverse effects caused by drugs
Allergic reactions
Immune responses to substances that are usually harmless
Select for resistant microorganisms
Encouragement of the growth of microorganisms that are resistant to treatment
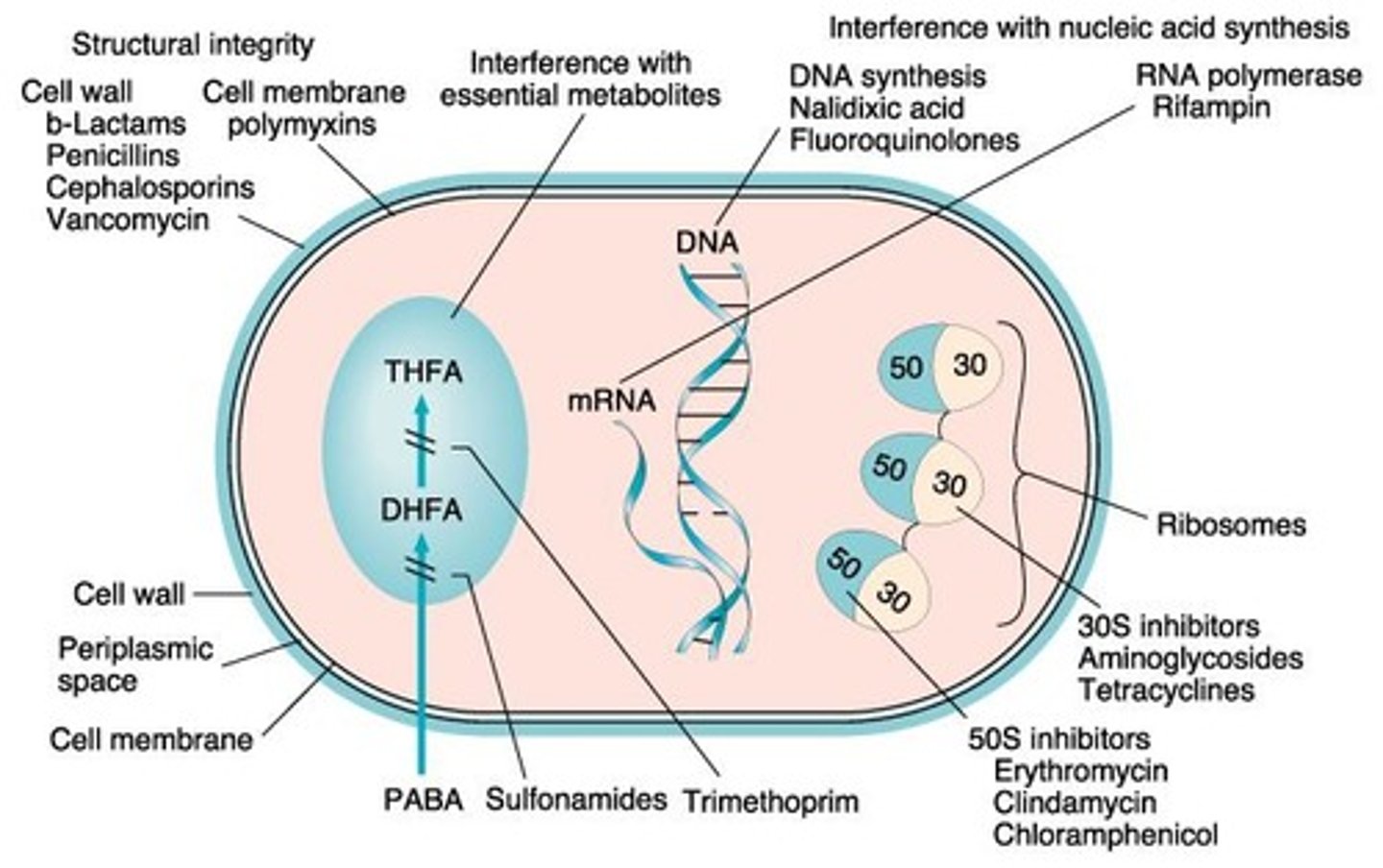
Cell wall biosynthesis
The process of forming the cell wall of bacteria
Folate synthesis
The process of producing folate, essential for DNA biosynthesis
DNA replication
The process of copying DNA
RNA transcription
The process of synthesizing RNA from a DNA template
mRNA translation
The process of translating mRNA into a protein
Beta-lactam antibiotics
Bind to penicillin binding proteins (PBP) on cell wall and interrupt cell wall synthesis
Natural Penicillin
Penicillin G, effective against gram-positive bacteria
Semi-synthetic penicillins
Methicillin, nafcillin, which are not inactivated by penicillinase
Beta lactamase inhibitors
Clavulanic acid and sulbactam, used to protect penicillins from degradation
Cephalosporins
Antibiotics that are resistant to penicillinase and have a broad spectrum
Carbapenems
Imipenem, which resist beta-lactamase activity and inhibit peptidoglycan synthesis
Glycopeptides
Do not bind to penicillin binding proteins but bind to cell wall precursors to prevent cell wall development
Inhibition of Folate Synthesis
Involves drugs like sulfamethoxazole and trimethoprim that are broad spectrum when combined.
tuberculosis infections
Infections caused by the bacterium Mycobacterium tuberculosis.
mRNA translation interference
The disruption of the process by which messenger RNA is translated into proteins.
Inhibit protein synthesis
Prevent the formation of proteins necessary for bacterial growth and function.
Agents that block translation
Substances that interfere with the process of translating mRNA into proteins.
30s subunit
A component of the bacterial ribosome that can be targeted by antibiotics to inhibit translation.
50s subunit
Another component of the bacterial ribosome involved in peptide bond formation and tRNA release.
Aminoglycosides
A class of antibiotics that bind irreversibly to the 30s subunit, causing misreading of mRNA.