Chapter 16: Practical Techniques + Synthetic Routes 🗺️
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
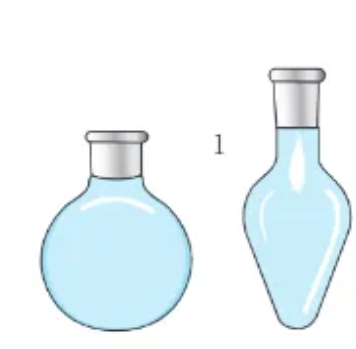
what is this?
round-bottom / pear-shaped flask
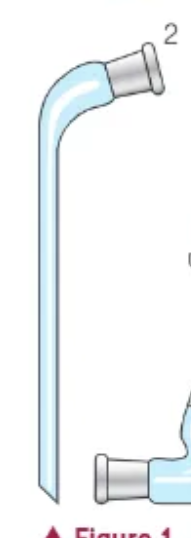
what is this?
receiver
what is this?
screw-tap adaptor
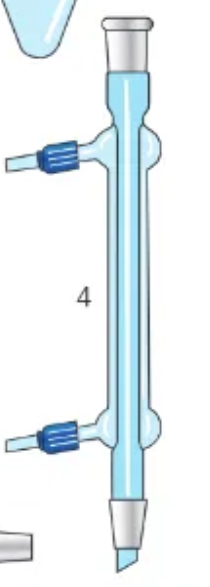
what is this?
condenser
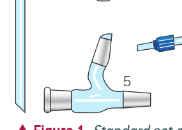
what is this?
still hero
in what temperature range can a water bath be used?
below 100oC
Before fitting a condenser, what needs to be added to the round-bottomed/pear-shaped flask?
anti-bumping granules → ensures that its contents will boil smoothly
→ prevent large bubbles forming
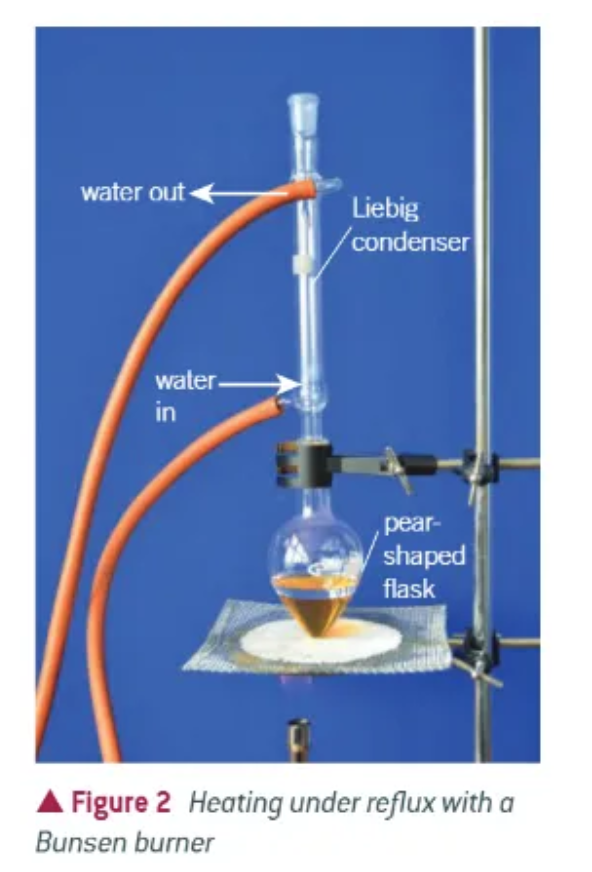
what does heating under reflux look like?
what should not be put in the top of a condenser?
a stopper → otherwise, it would be a closed system + the pressure would build up (the condenser would explode)
water enters condenser at the … → why?
bottom of the condenser → to ensure the outer jacket is full
what does heating at reflux ensure?
a liquid to be continually boiled whilst the reaction takes place
prevents volatile components from escaping (e.g. like a pot on a saucepan, preventing the steam from escaping)
prevents the flask from boiling dry
what is distillation used for?
separating pure liquid from its impurities
what does the setup for distillation look like?

in distillation, which liquid boils first?
the liquid with the lowest boiling point → it is the most volatile + will boil first
when organic liquids are produced, what may be produced too?
what does this look like?
water
2 liquid layers in your collection flask (1 organic, one aqueous/water)
how are 2 layers (one organic, one water) separated?
use a separating funnel
ensure tap of separating funnel is closed
pour mixture in → bung it → invert to mix
allow layers to settle
add some water (to see which layer is the water/aqueous layer)
place conical flask under separating layer → remove stopper → open tap
place second conical flask under separating funnel to collect other layer
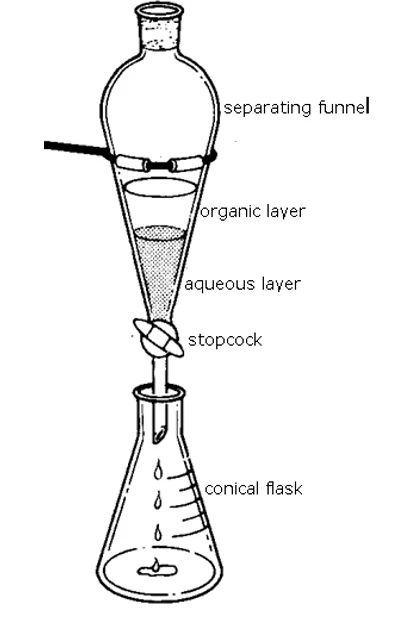
how do you know which layer is organic + which is water?
add water → the layer that gets bigger is water
how to remove acid impurities:
add aqueous sodium carbonate + shake mixture in separating funnel
acid present will react with the sodium carbonate + release CO2
hold the stoppered separating funnel upside down + open tap slowly to release the gas pressure
remove aqueous sodium carbonate layer
wash organic layer with water
run both layers into 2 separate flasks
which drying agent works for drying hydrocarbons?
anhydrous calcium sulphate
CaCl2
which 2 drying agents can be used for general drying of organic products?
Anhydrous calcium sulphate → CaSO4
Anhydrous magnesium sulphate → MgSO4
procedure for drying an organic liquid (with a drying agent)
add organic liquid to a conical flask
spatula some drying agent in → swirl
place stopper on flask → leave for about 10 minutes
add drying agent until solid is dispersed in solution as fine powder
decant the liquid into another flask
what is redistillation for?
when the first distillation doesn’t work entirely (because organic liquids have boiling points that are relatively close together)
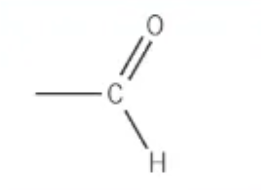
which functional group is this?
aldehyde
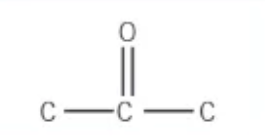
which functional group is this?
ketone
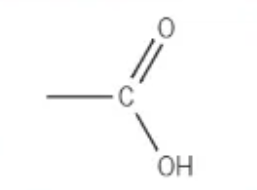
which functional group is this?
carboxylic acid
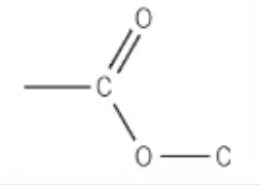
which functional group is this?
ester
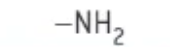
which functional group is this?
amine
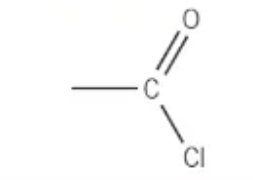
which functional group is this?
acyl chloride
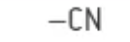
which functional group is this?
nitrile
what is needed for this reaction: alcohol → haloalkane
sodium halide + H2SO4
what is needed for this reaction: alkane → haloalkane
halogen + UV light
what is needed for this reaction: alkene → haloalkane
hydrogen halide
what is needed for this reaction: haloalkane → alcohol
NaOH (aq) under reflux
what is needed for this reaction: alkene → alkane
H2 and Nickel catalyst
what is needed for this reaction: alcohol → alkene
concentrated H2SO4
what is needed for this reaction: alkene → alcohol
H2O (g) / H3PO4 catalyst
what is needed for this reaction: 1y alcohol → carboxylic acid
K2Cr2O7 / H2SO4 under reflux
what is needed for this reaction: 1y alcohol → aldehyde
K2Cr2O7 / H2SO4 under distillation
what is needed for this reaction: 2y alcohol → ketone
K2Cr2O7 / H2SO4 under reflux
what is a target molecule?
the compound that a chemist is trying to prepare by organic synthesis