NUCLEAR CHEMISTRY UNIT TEST STUDY GUIDE - CHEMISTRY CORNER
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
88 Terms
What Is the difference between a chemical reaction and a nuclear reaction?,
Chemical reactions involve the electrons of an atom - gaining, losing, or sharing electrons. Nuclear reactions involve the nucleus of the atom - changes in the numbers of neutrons and/or protons, and transmutations,
Explain why all elements above element #83 are radioactive (unstable).
Elements #84 and up have an unstable number of neutrons. The ratio of neutrons to protons is too great. Also, with more and more protons, the electrostatic force that repels protons from other protons increases and begins to be stronger than the strong nuclear force.
Using the Band of Stability graph, what is the ratio of neutrons to protons that is most stable for elements 1-20?
1:1
Using the Band of Stability graph, what is the ratio of neutrons to protons that is most stable for elements above element H20? What is the neutron-proton ratio for nitrogen-14?
1.5:1 Nitrogen: 1:1
What two opposing forces are working in the nucleus of an atom. Name them and briefly describe the force?
The Strong Nuclear Force and the electrostatic force. The strong nuclear force is the force of the neutrons to protons, It holds the nucleus together, and is much stronger than the electrostatic force. The electrostatic force present in the nucleus is the natural repulsive force between positively charged protons.
What is radioactivity, and is radioactivity a natural process or a man made process?
The process by which an unstable nucleus spontaneously emits high energy particles or rays from the nucleus in order to attain a more stable nuclear state, Radioactivity is a natural process.
List the three most common types of radioactive decay in order of heaviest mass to lightest mass.
decay in order of heaviest mass to lightest mass.
Alpha decay is represented by the symbol 4-2 He
Remember this
Complete the following statement. Some (No, All, or Some) naturally occurring isotopes are radioactive.
Why do very large nuclei tend to be unstable?
As the number of protons increase, the electrostatic repusion strong nuclear force.
between the positively charged
Of the three most common types of radiation, alpha, beta, and gamma, which is the most harmful to humans?
gamma rays
An electron emitted from the nucleus during radioactive decay is called a…
beta particle.
Give the charge and mass number for each of the following:
alpha particle:
beta particle:
gamma ray:
neutron:
positron:
charge +2 mass 4
charge -1 mass 0
charge 0 mass 0
charge 0 mass 1
positron +1 mass number 0
match the common types of radiation to their attraction
negatively charged plate
positively charged plate
attracted to neither
alpha
beta
gamma
Which type, or types, of radiation can wood protect you from?
alpha & beta
beta decay of iodine-131
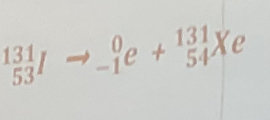
the radioactive decay of radon-212 decays into polonium-208 and what other particle?
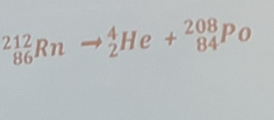
Alpha decay that results in hassium-267
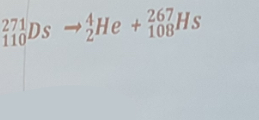
The electron capture of argon-37

The decay of potassim-38 by positron emission
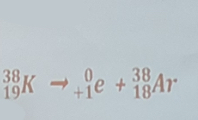
The neutron bombardment of plutonium-239 yields americium-240 and another particle.

The half-life of a radioisotope is the time required
for ½ of the atoms in a radioactive isotope to decay.
What will be the mass of a 35.0 g sample of curium-238 (half-life = 2.4 hr) after 9.6 hours?

Phosphorus-32 has a half-life of 14.3 days. If an initial sample has a mass of 4.00 mg, how many milligrams will remain after 71.5 days?

Radium-224 has a half-life of 3.66 days. What was the mass of the original sample of radium-224 if 0.0500 g remains after 7.32 days?
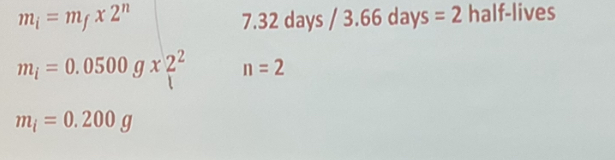
Steam is produced here fromthe hot water coming from the reactor core.
E (steam generator)
These are used to control the chain reaction produced by the fission reaction.
C (control rods)
Steam turns this which then produces electricity.
(This diagram shows two things together as one)
G (turbine generator)
The strong dome-shaped structure that houses the
reactor.
A (containment structure)
Steam is cooled here and returned to the water
phase.
H (condenser)
Critical mass:
mass required to sustain a chain reaction
chain reaction:
the material that starts the reaction is also one of the products and can start another reaction.
Nuclear reactors and the atomic bomb are both fission reactions. How are they different?
The fission reaction that takes place in nuclear reactors is a controlled chain reaction. The reaction in the atomic bomb is an uncontrolled reaction.
Nuclear Fission:
splitting of the nuclear. Heavy nuclei are split into lighter weight nuclei and other particles
Nuclear Fusion:
combining lighter nuclel into a larger, more stable nuclei
Which of the above is the process fuels the energy of the sun?
nuclear fusion
What particle begins the fission reaction?
neutron
How does the fission reaction of uranium-235 produce a chain reaction?
neutrons begin the reaction, and are also one of the products of the reaction. So, neutrons can go on to begin more reactions
Describe some of the problems that prevent fusion from being used to produce energy. If we could produce fusion as an energy source, what would some of the benefits be in using fusion.
Due to the incredible amount of temperature and pressure necessary, the reaction is not yet sustainable. If fusion could be used in the future as an energy source, there would be no danger of a meltdown, no toxic waste, the fuel for the reaction is abundant, and it produces thousands times more energy than fission, and millions times more energy than coal.