Physio 2: Fluids and Electrolytes
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Where is water contained within the human body?
Mostly in the cells, eyes are the organ with the highest water content

What body systems are involved in maintaining fluid balance?
Lungs, GI, Kidneys, skin, and cardiovascular
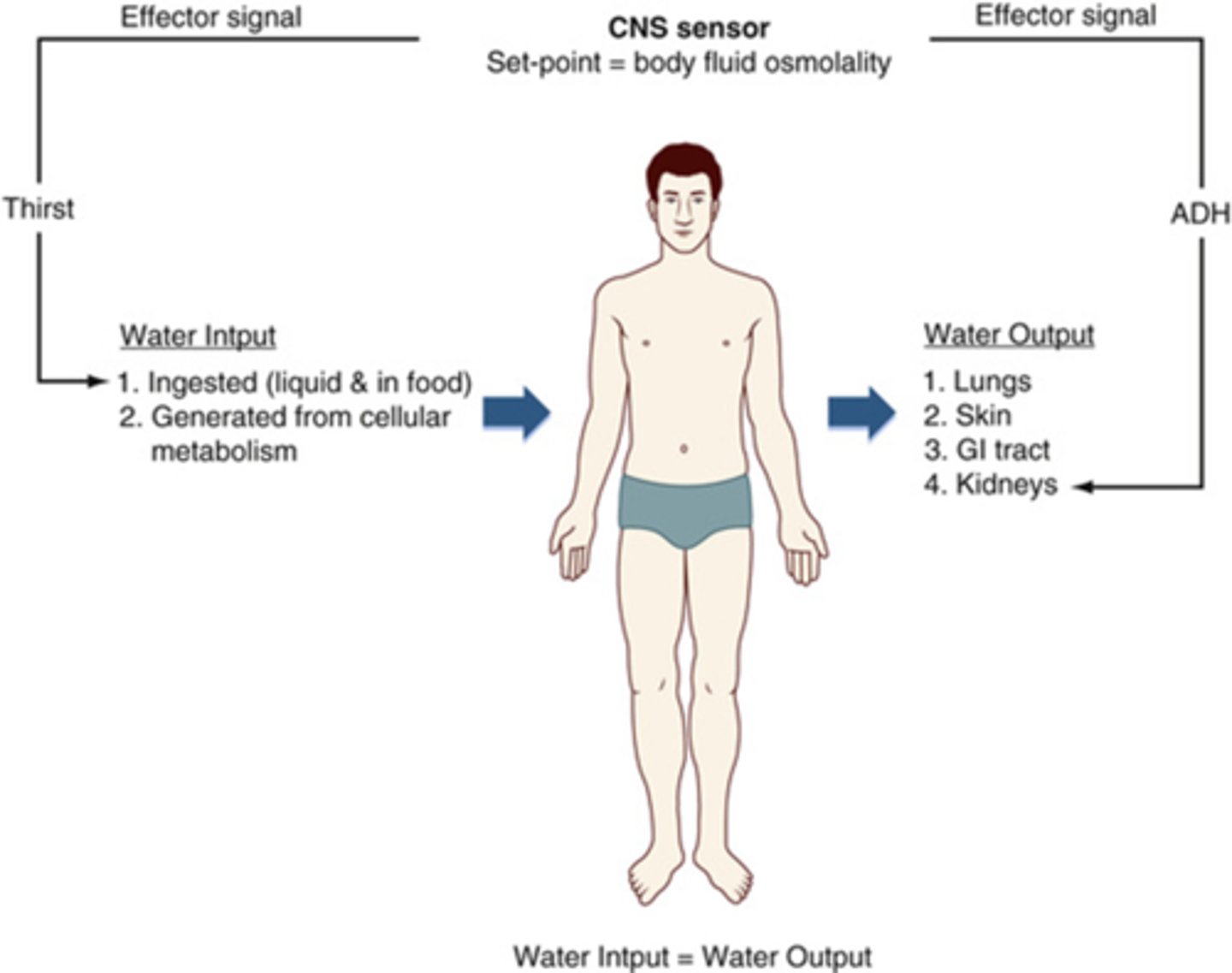
How is homeostasis of water maintained?
output=input
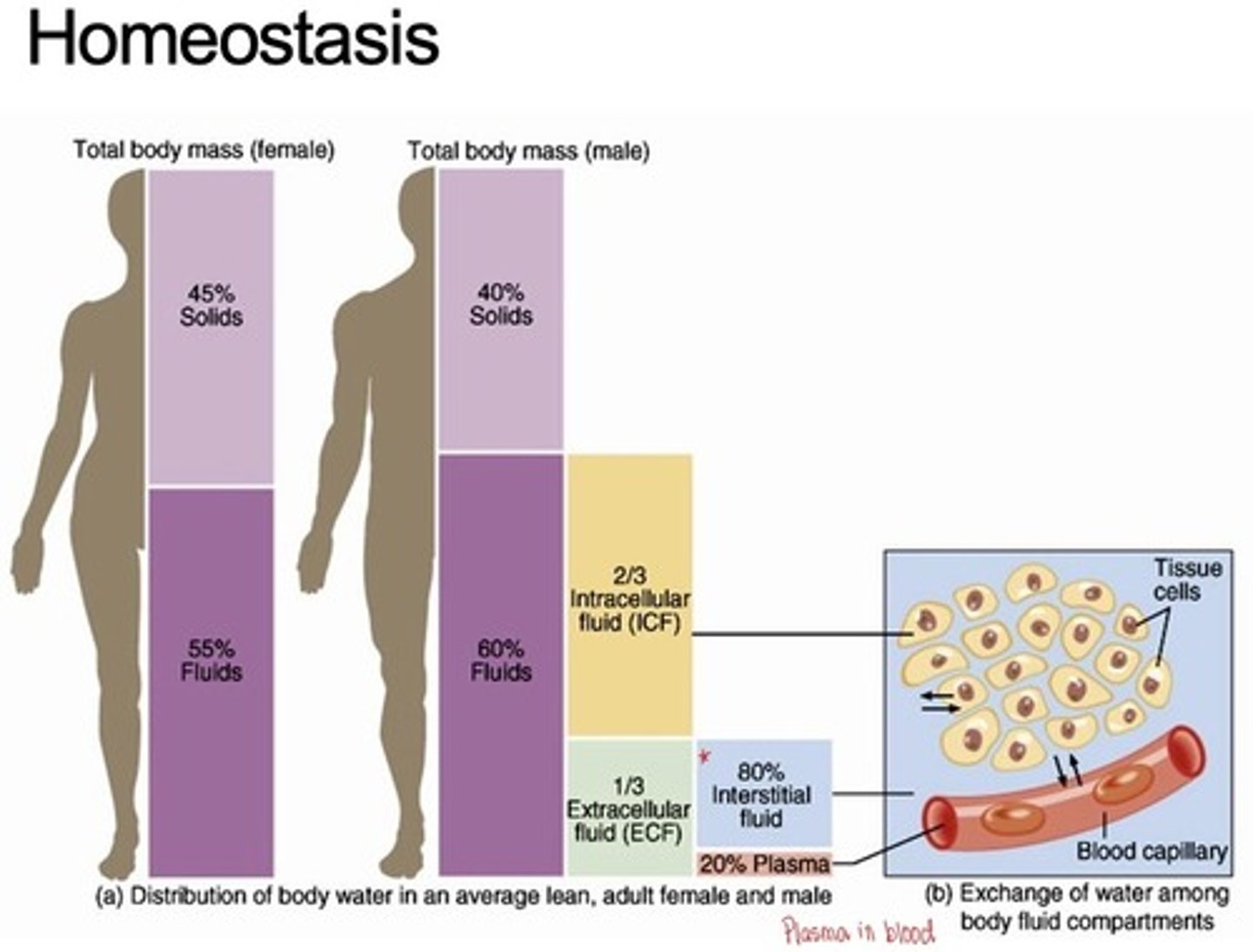
Total body mass female vs male in homeostasis
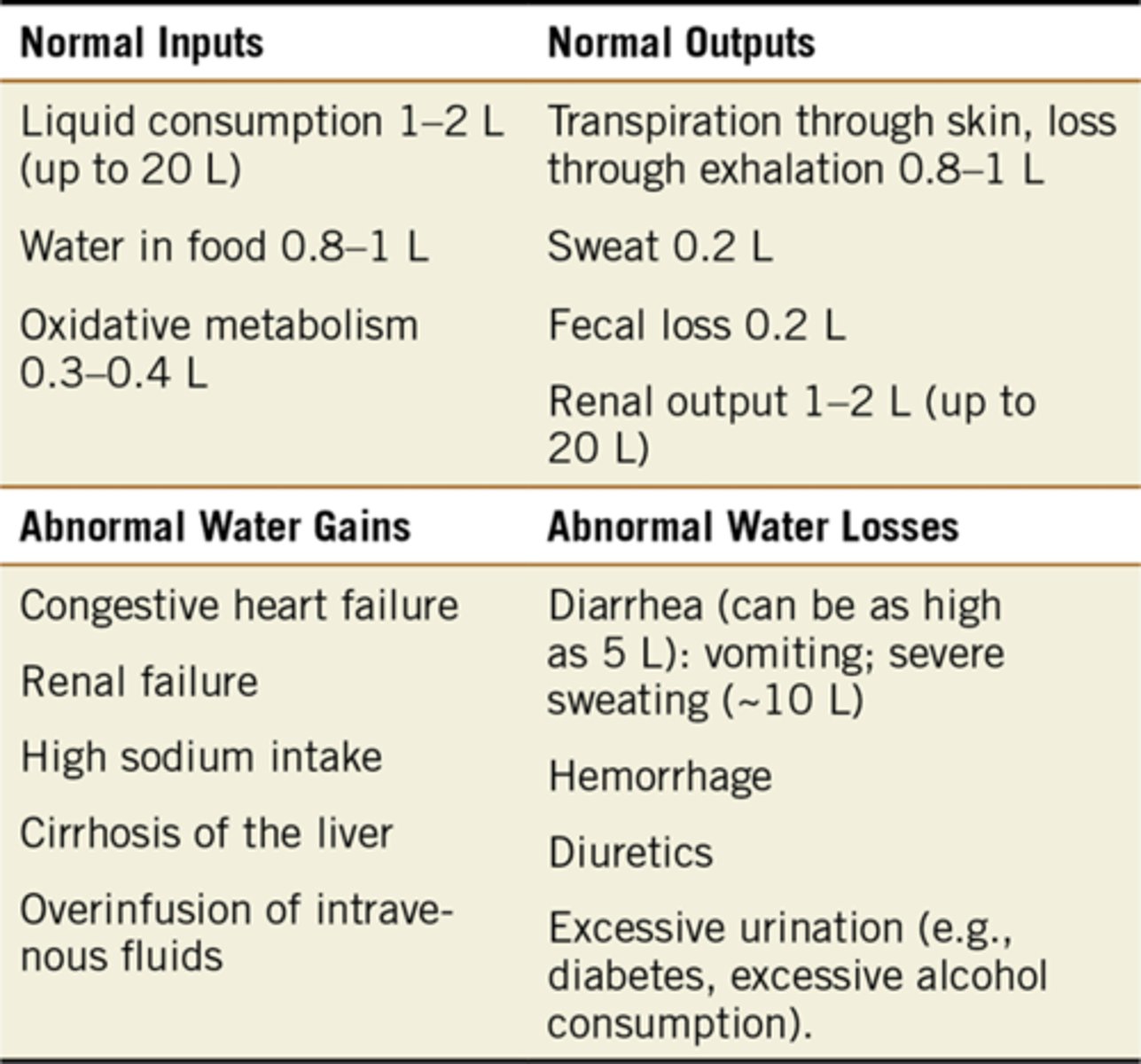
What are the normal and abnormal inputs and outputs of water that contribute to homeostasis?
Normal Input: 2.1-3.4L per day
Normal output: 2.2-3.4L per day
What is transpiration?
loss of water as the water moves between cells
What hormones are made by the body to alter water levels?
Antidiuretic hormone (ADH) and Renin and and Angiotensin 2
How might water loss be altered by environmental changes?
different factors can alter water loss, thus we will modify our intake as a reflection of those changes (Know numbers)
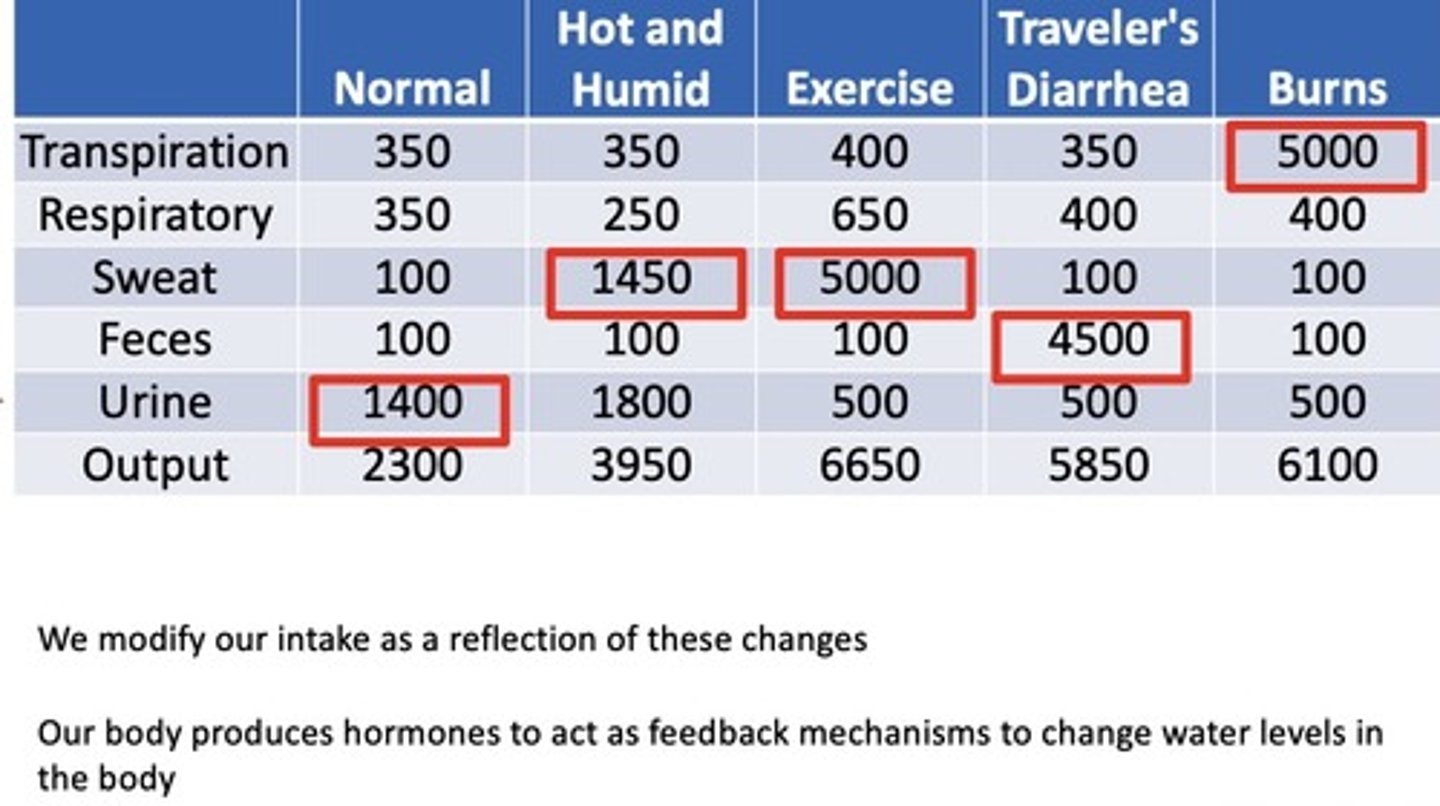
What form of fluid loss is of special significance in burn victims?
transpiration, will need extra hydration to compensate
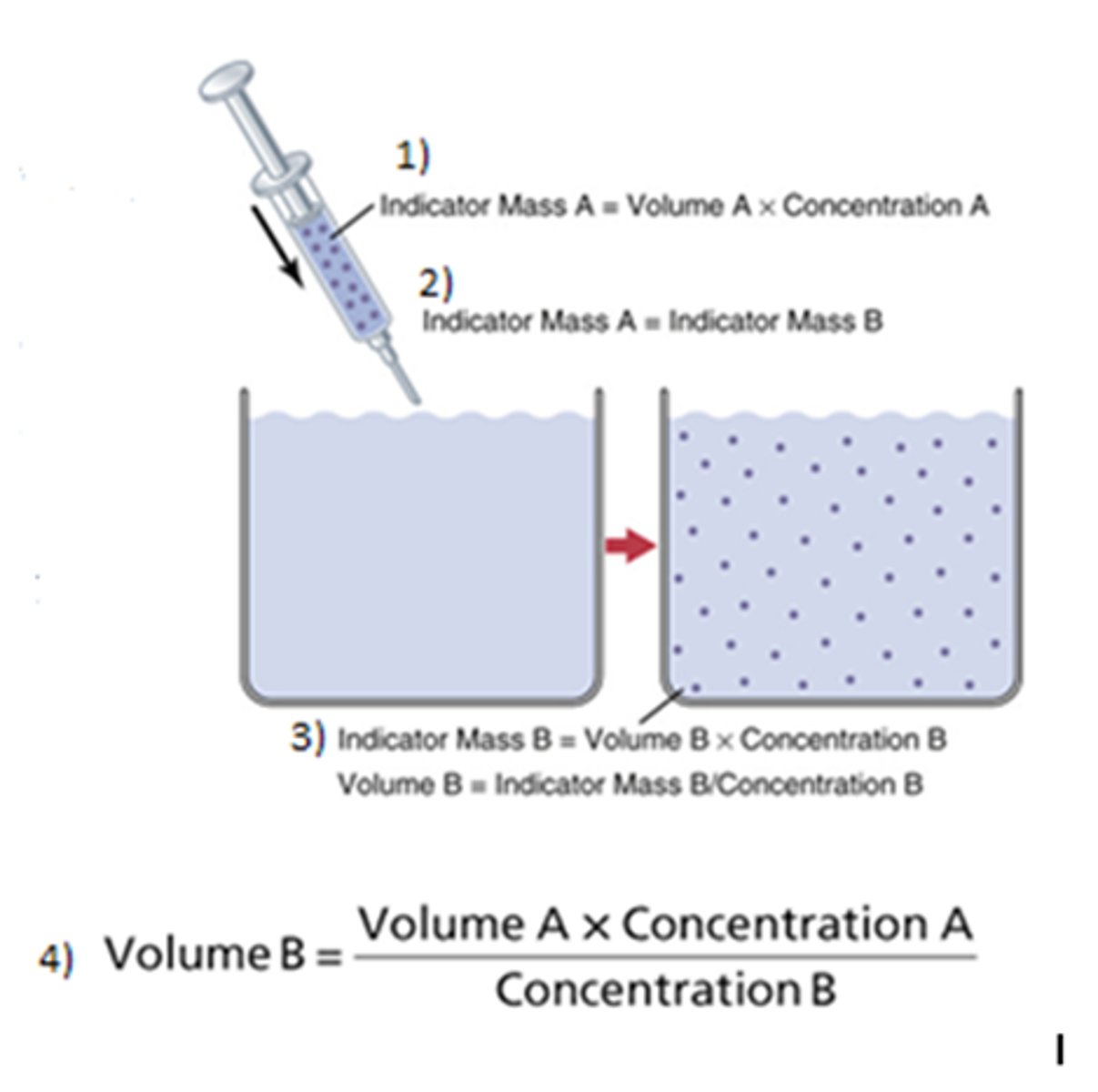
How is the indicator dilution method used?
Used to measure water within compartment of the body, indicator must be confined to that specific compartment, allow indicator to disperse then take a plasma sample (must account loss)
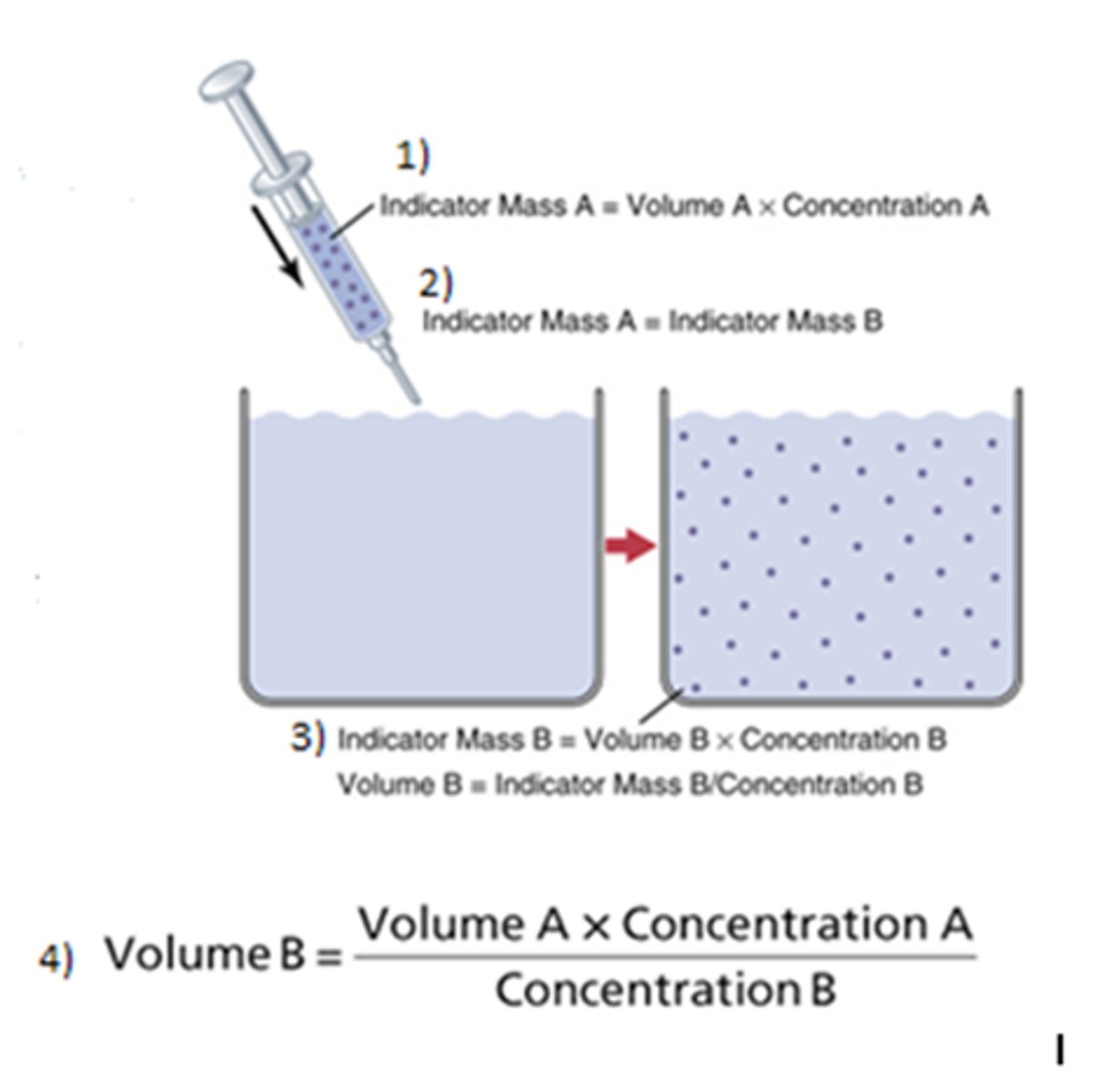
What is the equation for the indicator dilution method of measuring water?
C1V1=C2V2 (because the fluid injected can only come from external source!) Mass A = Mass B
What indicators can be used to identify the volume of the various compartments?
right indicator must be used for the right compartment*!
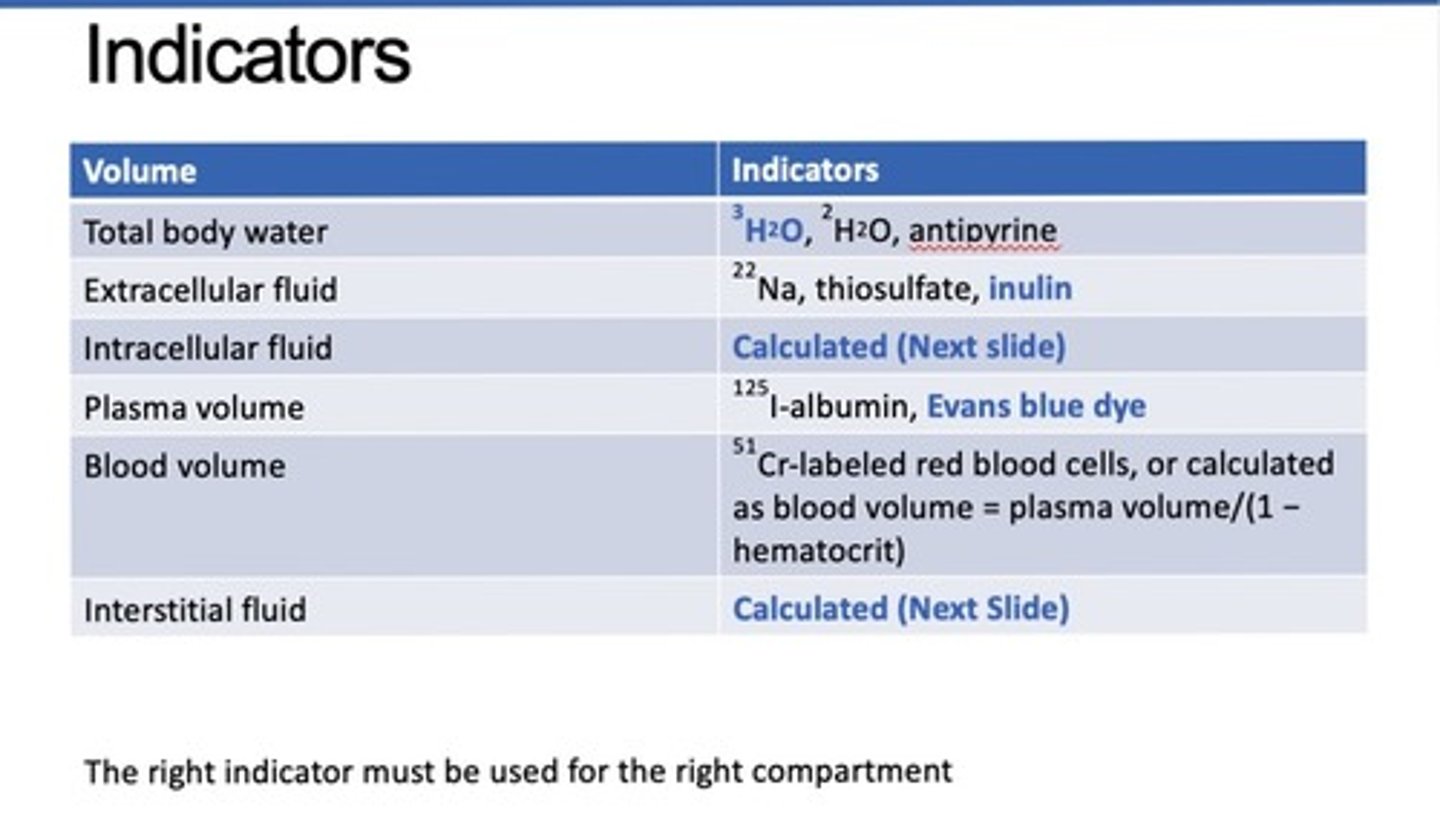
What indicators can be used for TBW?
3H2O, 2H2O, antipyrine
What indicators can be used to measure ECF?
22Na, thiosulfate, inulin
What indicators can be used to measure plasma volume?
125I-albumin, Evans blue dye
Evan always measures plasma
What indicators can be used to measure blood volume?
51Cr-labeled red blood cells, or calculated as blood volume = plasma volume/(1 − hematocrit)
Which volumes cannot be directly measured and must be calculated?
Intracellular fluid, Interstitial fluid, and Blood volume
How is intracellular fluid calculated?
ICF=TBW-ECF
How is interstitial fluid calculated?
ISF=ECF-Plasma
How is blood volume calculated?
BV=Plasma/1- Hematocrit
100 ml of antipyrine is injected at a concentration of 60 mg/l into a 60 kg woman. After a two-hour equilibration, a venous sample is obtained and analyzed for antipyrine. A concentration of 0.2 mg/L of antipyrine was measured. Determine total body water?
Weight can be used to check reasonableness.
30L/60 kg = 0.5kg/L (total fluid mass of female body)
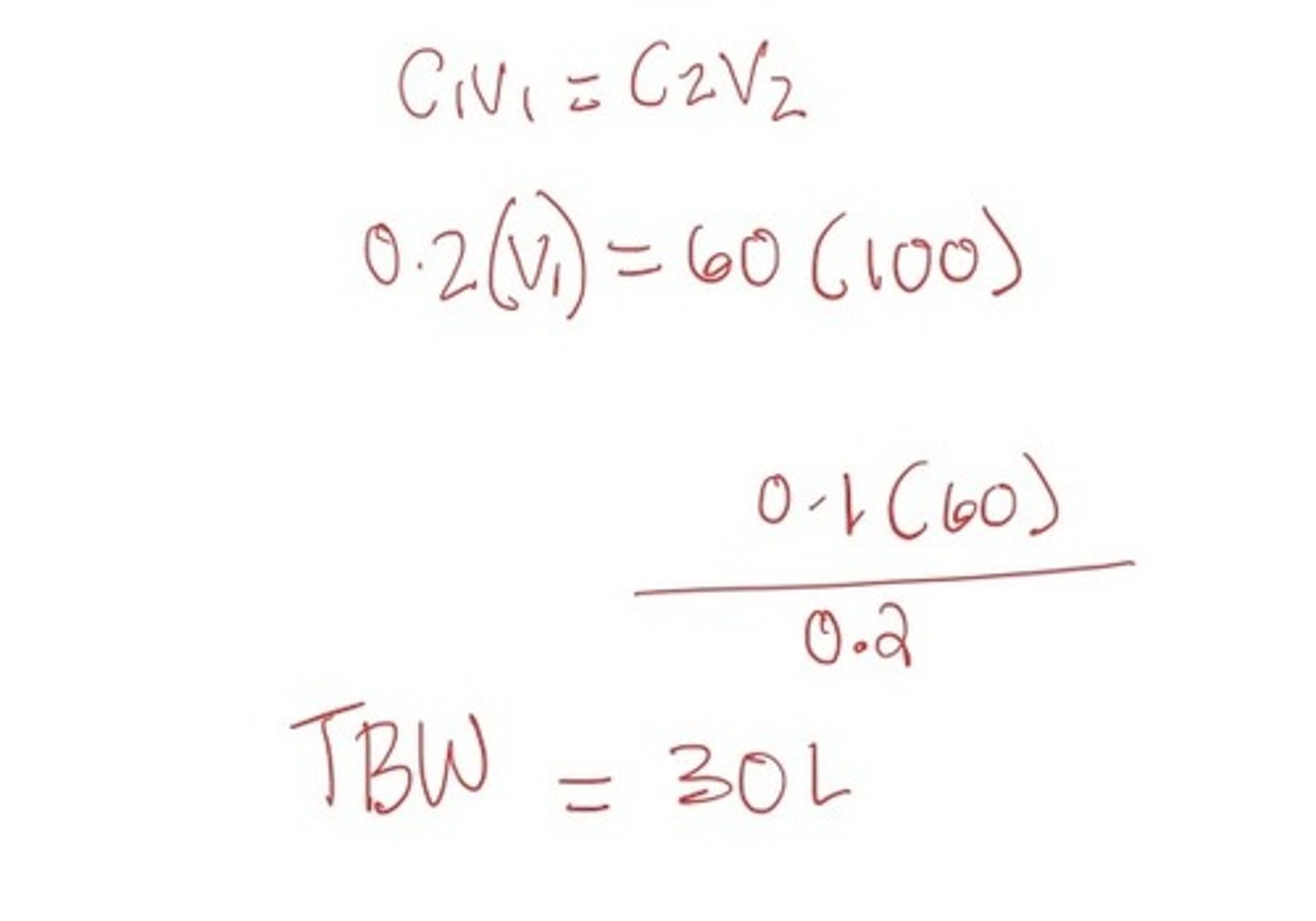
A 100 ml injection of insulin is then given at a concentration of 50 mg/L. After the same equilibrium, a venous sample shows that fluid concentration is 0.5 mg/L. What are the estimates of her ECF and ICF
ECF: 10L
ICF: TBW - ECF --> 30-10 = 20L

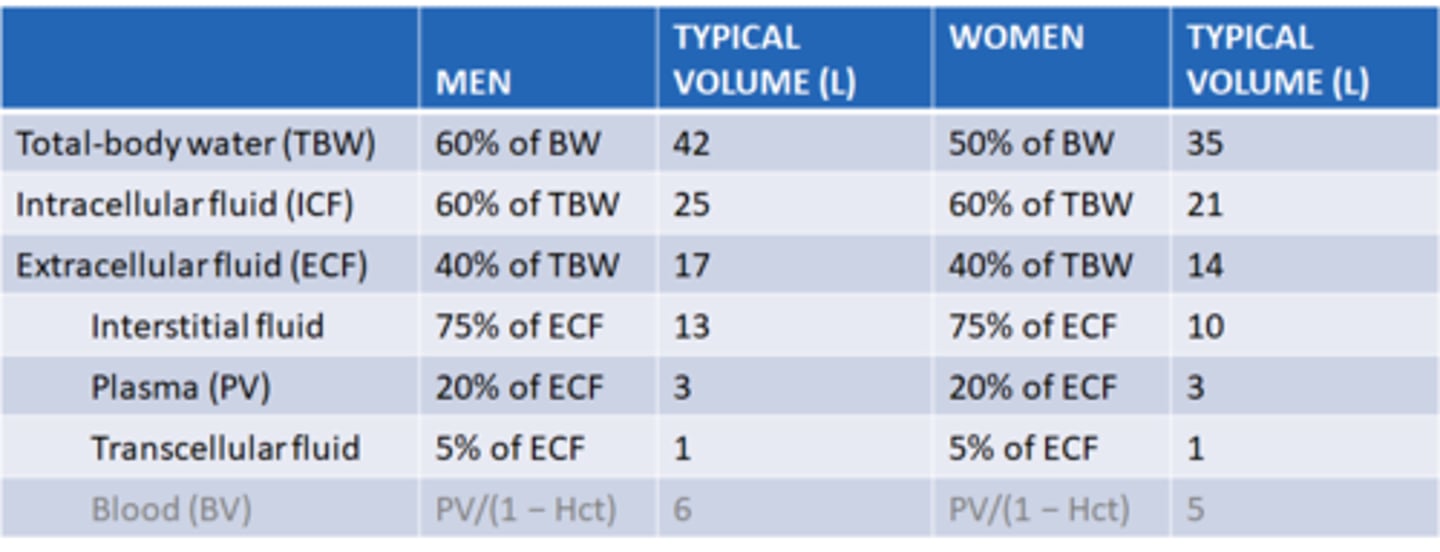
What are the typical volumes of the compartments of the body in men and women?
most notable difference between ICF and ECF
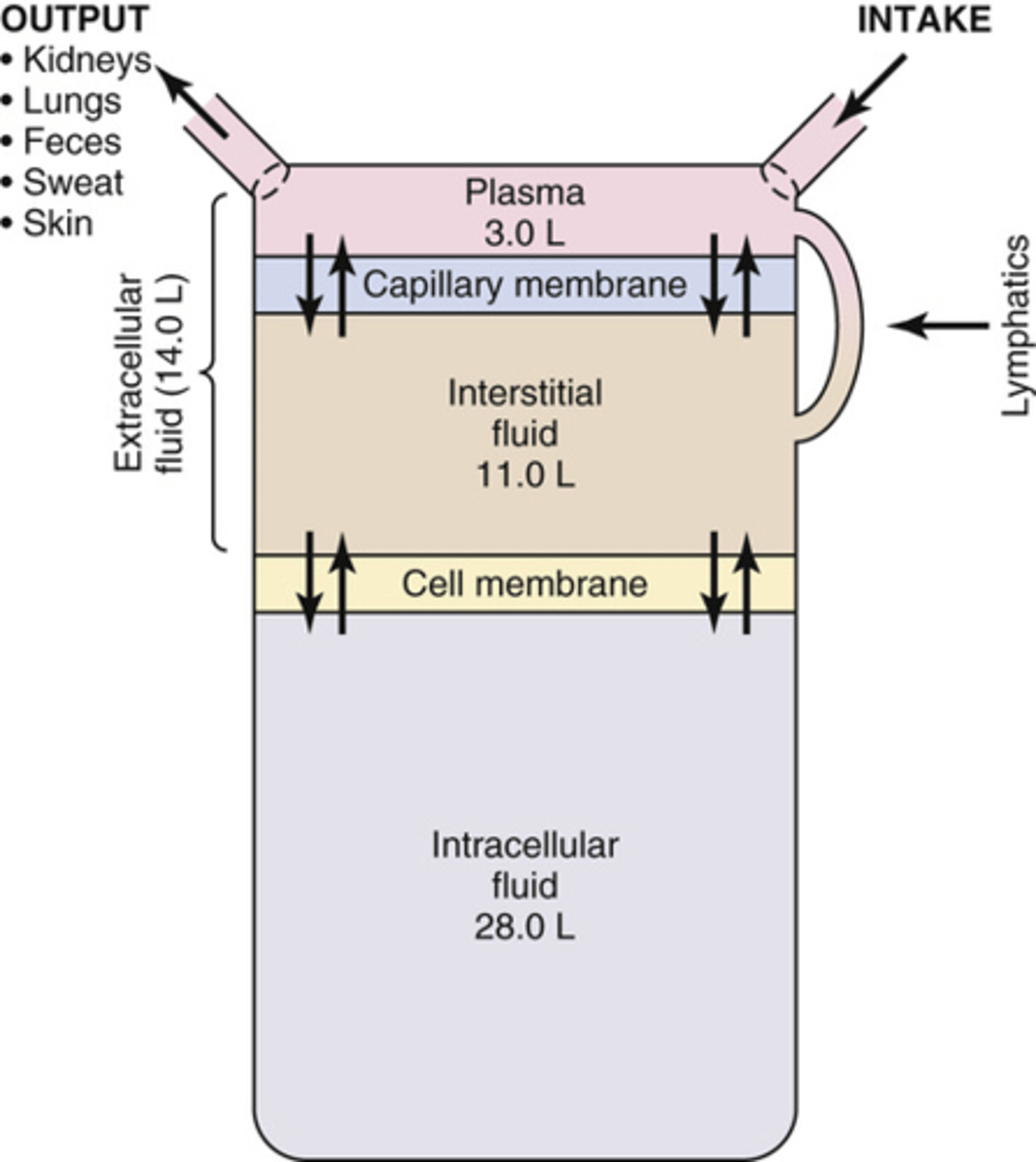
Why must fluids be moved throughout the body?
to maintain homeostasis
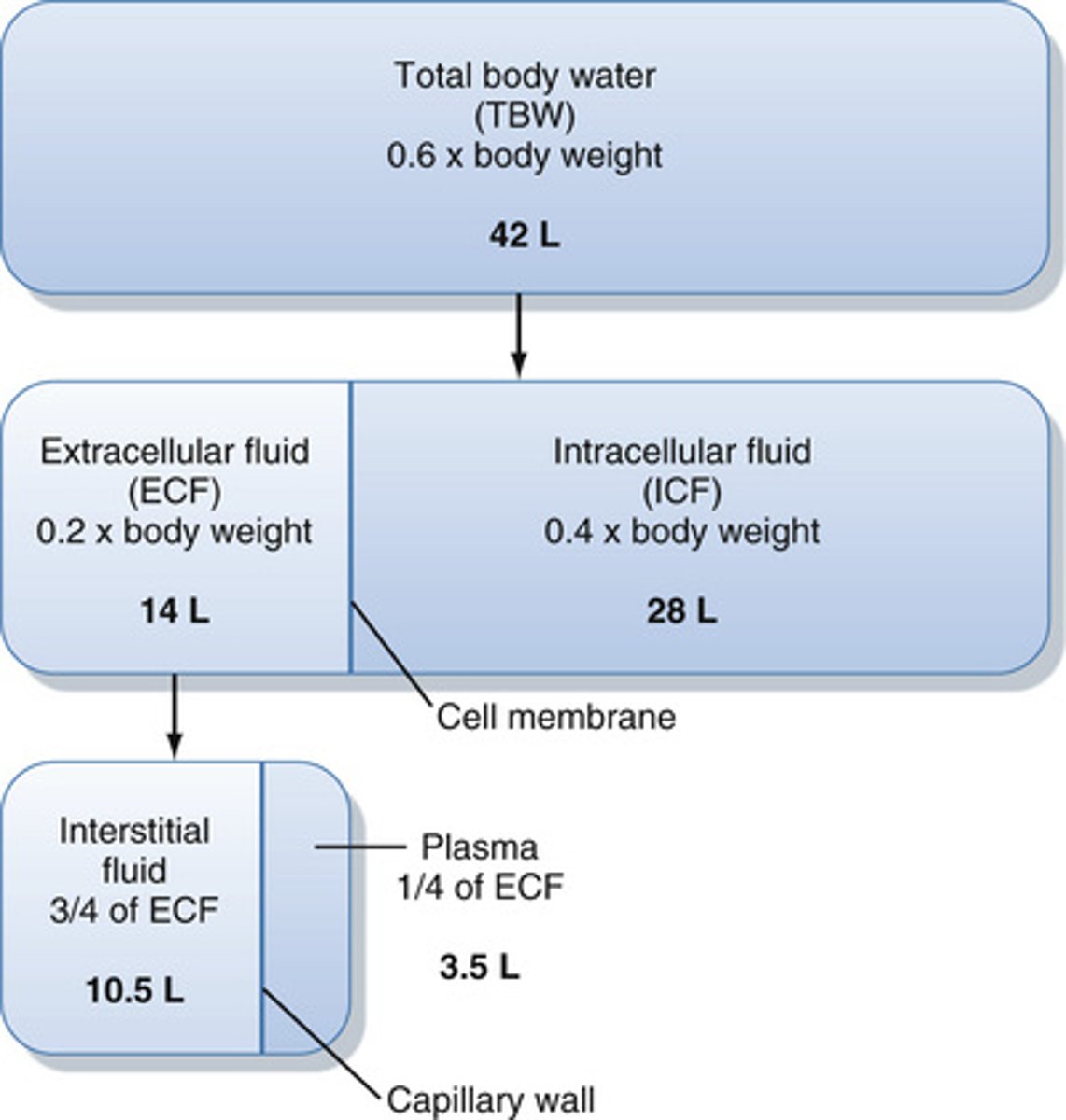
What portion of the water in the body is in the ECF and ICF?
ECF: 1/3
ICF: 2/3
What is the normal osmolarity of the ECF and ICF?
275-300 mosmol/L (he uses 290 alot)
What portion of the ECF will be in constant motion?
~1/5
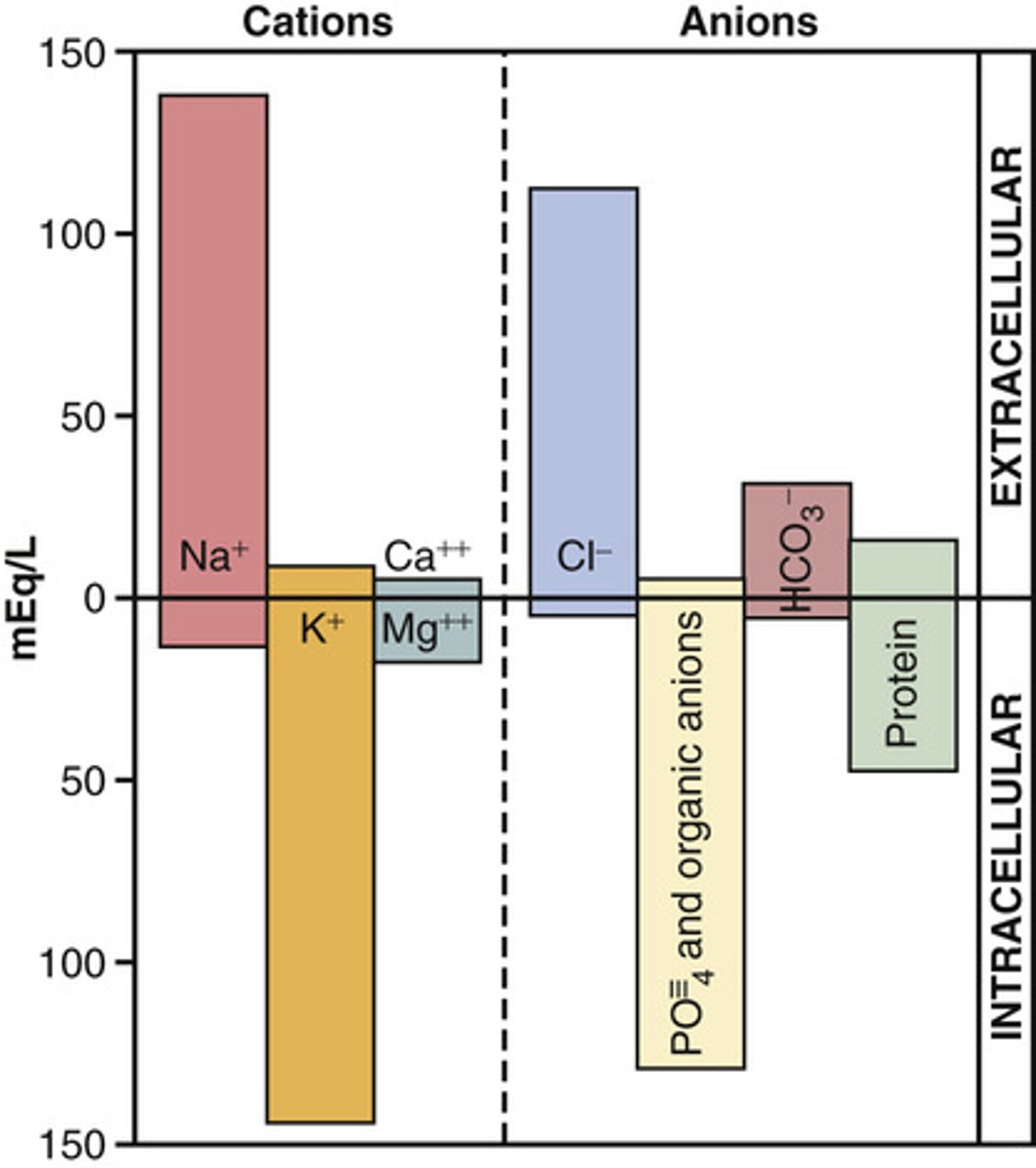
Know the level of different electrolytes in different body compartments (picture)
know this image for volumes of compartments (plasma and interstitial are about the same for testing purposes)
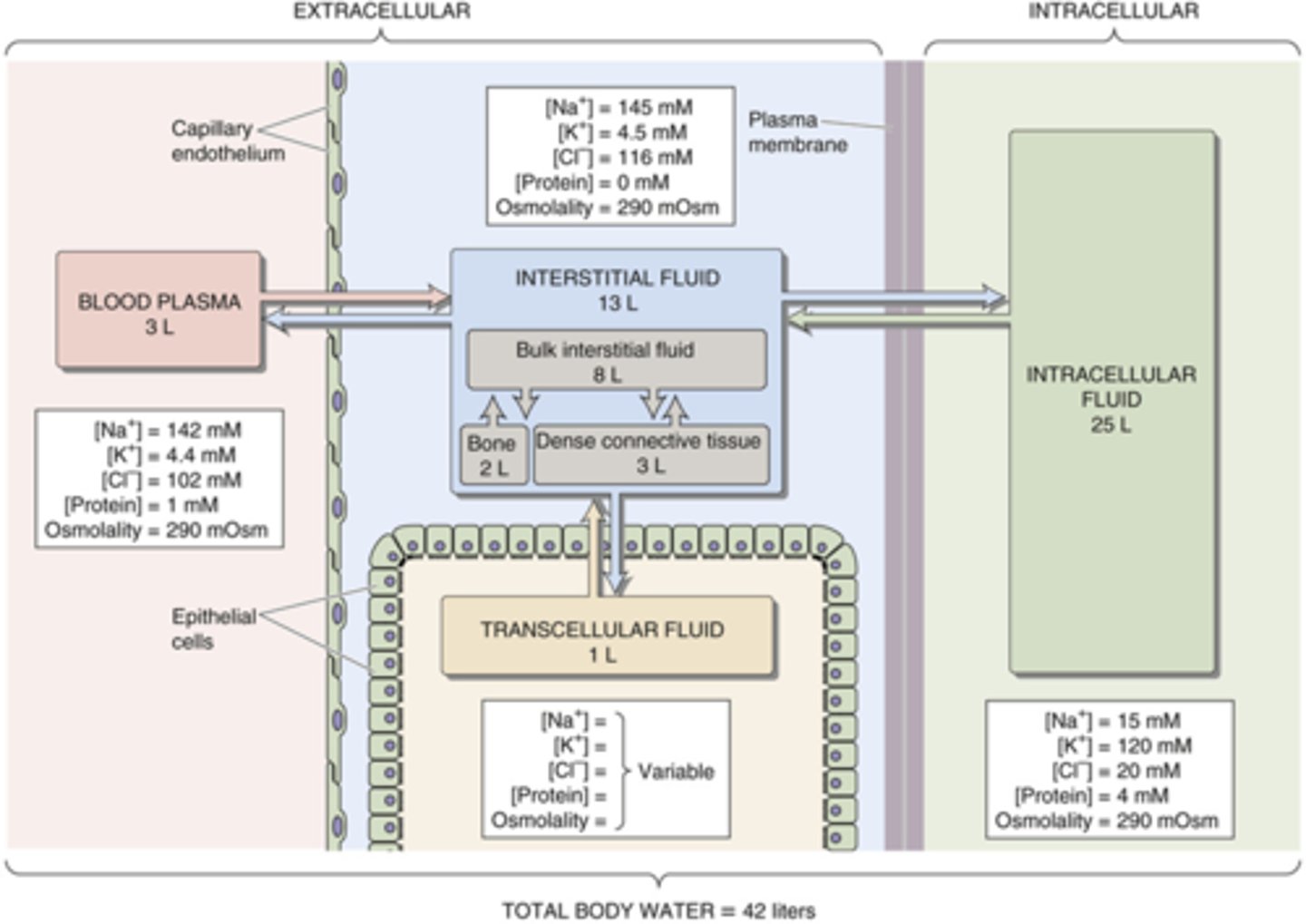
What allows a difference in concentration of ions in the ECF and ICF?
transporters that are selective for ions
Know the level of solutes in the different compartments of the body
know all

What allows for different concentrations of solutes in the ECF and ICF?
cellular transport across plasma membrane
How do sodium and potassium differ in concentration inside and outside of the cell?
K+ higher inside
Na+ higher outside
*Na+/K+ transporter on every single cell in the body
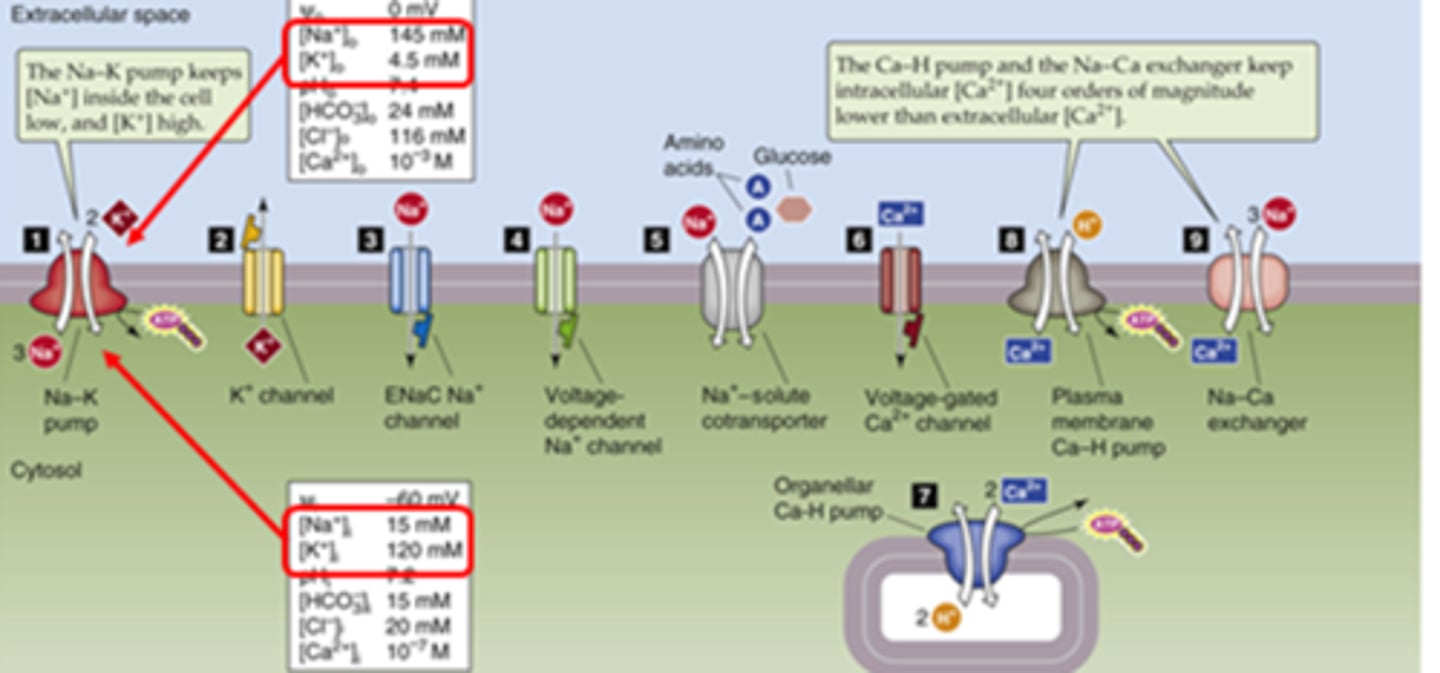
Be familiar with some of the main transporters on cells
Cl-/bicarb seen in blood cells and lungs
NKCC target of diuretics
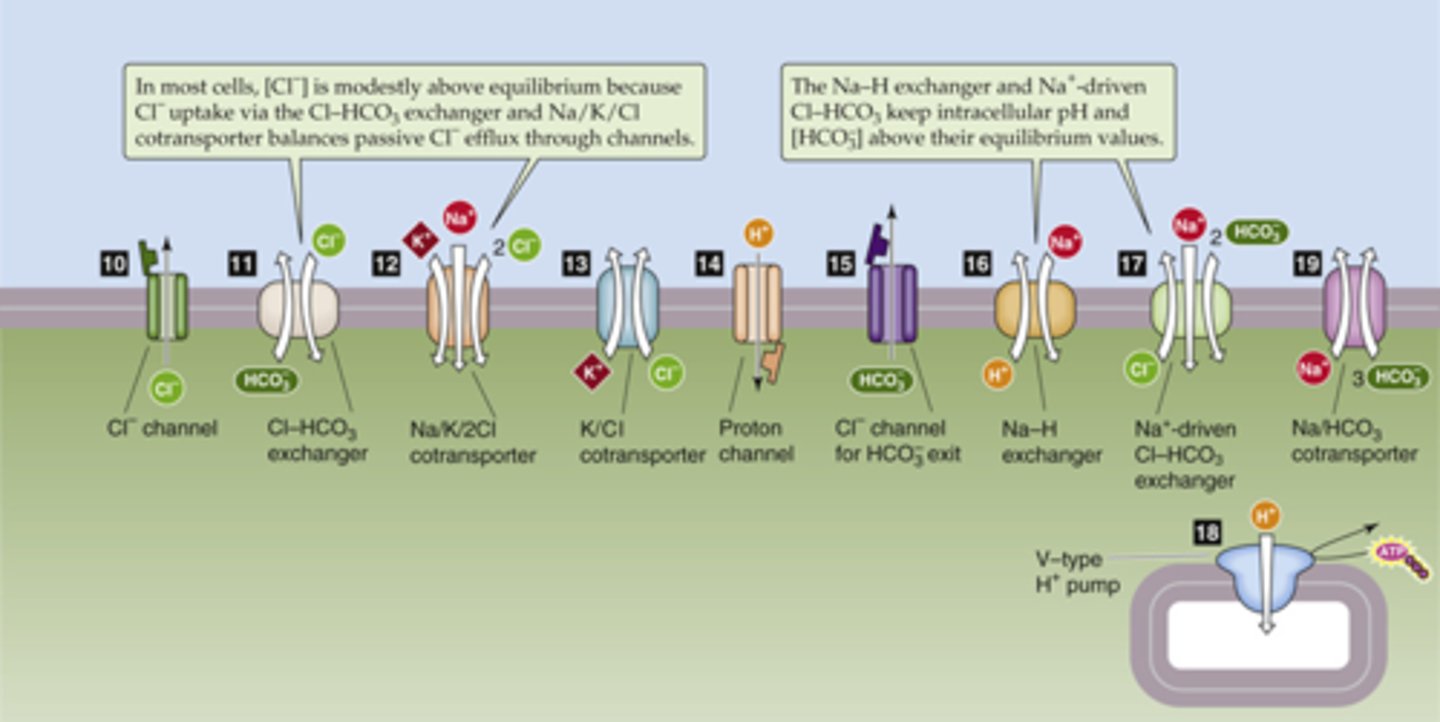
How can the steady state of a cell be altered?
deviations in homeostatic set points, environmental factors, or activation of channels and transporters
What part of the cell is especially important in steady state?
active channels and transporters (things are moving in steady state!!)
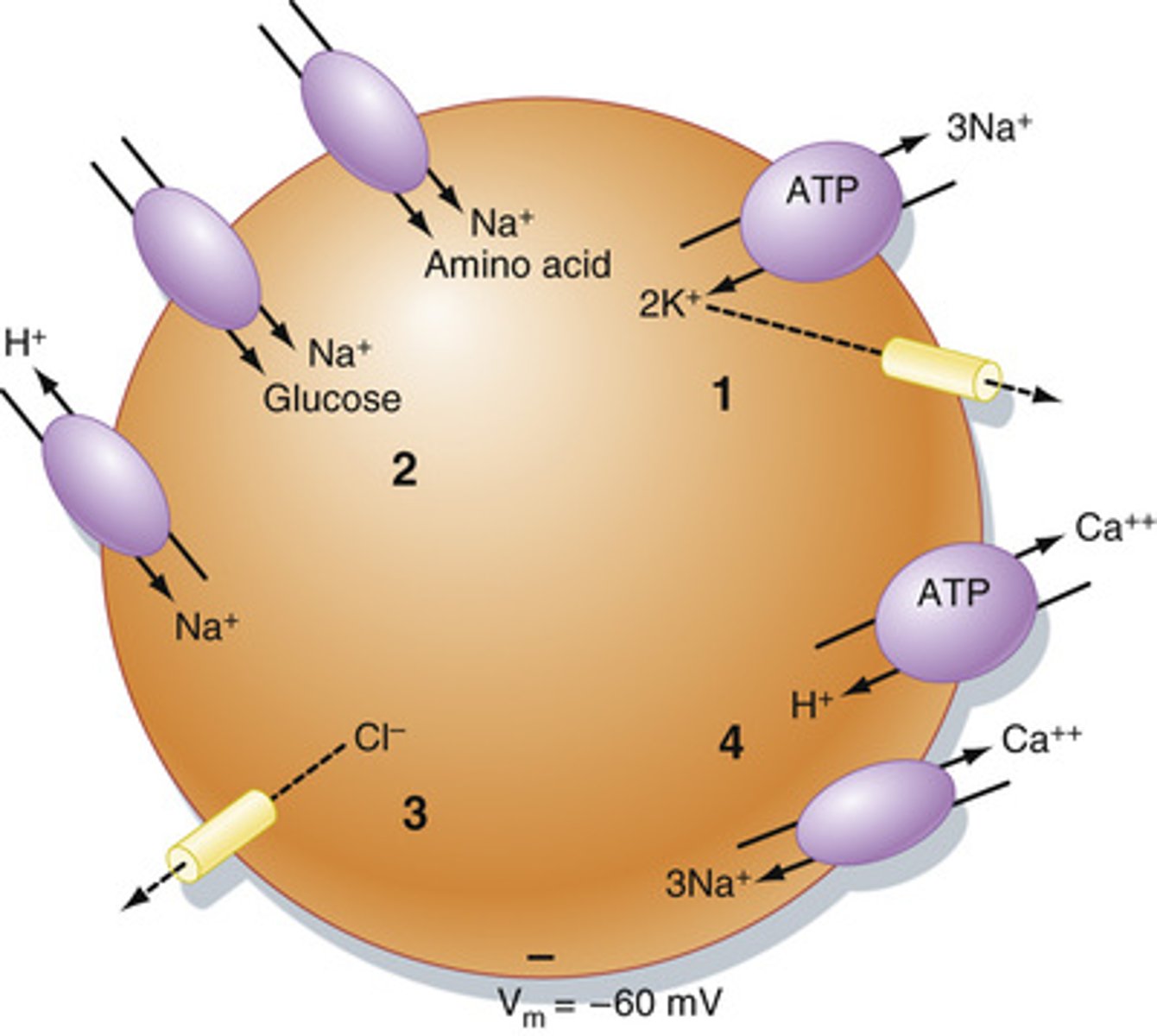
What is the function of Na+/K-ATPase in maintaining steady state?
Keeps Na+ high outside the cell (3 out) and K+ high inside the cell (2 in)
What will calcium levels be like inside a cell and why are they kept like this?
low, Ca2+ is a potent secondary messenger and will cause intracellular events and growth if free within the cell
How are positive and negative charges of electrolytes in the body offset?
small negative net charge inside the cell (Na+ balances K+, Cl- balances protein which has negative aa)
How are gradients of electrolytes established?
through the actions of pumps and transporters
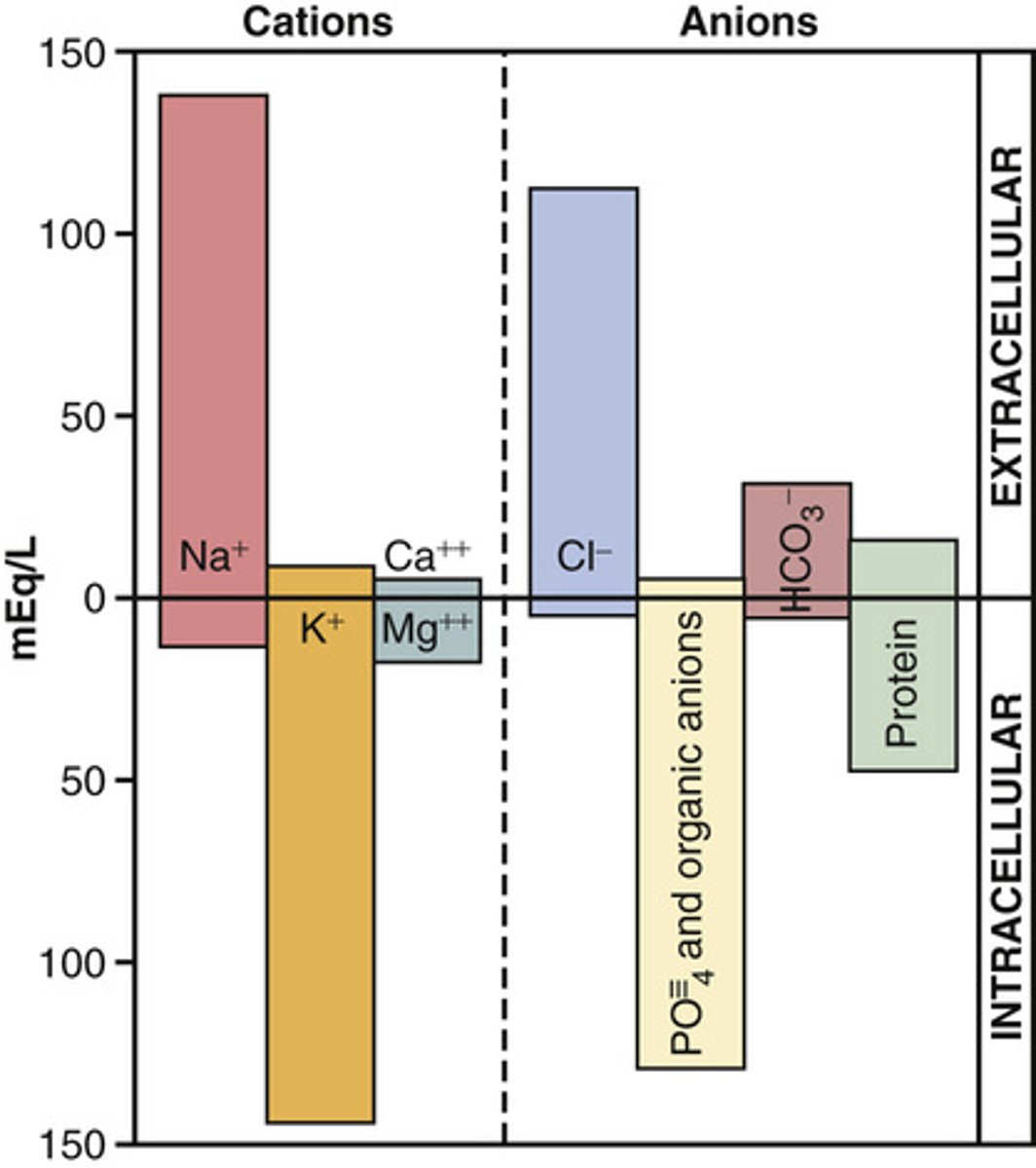
What is the normal concentration of ions in the ECF and ICF?
Note: when we refer to ion concentrations we are referring to concentration in the plasma unless noted
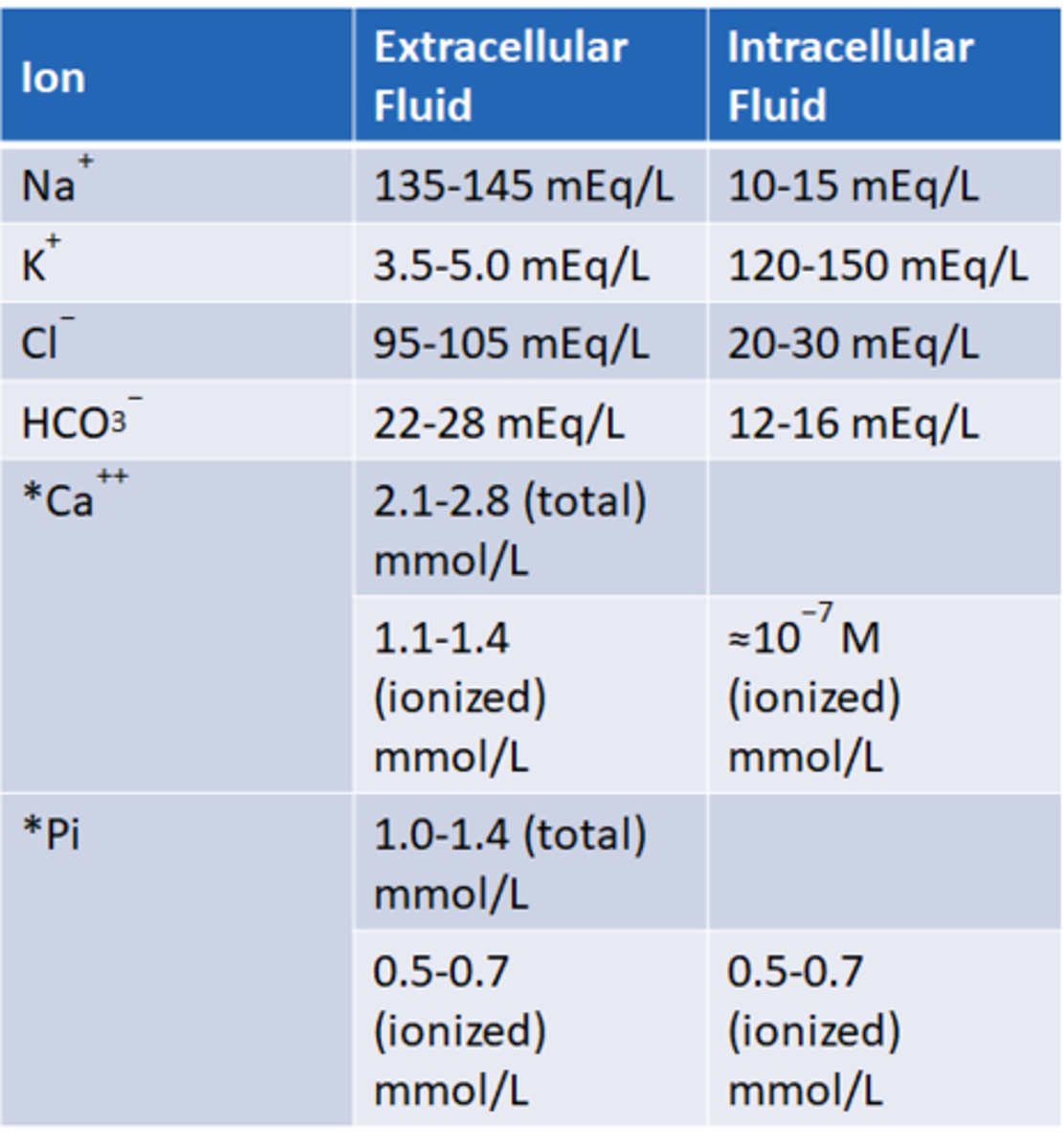
Na+ extracellular fluid normal concentration
135-145 mEg/L
K+ extracellular fluid normal concentration
3.5-5 mEg/L
Cl- extracellular fluid normal concentration
95-105 mEg/L
HCO3- extracellular fluid normal concentration
22-28 mEg/L
What are possible serum electrolyte imbalances?
Know the table!!
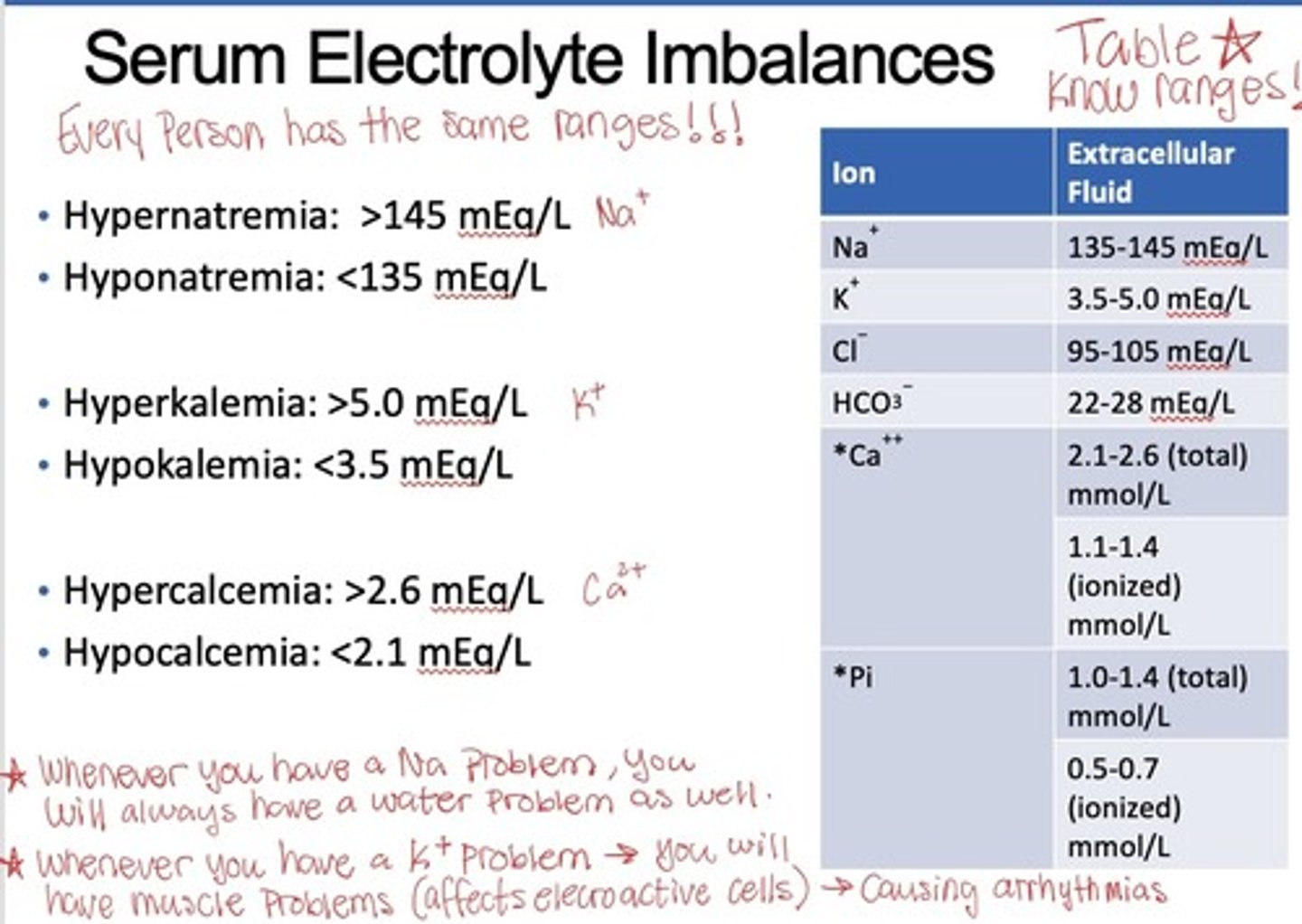
What will water follow?****
Sodium (sodium imbalance=water imbalance!!)
What does potassium cause cellularly?***
Affected electro-active cells (Excitability -- MI, cardiac arrhythmias)
(people on POT are EXCITED)
What will result from excess sodium in the ECF?
water will leave the cell and enter the ECF causing edema (this will cause a driving force for Na+ to enter cell which will mess with potassium)
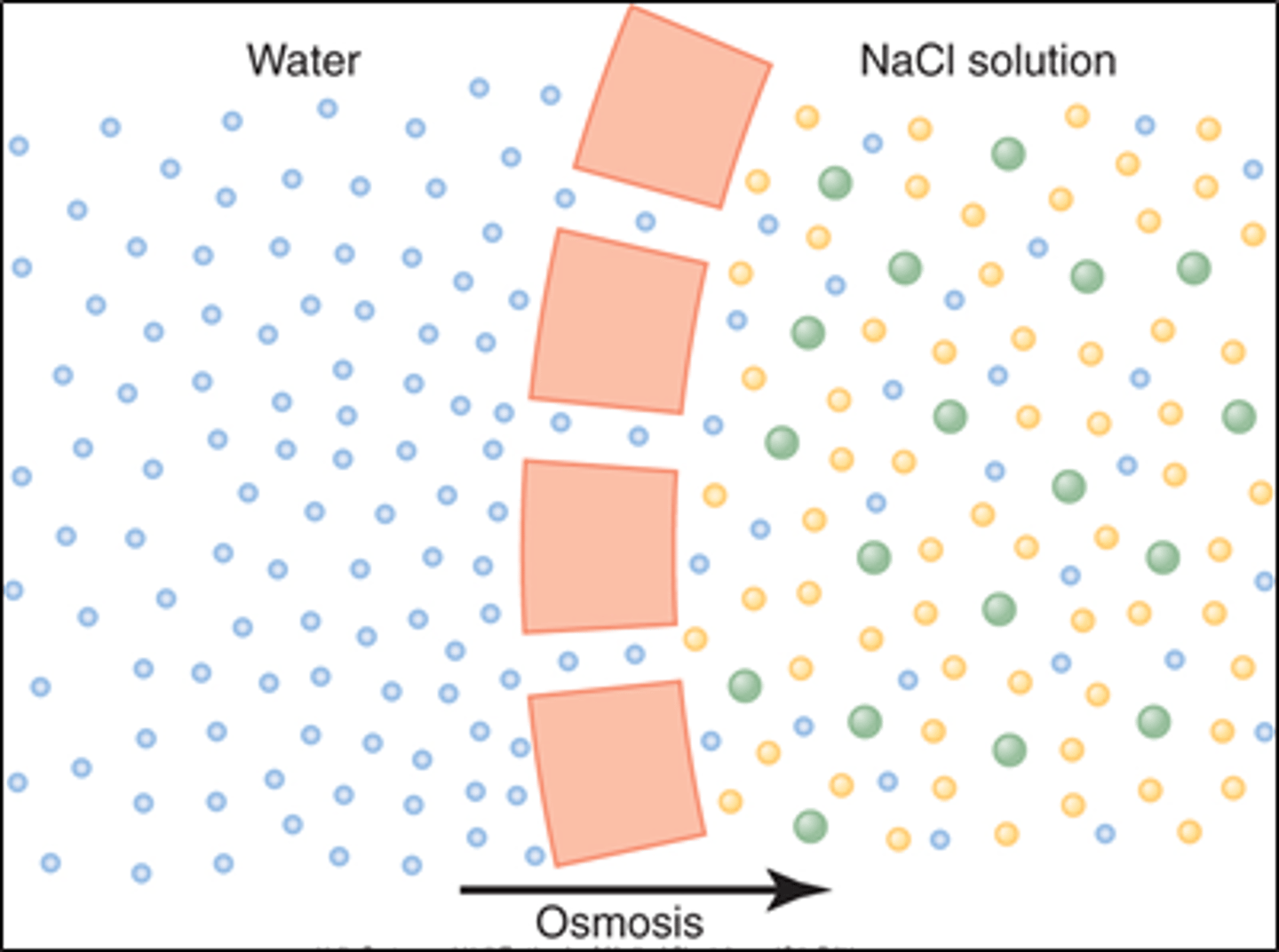
What is osmosis?
diffusion of water across a selectively permeable membrane from area of lower solute concentration to area of higher solute concentration
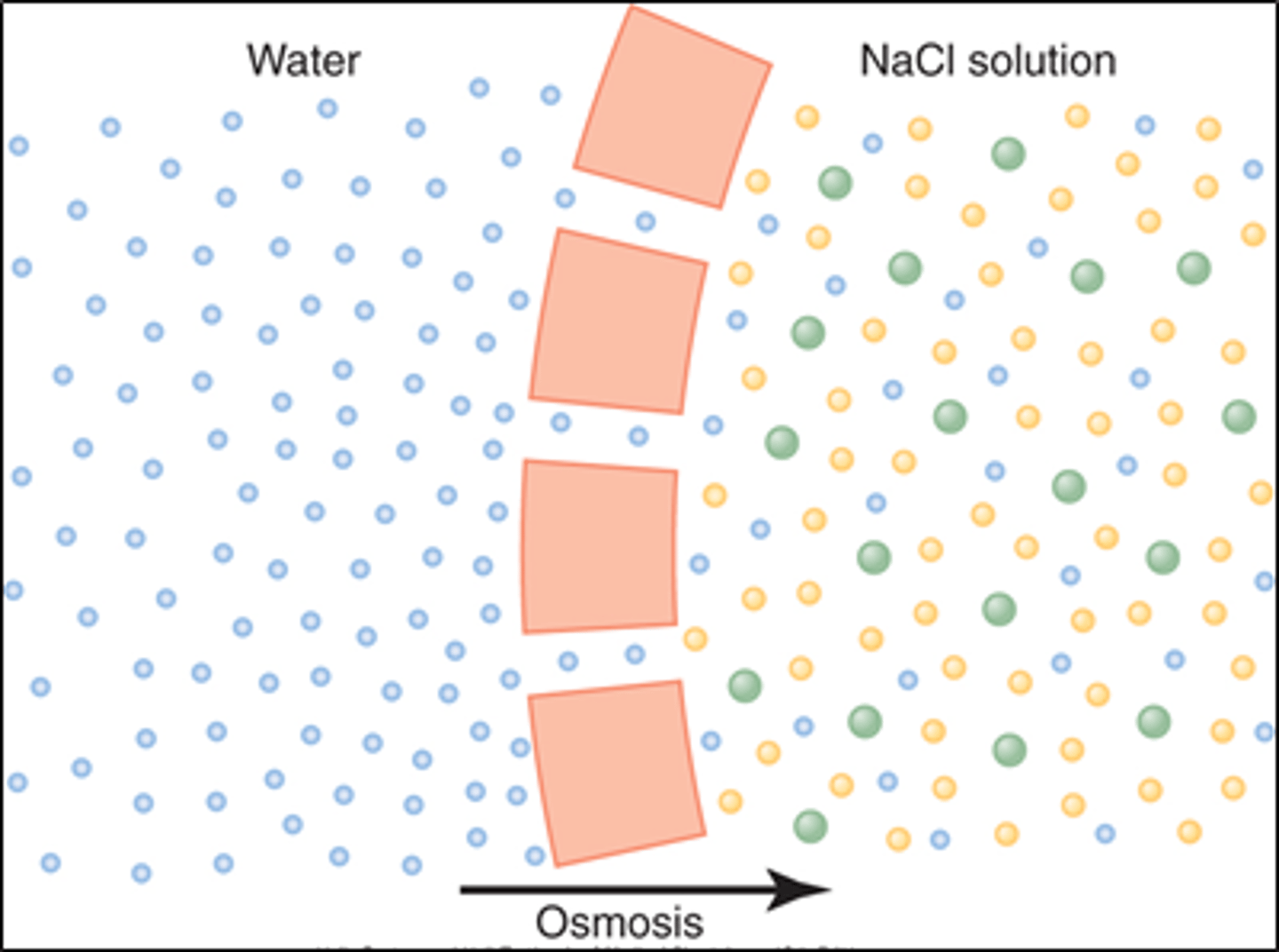
How is the concentration of solute determined in osmosis?
by the number of particles (higher # particles=lower water concentration)
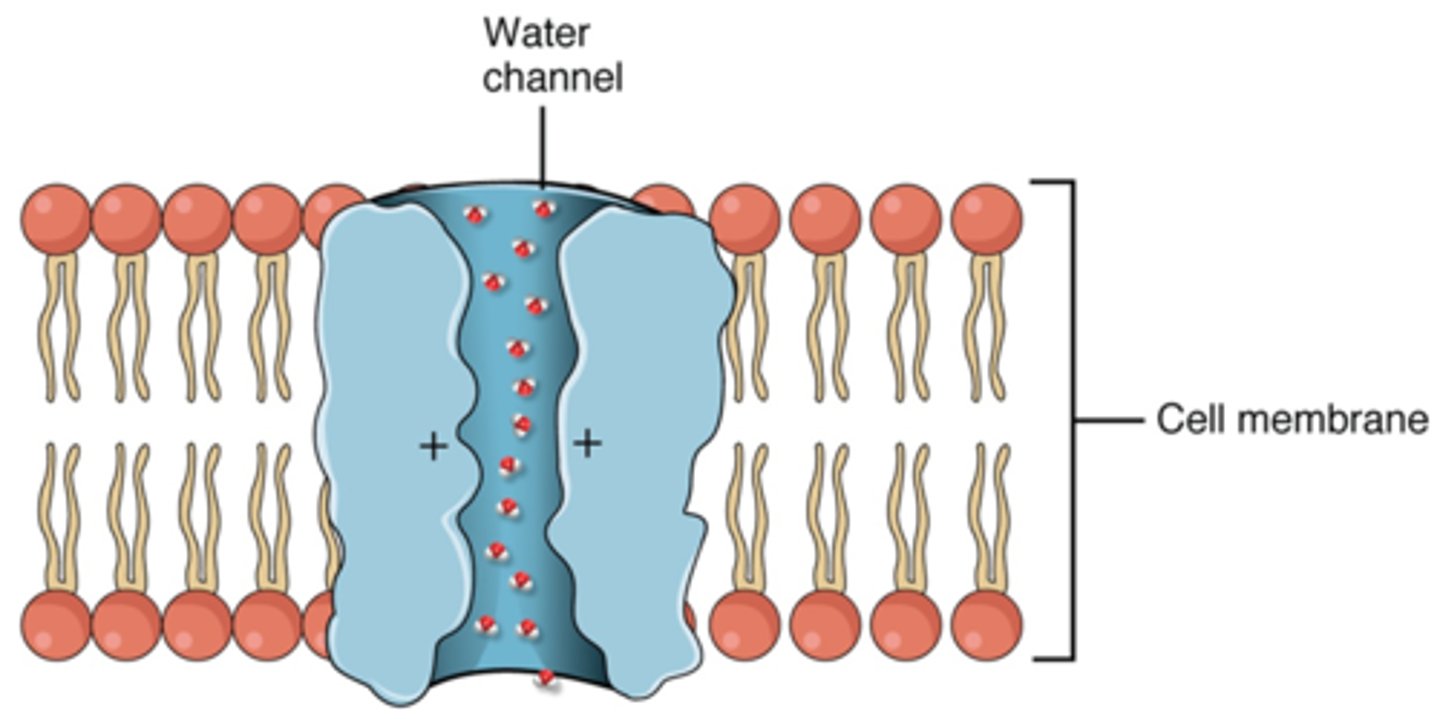
What allows the passage of water from one side of a membrane to another?
aquaporins (if absent solute concentrations will become difficult to manage), can be altered by the cell to alter the amount of water that can enter
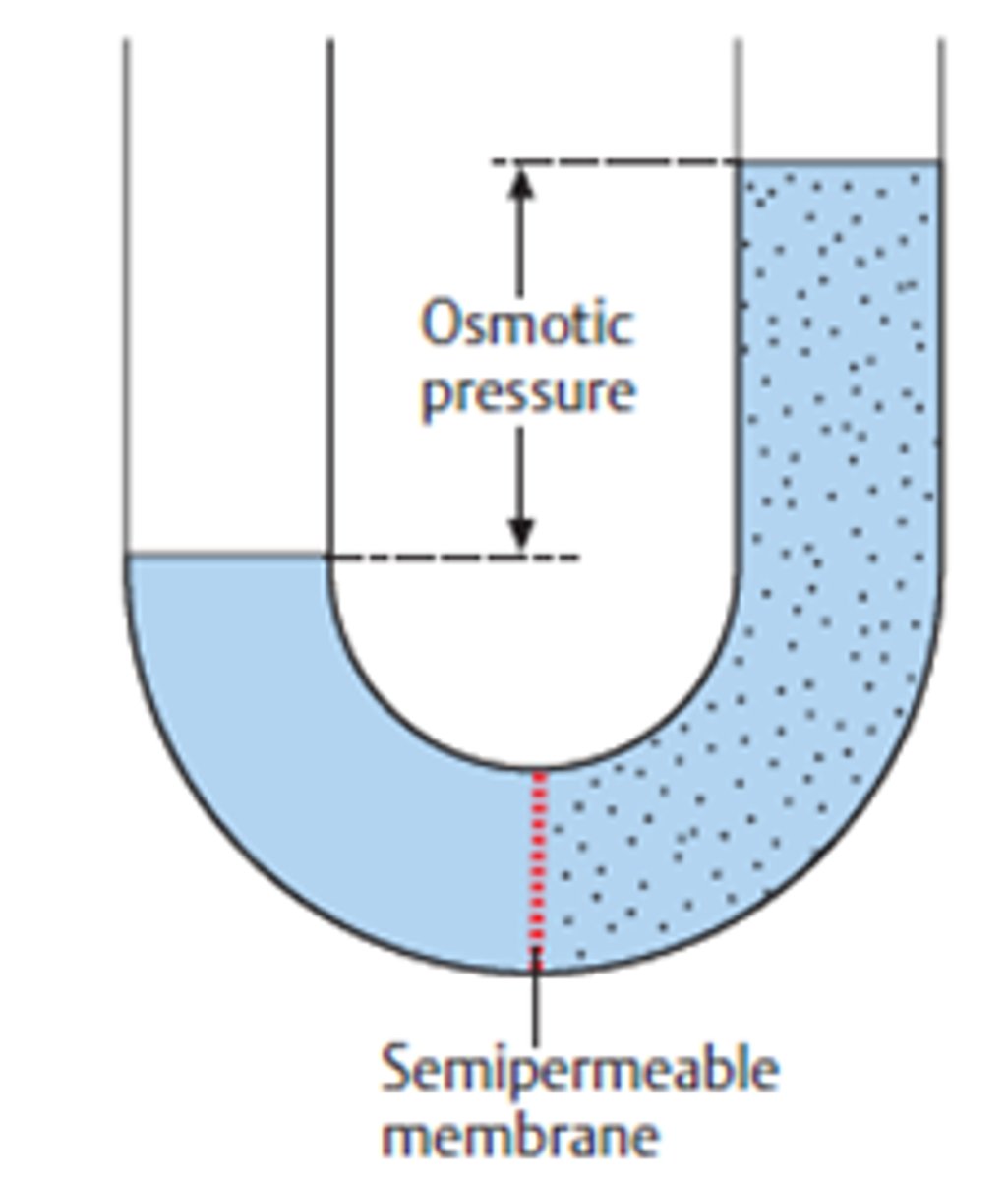
What is osmotic pressure?
The amount of pressure required to stop the osmotic flow of water across a membrane that separates a solution from pure water
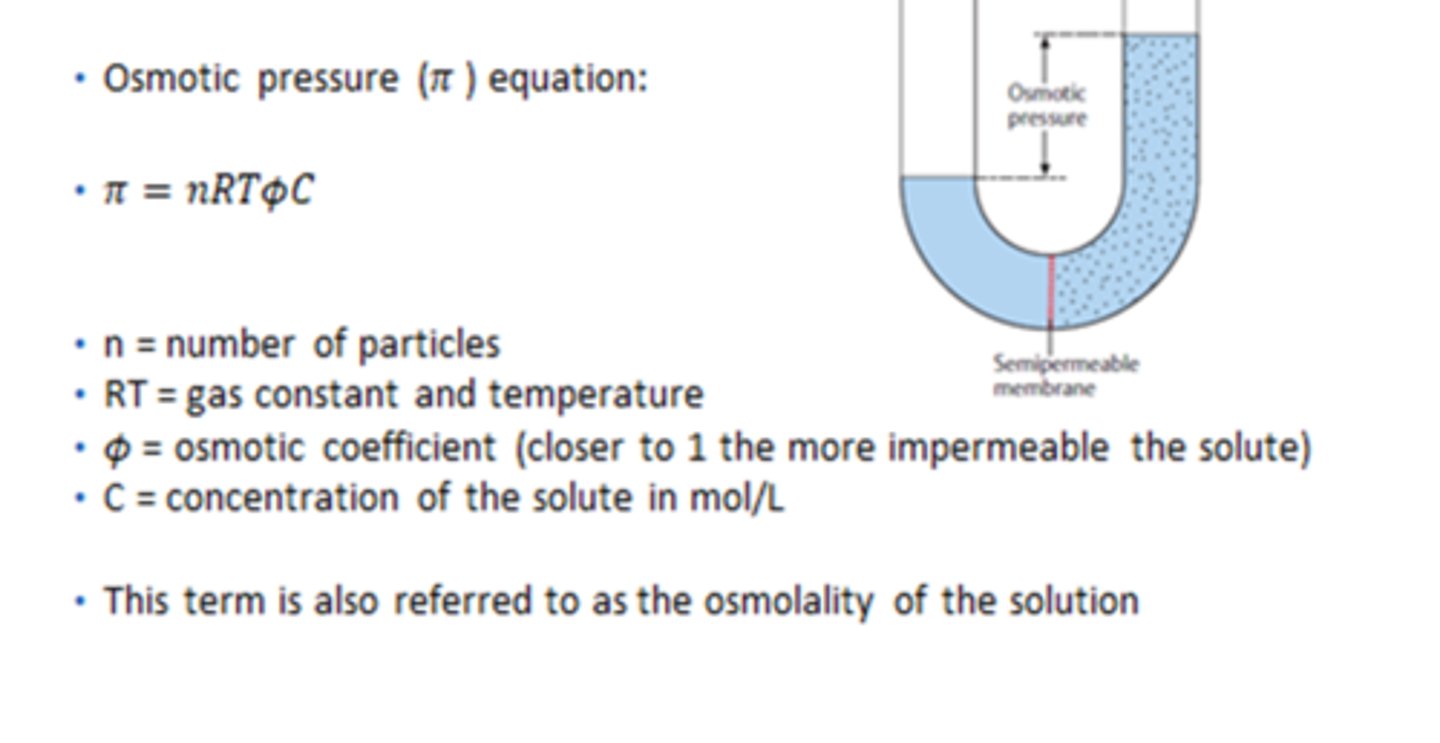
What is the equation for osmotic pressure?
Note: changing the amount of solute will change the amount of pressure!
What solutes are taken into account in osmolarity?
all solutes including membrane penetrating solutes and non-penetrating solutes (non-penetrating are most important!!)
How can solute be categorized based on osmolarity?
Isosmotic, hypoosomotic, and hyperosmotic (refer to relative amounts of solutes in one solution compared to another)
What is the difference between osmolality and osmolarity?
Osmolality: number of osmoles in a kilogram of solvent (Osm/Kg)(weight)
Osmolarity: number of osmoles in a liter of solution (concentration of an osmotic solution) (osm/L)(volume)
-In general 1L=1Kg, consequently osmolarity and osmolality can almost be used synonymously.
What is tonicity?
the ability of an extracellular solution to make water move into or out of the cell by osmosis (related to osmolarity)
What determines cell volume?
tonicity
What is tonicity influenced by?
only by solutes that cannot cross the membrane, these can exert effective osmotic pressure
What are the different classifications of tonicity?
isotonic, hypertonic, and hypotonic
What will be the units for osmolarity, osmolality, and tonicity?
osmolarity: Osm/L
osmolality: Osm/Kg
Tonicity: Osm/ volume of solution
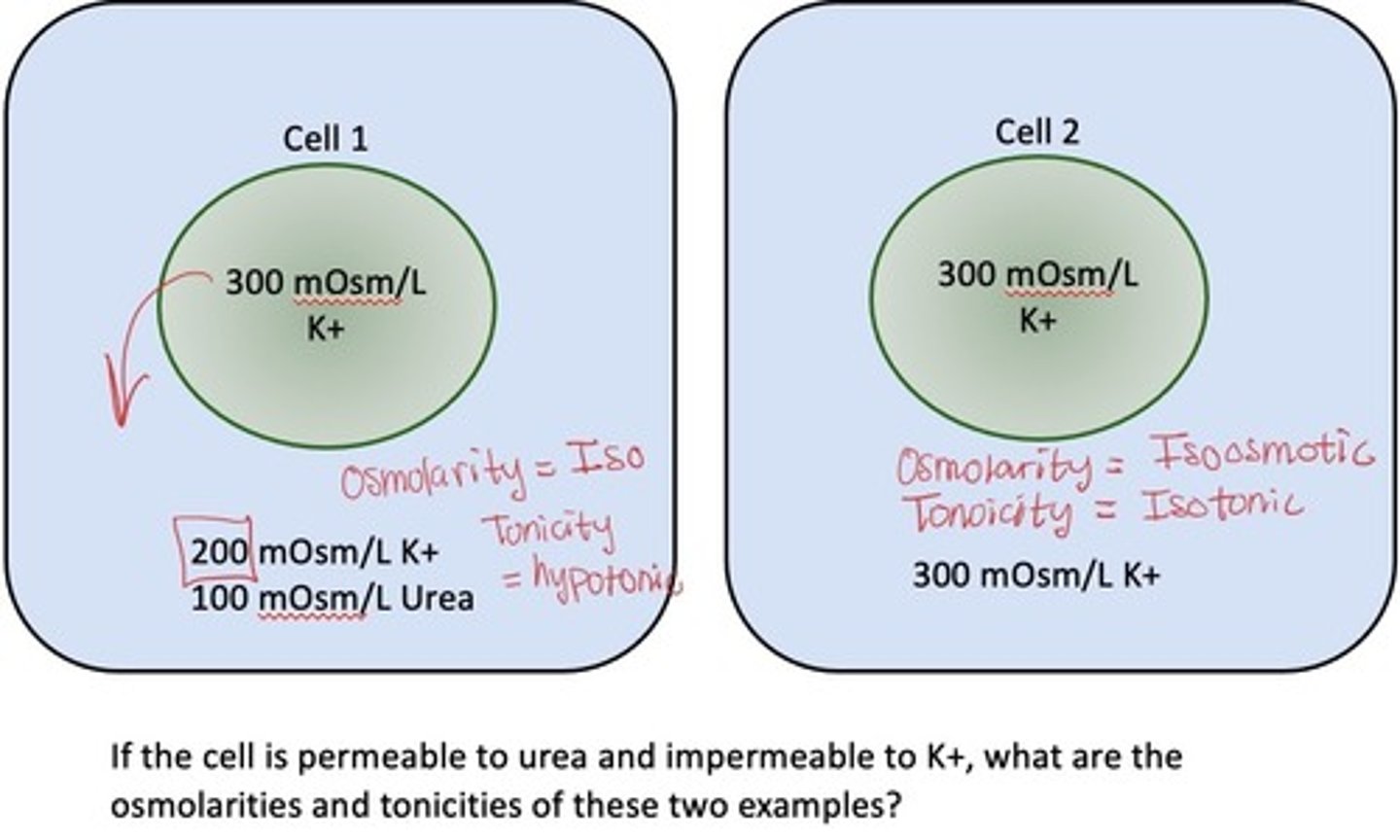
How will cell volumes differ if they are isotonic or isoosmotic?
Cell 1 is isotonic: has potential to have driving force as urea is impermeable
Cell 2 is isoosmotic: no driving force
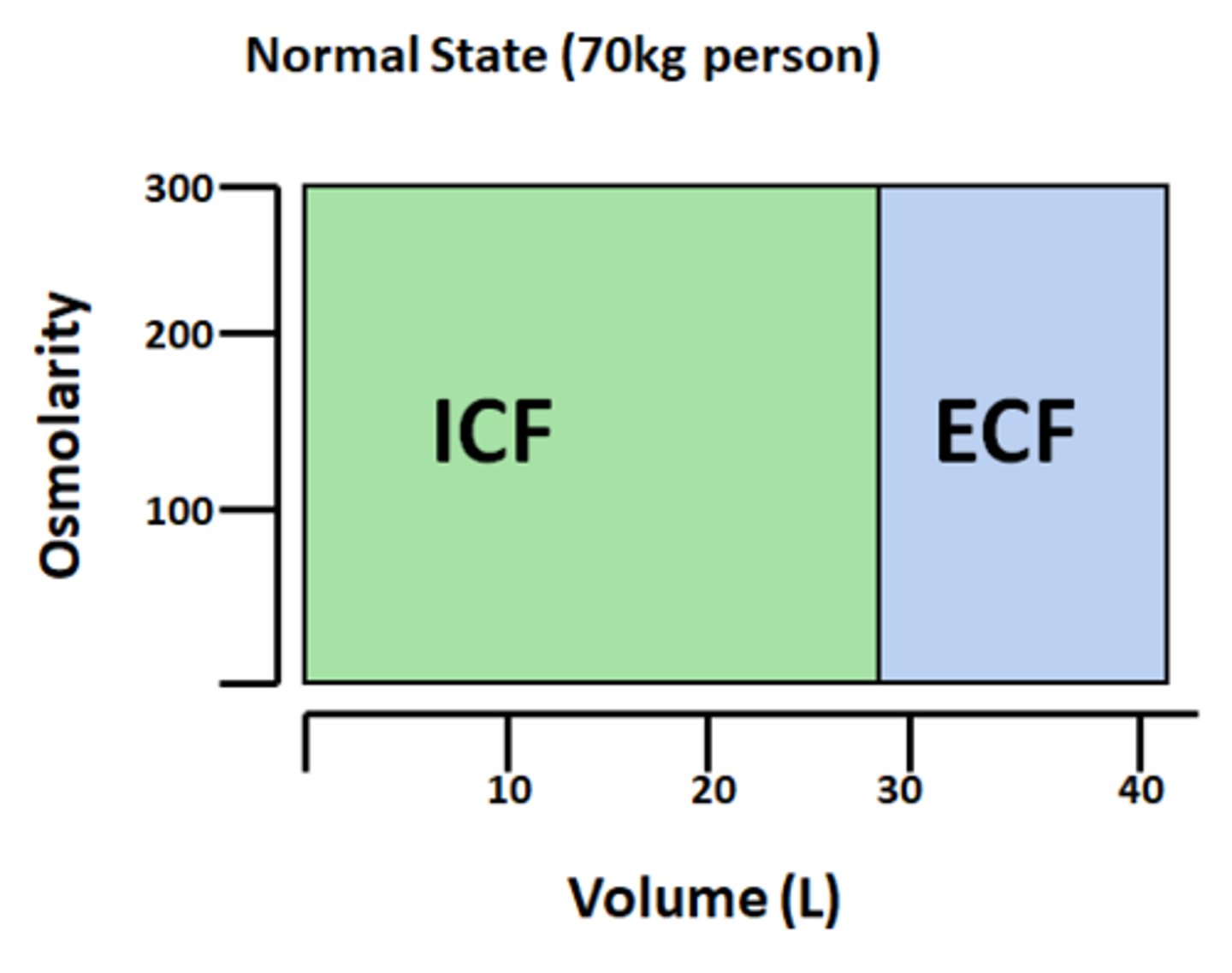
How will the osmolarity differ based on fluid compartment?
will be very similar actoss compartments

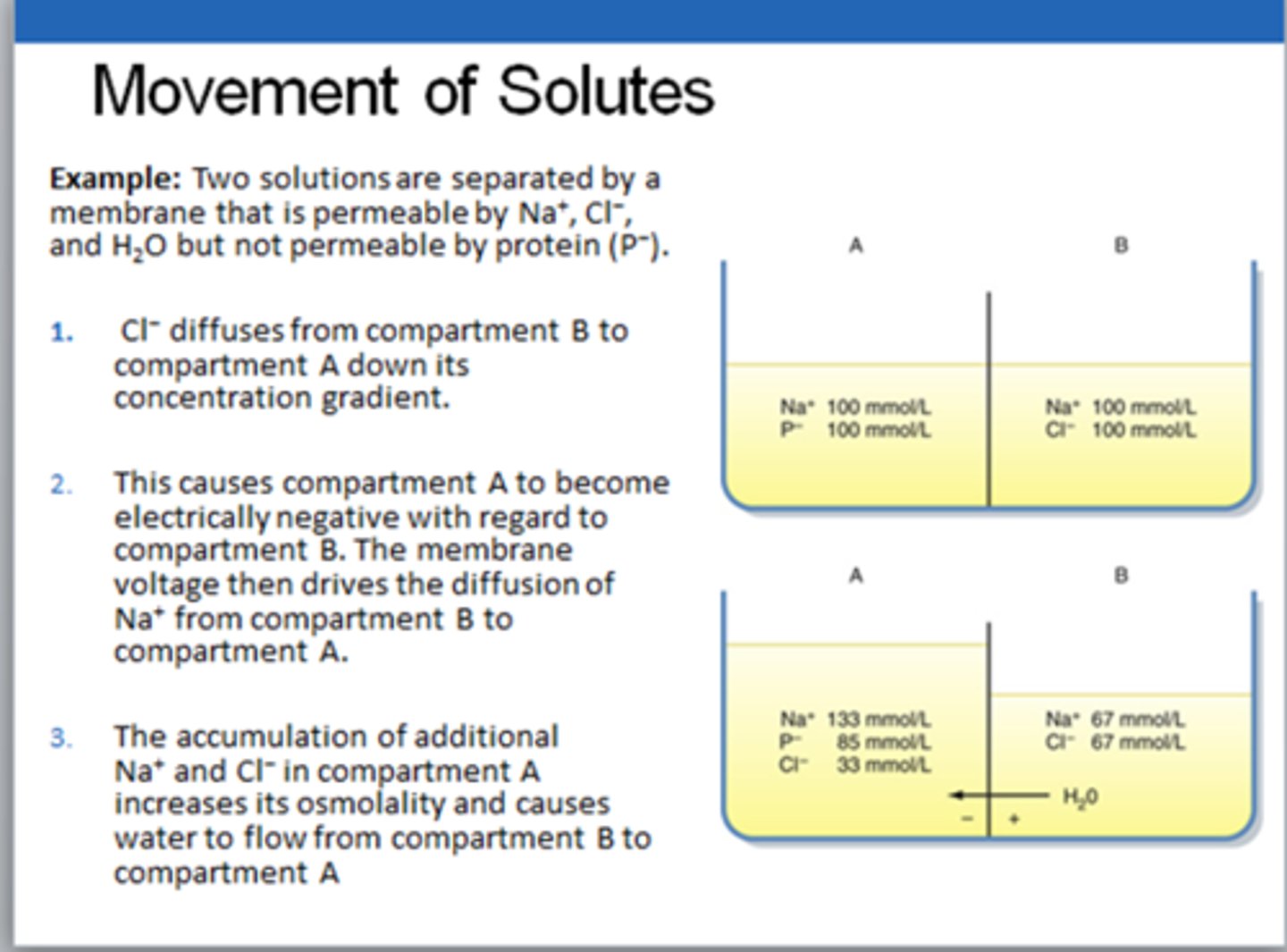
Understand how solutes will move across membranes (beaker example)
ions will follow their individual concentration gradient!!
Cl- will bring water, sodium will follow to balance the difference and will bring more water
What will happen to a cell when it is placed in a hypotonic solution?
it will swell
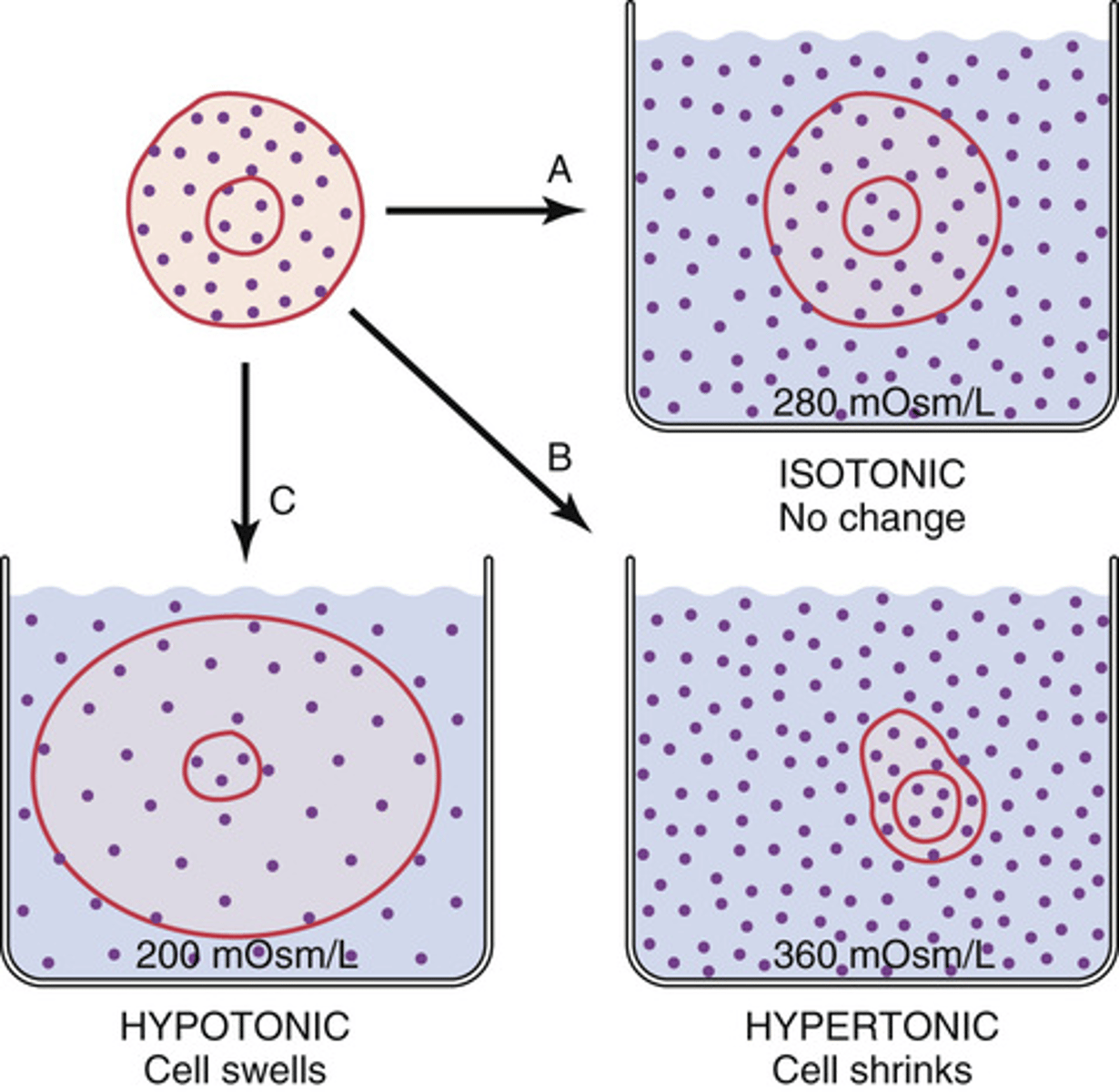
What will happen to a cell placed in a hypertonic solution?
will shrink
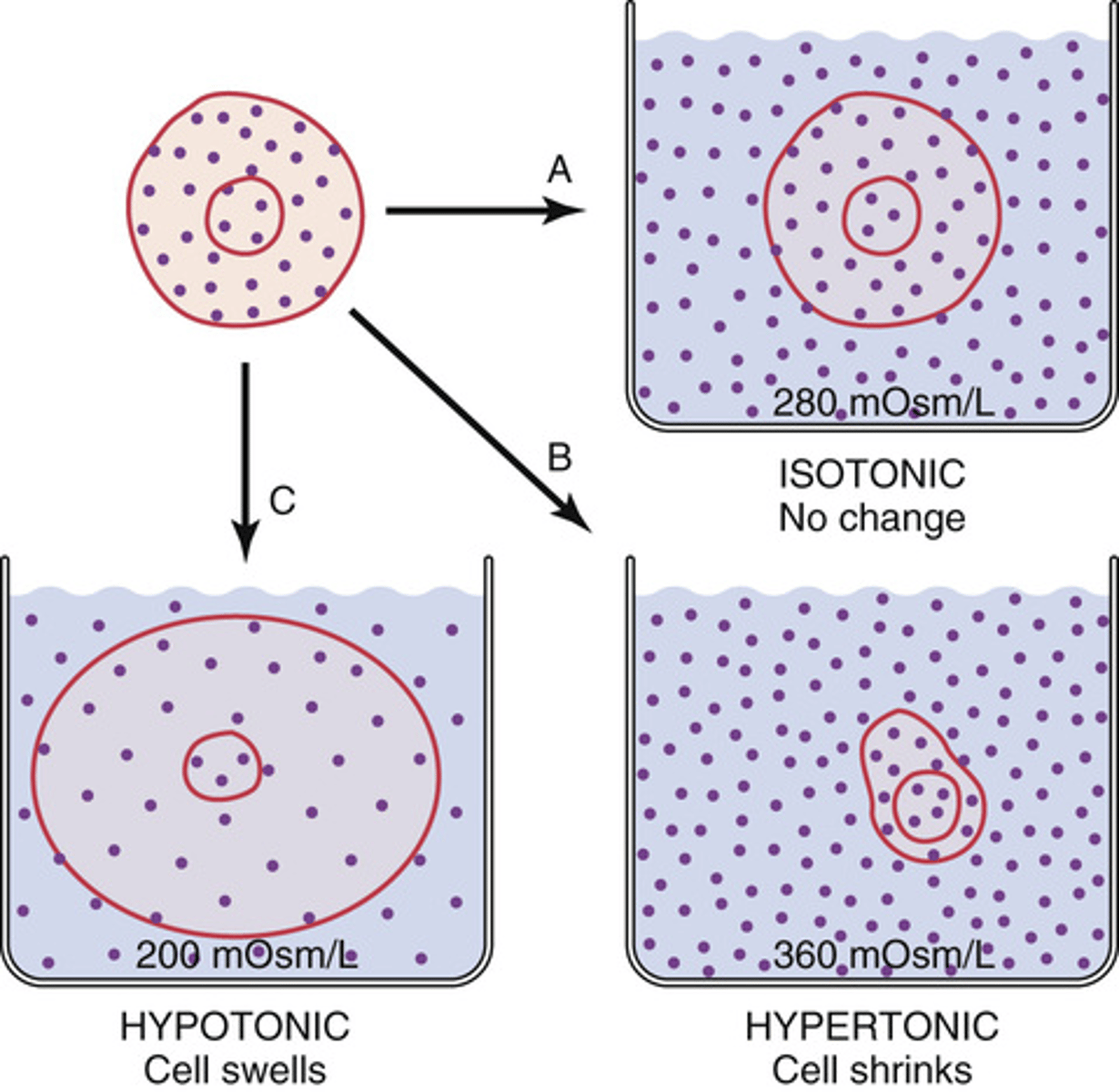
What will happen to a cell placed in an isotonic solutions?
will remain the same size
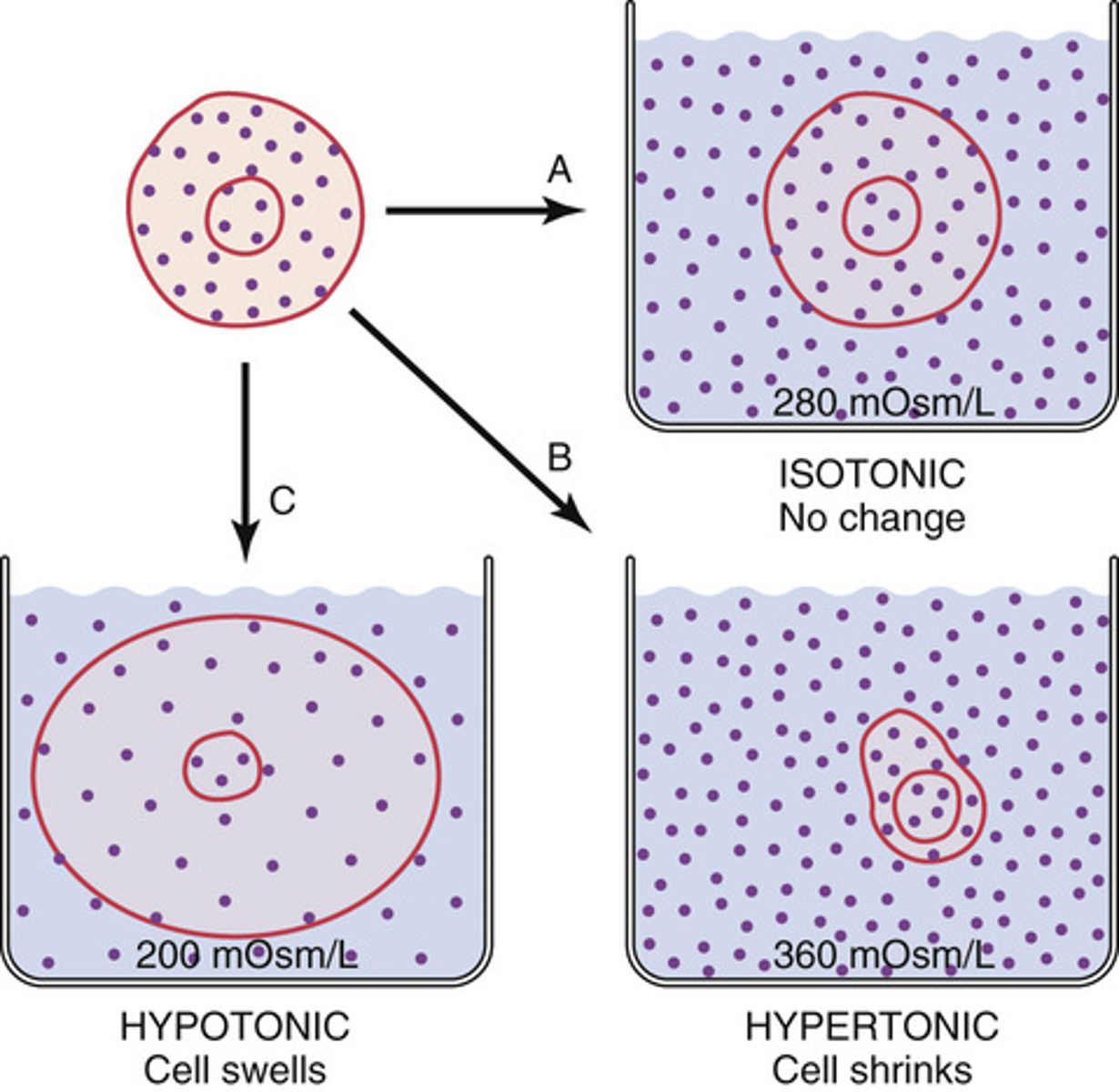
What type of solutes must be present for cells to change size?
impermeable solute
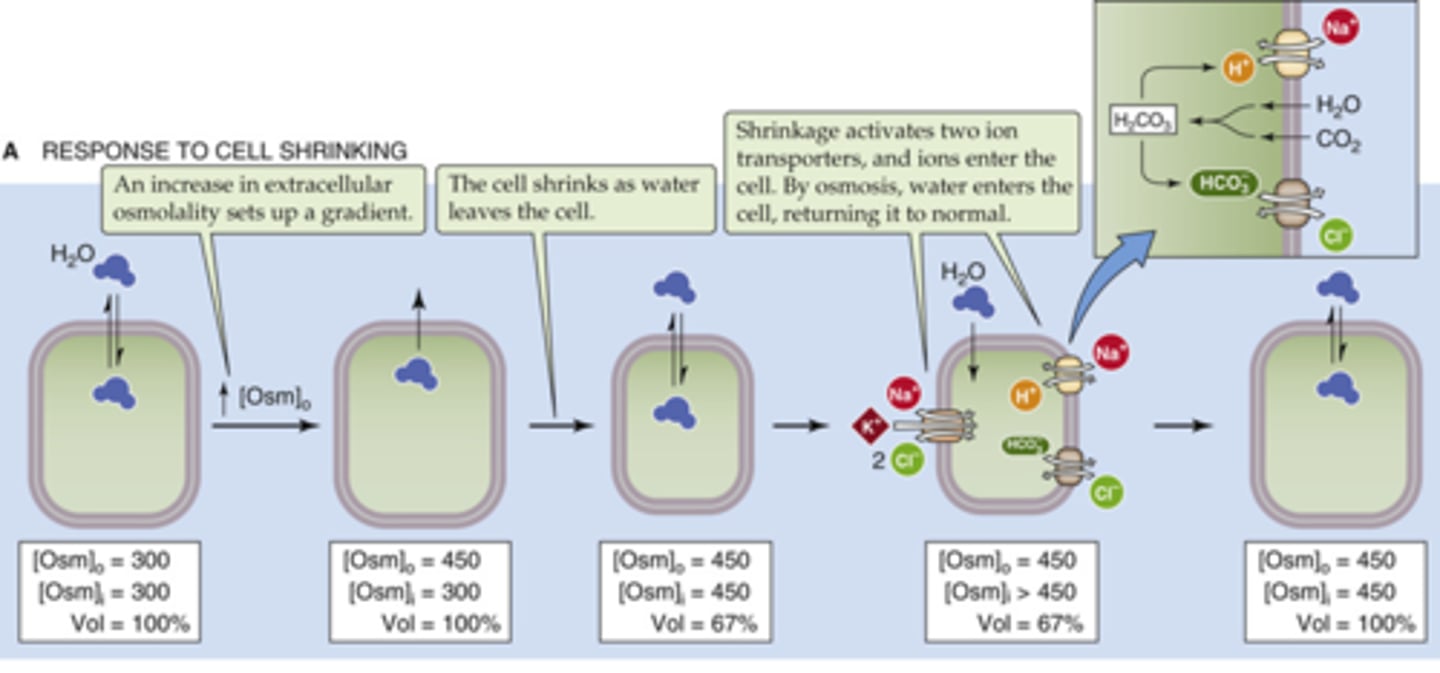
How will cells respond to shrinkage?
brings ions in and water will follow, uses permeable solutes to achieve this (bringing in solutes can be detrimental to the cell)
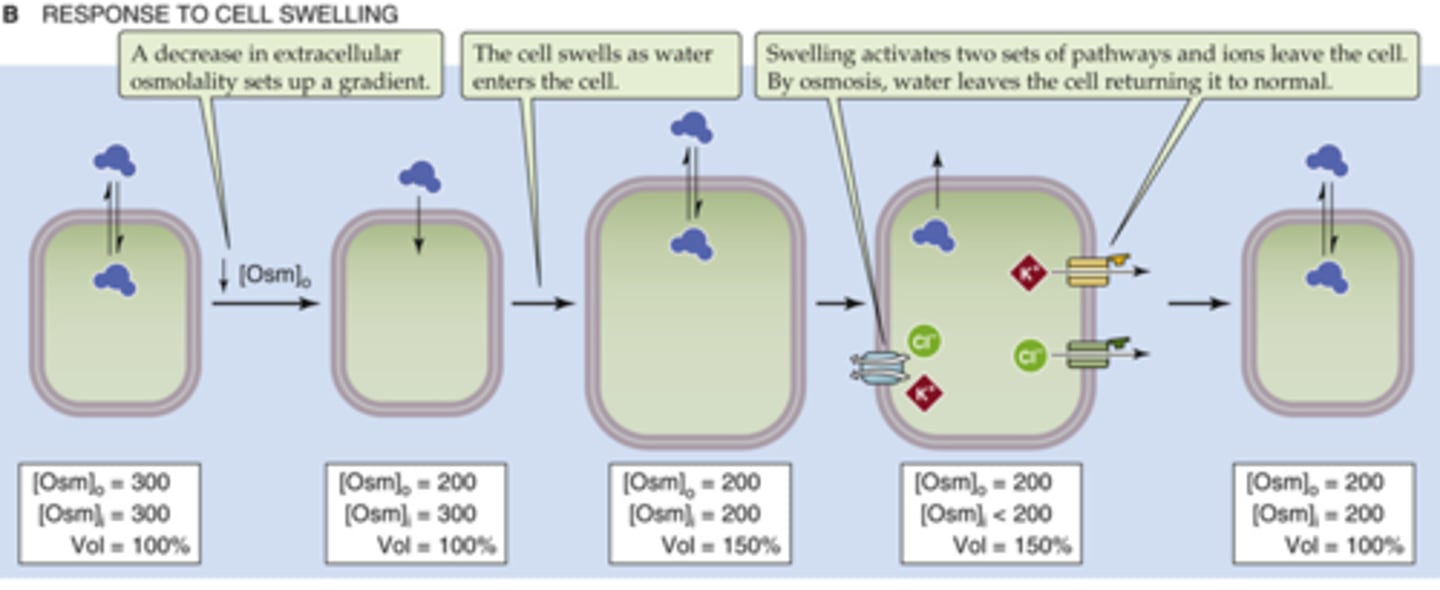
How do cells respond to swelling?
pumps ions out and water will follow
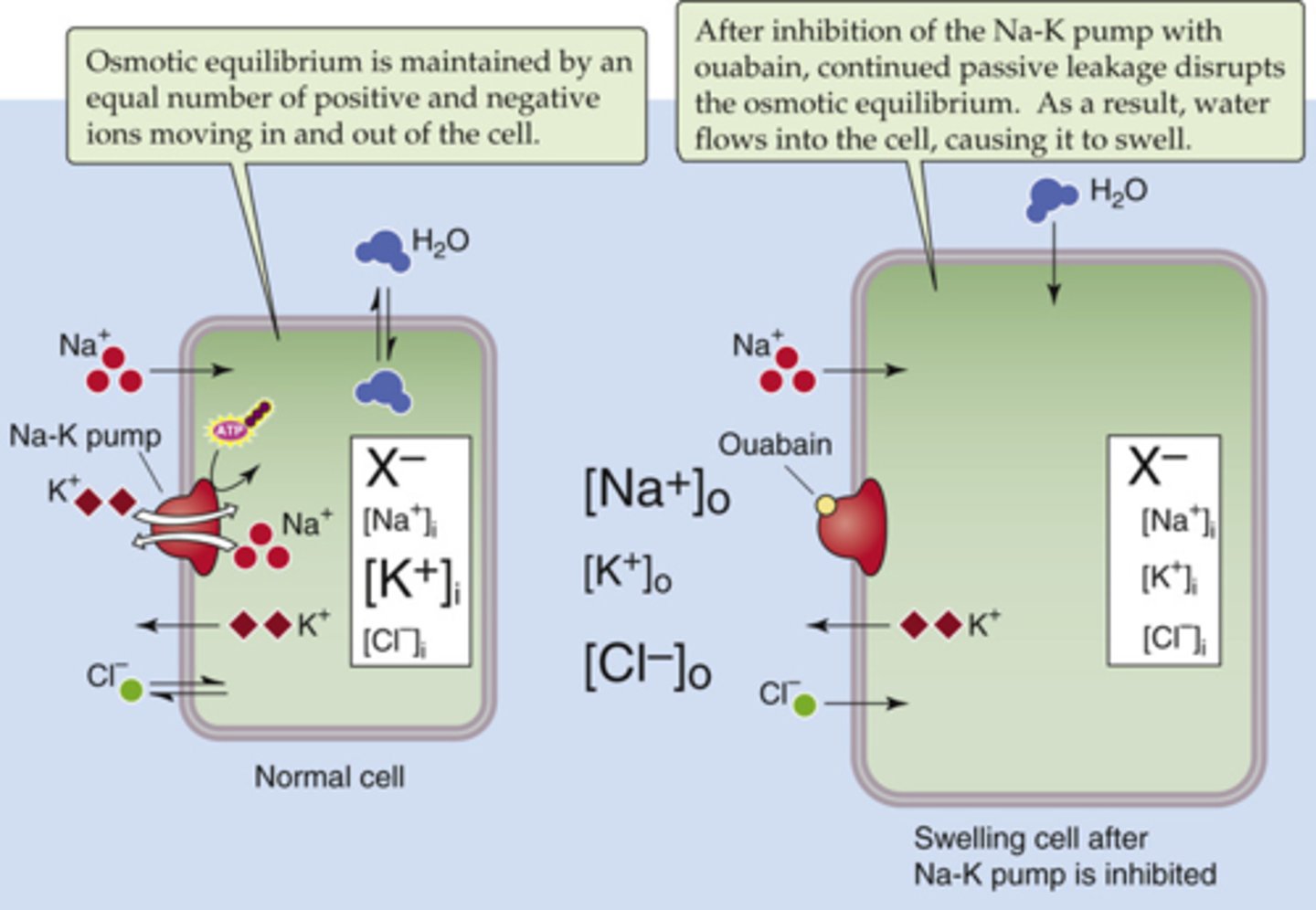
What effect will the drug ouabain have on a cell?
blocks Na-K-ATPase thus causing the cell to swell (water follows sodium which will be high in the cell)
What fluid compartment can be manipulated to alter homeostasis?
ECF (through IV)
What is the ECF composed of?
plasma and interstitial fluid
What facilitates transport between ICF and ECF?
capillary exchange
Plasma->ISF->ICF
How is fluid exchanged between plasma and interstitial fluid?
via capillaries
How can the interstitial space be altered?
by changing plasma concentration of solutes
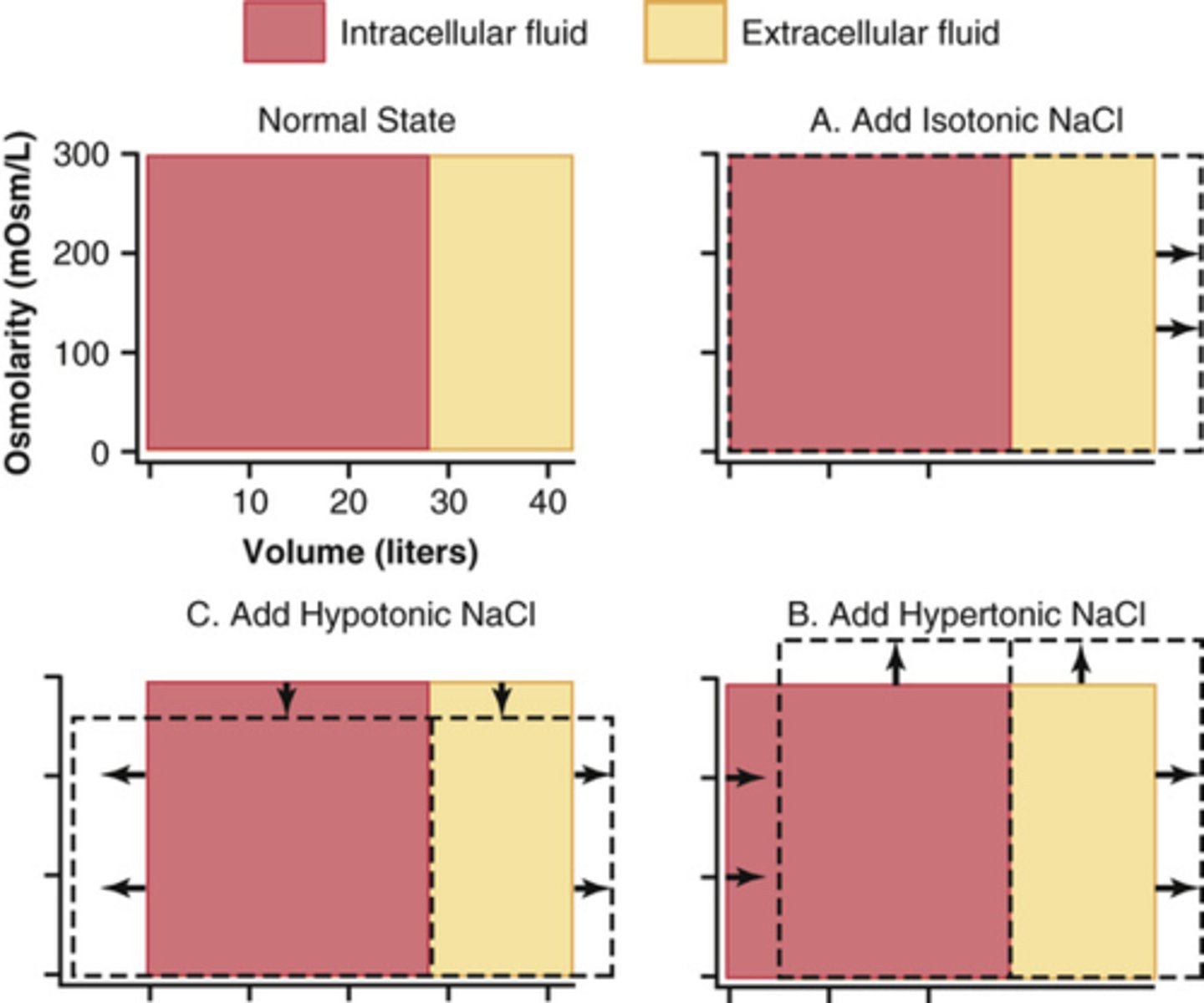
What is the purpose of Darrow-Yannet diagrams?
illustrates the body compartements and visualize changes to fluid concentrations and volumes (right box first then left box)
Wht type of solution can be given to lower the concentration of ECF?
hypotonic, water will go into the ICF
How will adding isotonic, hypertonic, and hypotonic solutions be showed in a darrow-yannet diagram?
Isotonic: won't change osmolarity, just adding volume to ECF
Hypertonic: will increase osmolarity, add volume to ECF decrease volume in ICF, water from ICF will come out and add to ECF
Hypotonic: decrease osmolarity, increase volume in ECF and ICF, loss of driving force outside will slow down the movement of water outside to inside
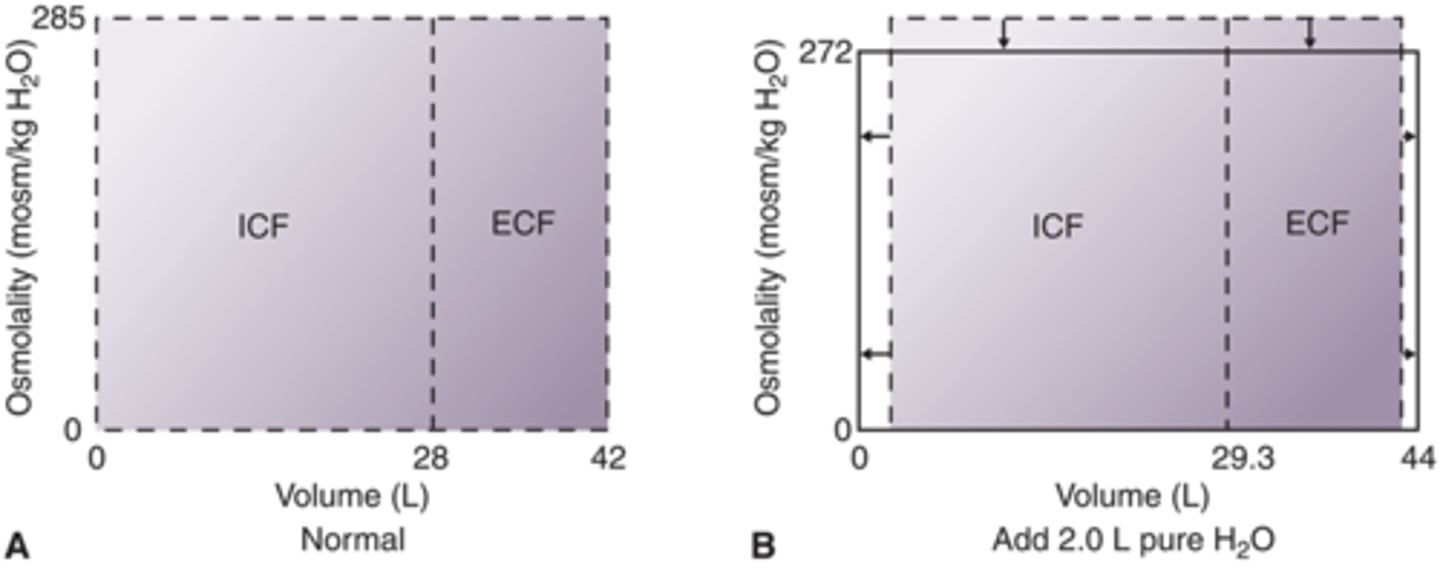
How will fluid compartments change when 2.0 L of pure H2O is added IV? (Draw)
hypotonic, decrease osmolarity, increase volume in ECF and ICF (more so in ICF) contains 0 solutes (don't give)
How will fluid compartments change after addition of 2.0 L isotonic solution IV?
Isotonic, will just add volume to ECF, will not alter osmolarity
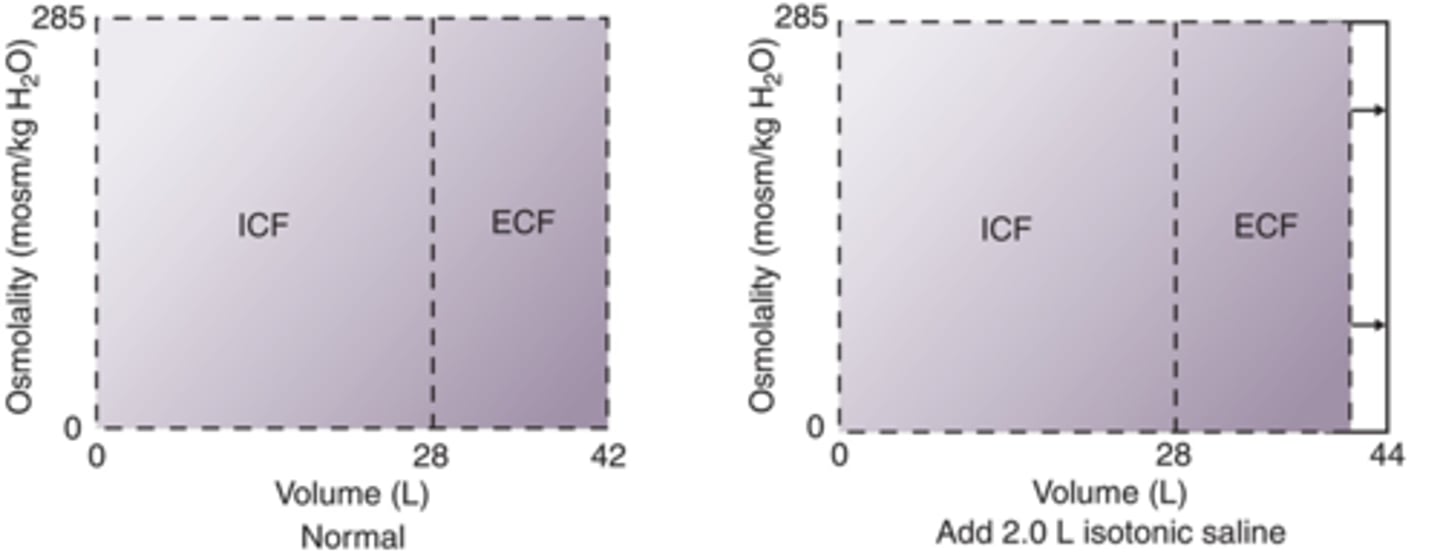
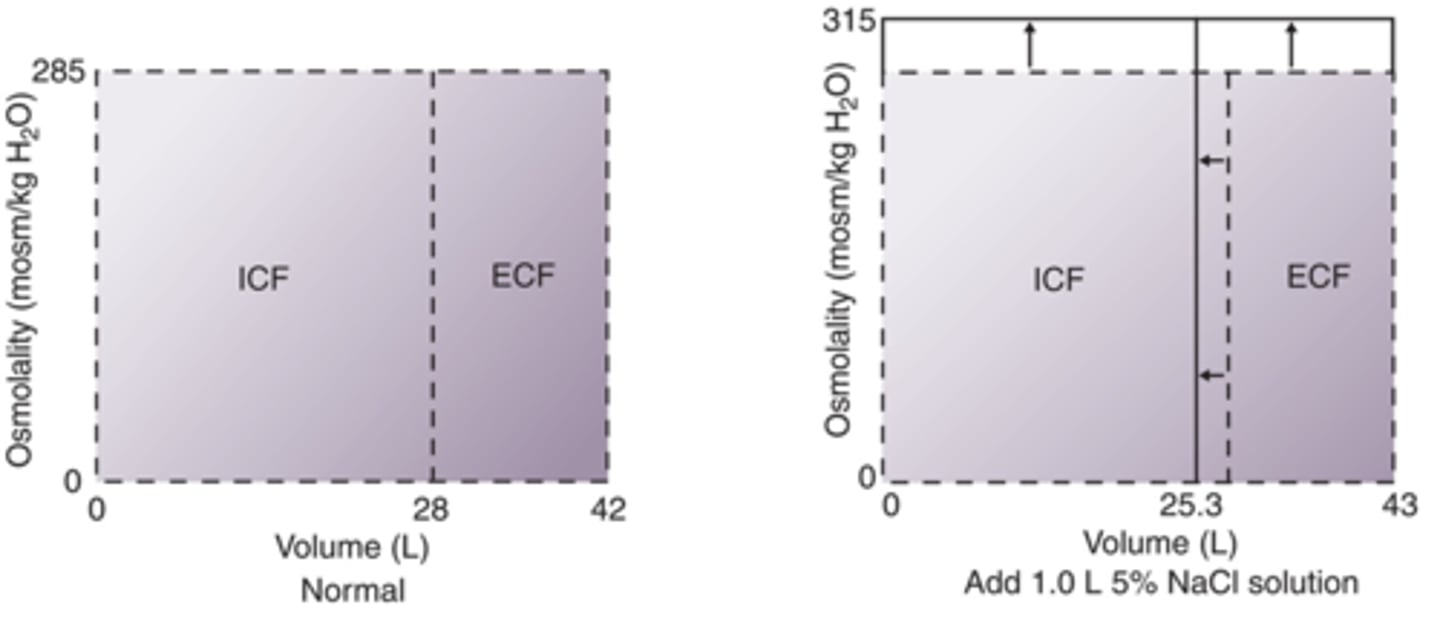
How will fluid compartments change after adition of 1.0 L 5% NaCl IV?
Hypertonic (0.9% is isotonic normal saline), increases osmolarity, increases ECF, decreases ICF
What are common volume expanders?
D5W (5% dextrose) starts as isotonic but quickly becomes hypotonic because body uses glucose right away.
1/3 NS and half normal saline both are hypotonic.
Normal saline - isotonic
Ringer's lactate (closest to normal levels) used for cardiac and renal patients (best solution to give but most expensive)
D5NS (5% dextrose, NS) isotonic solution.
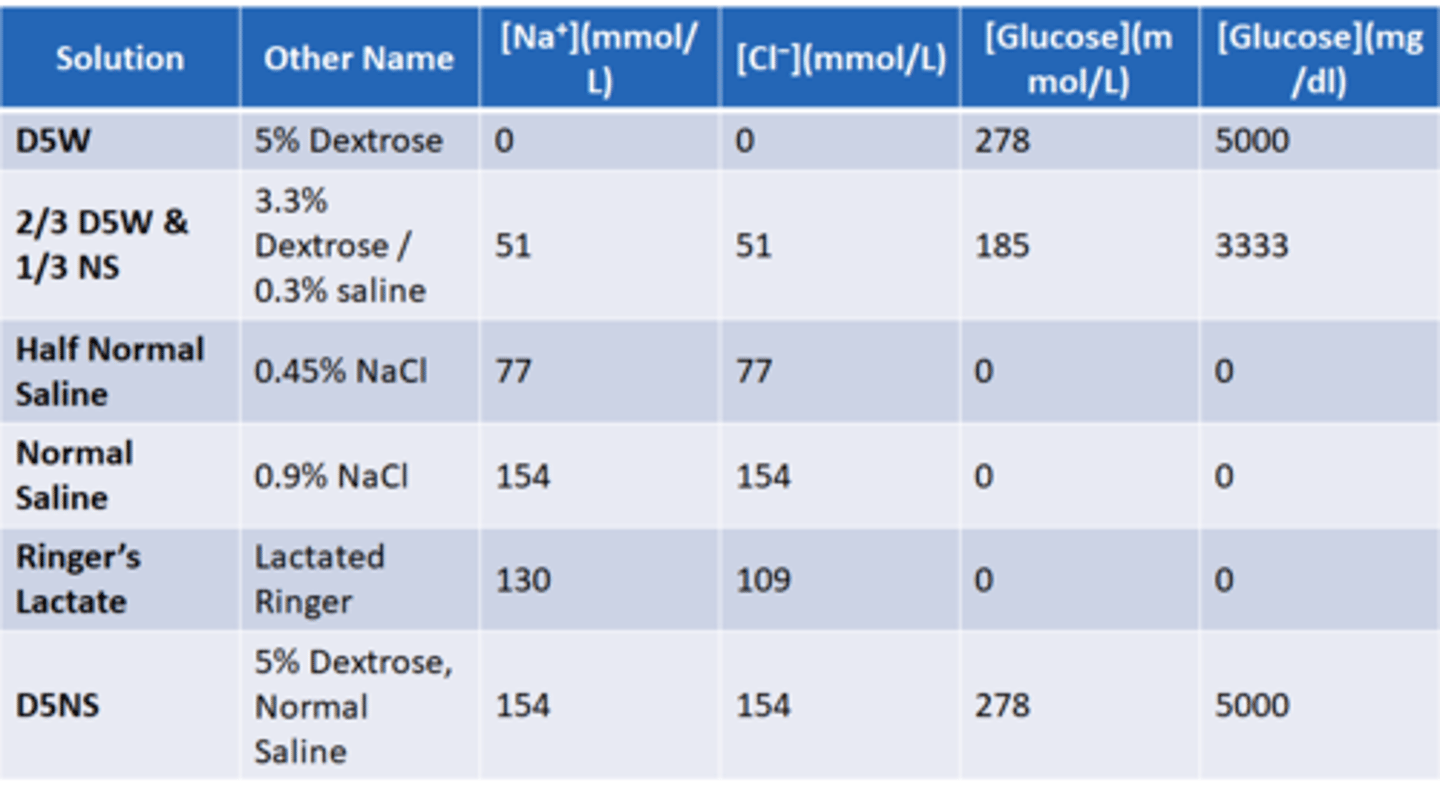
What changes can result from volume expanders (chart)
know the numbers

How is dextrose absorbed and what effect does it have?
slowly over time, added benefit of adding energy to the system that can be used to accomplish the movement of solutes and water necessary to expand volume
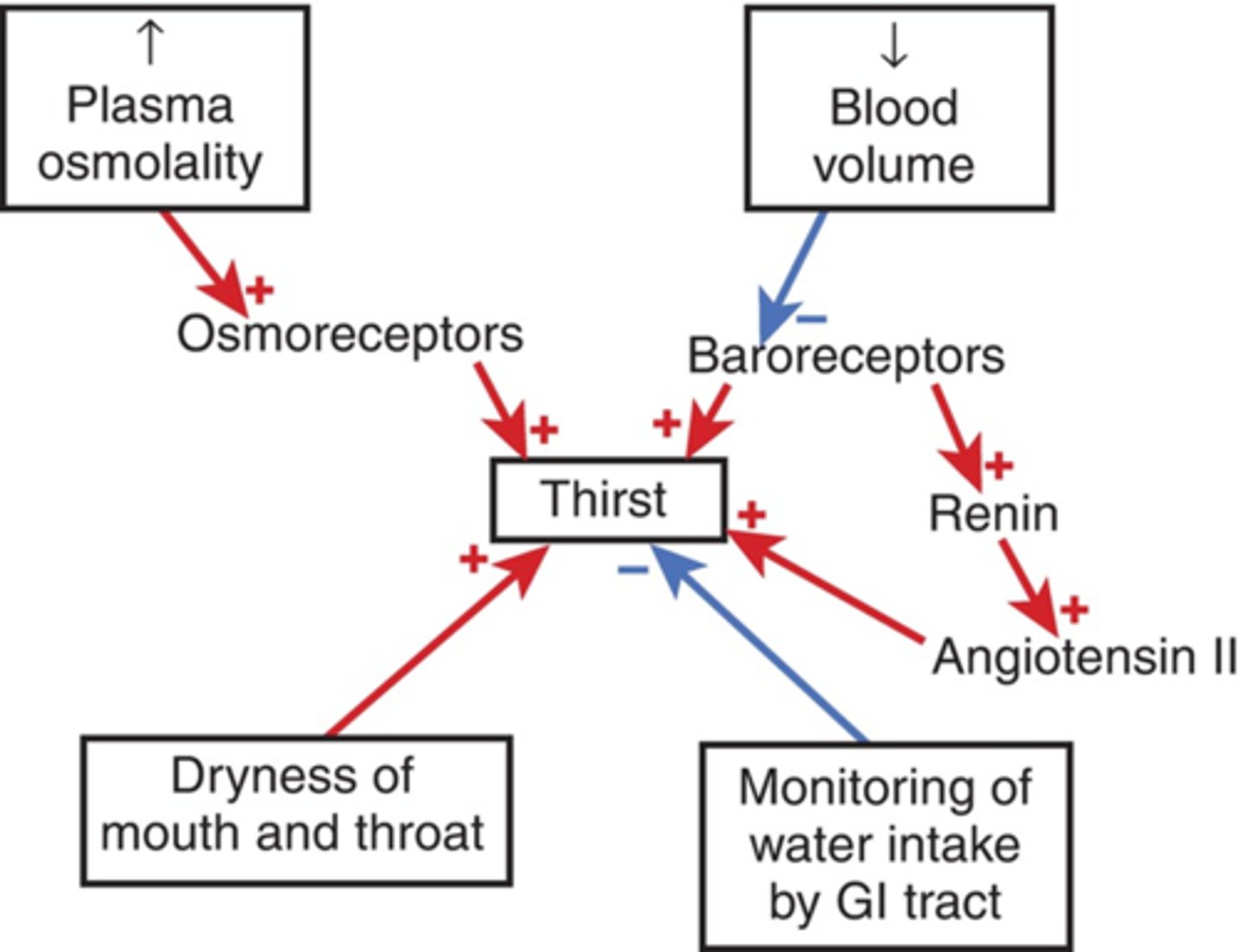
Where does ADH act?
Antidiuretic hormone: acts on collecting duct of nephron to increase water retention (also known as arginine vasopressin) (AVP)
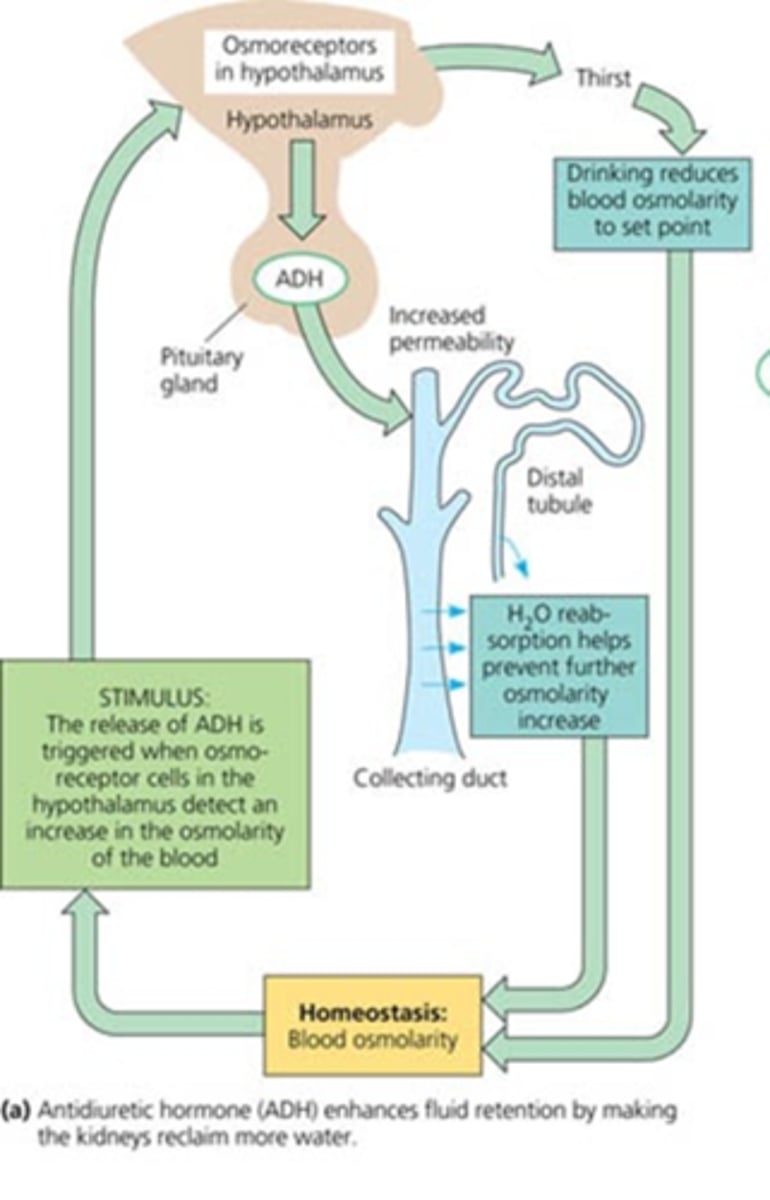
What is responsible for the release of ADH?
osmoreceptors in the hypothalamus that monitor blood osmolarity
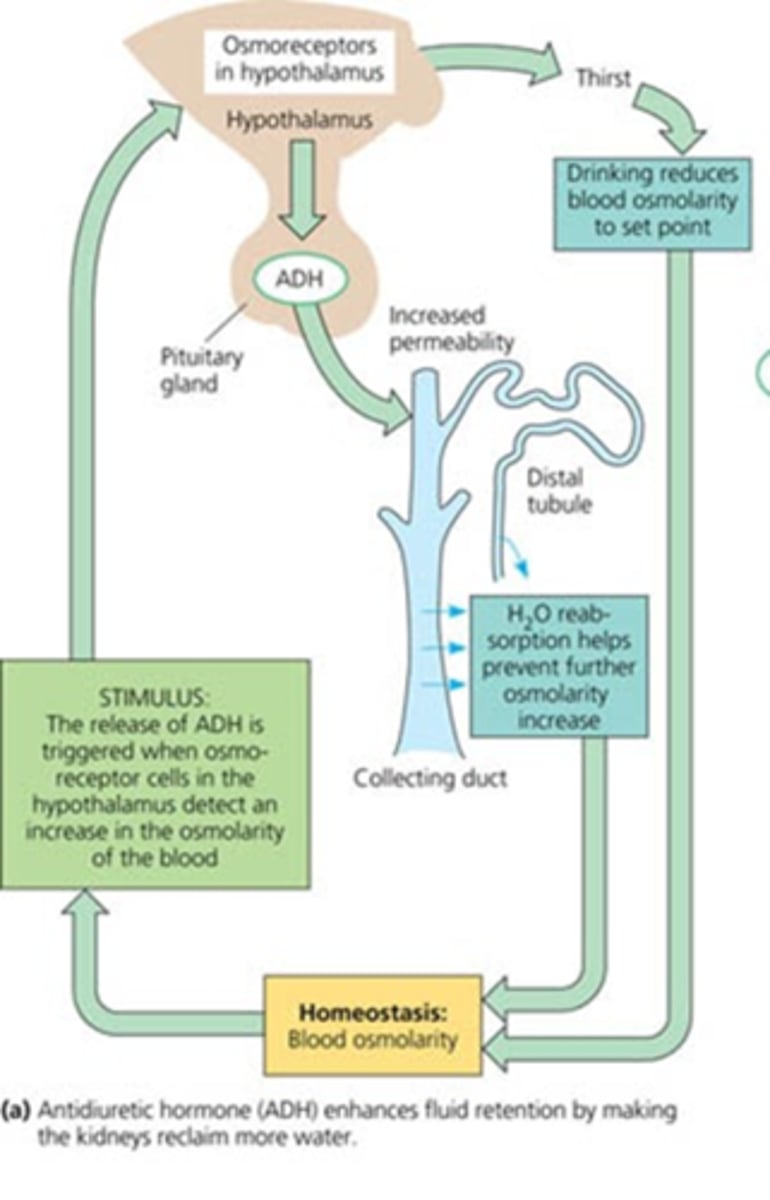
What would severe loss of blood volume stinulate?
ADH
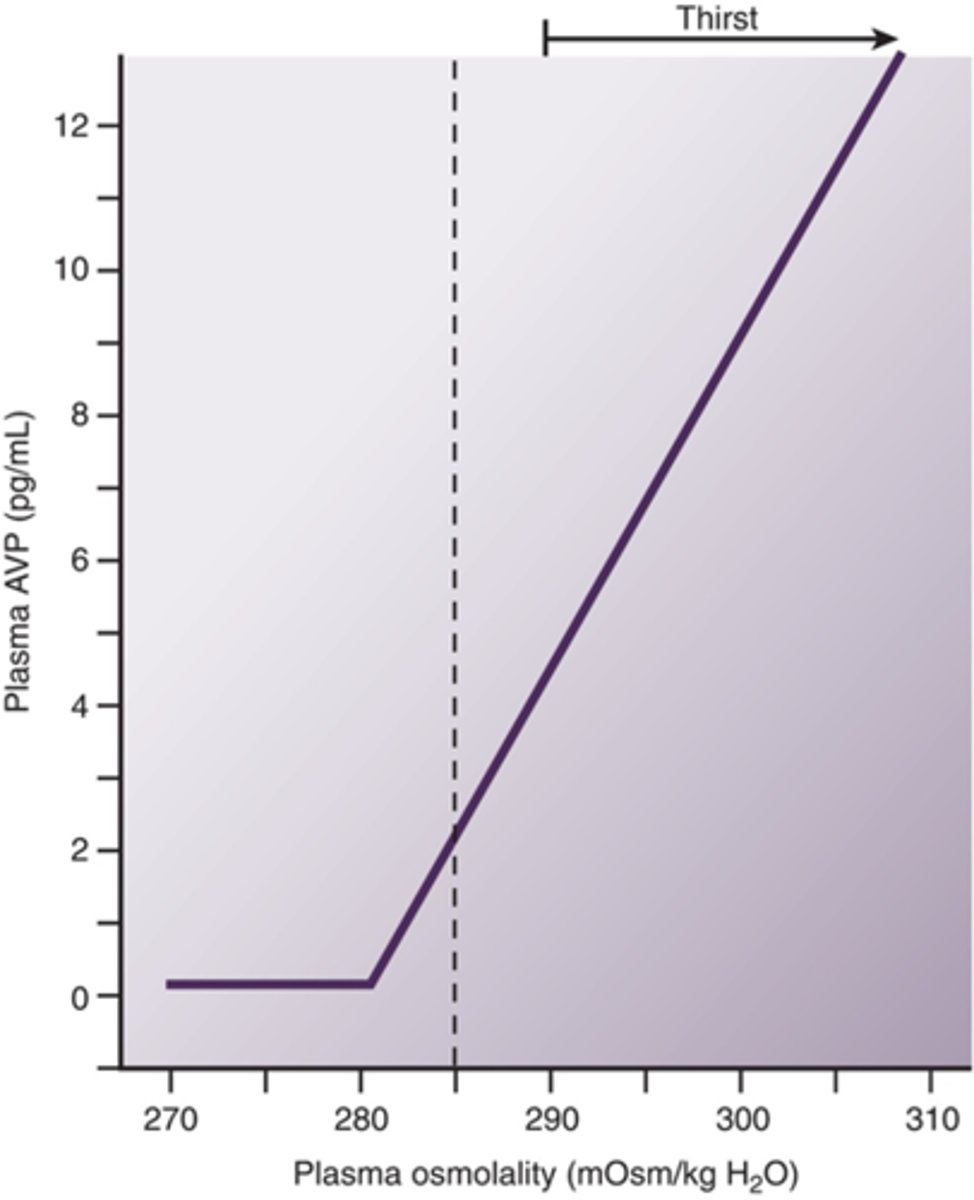
Be able to decribe the relationship between ADH and plasma osmolality
as osmolality increases ADH increases (causing thirst)
Describe the releationship between ADH and urine osmolality
urine osmolality increased as ADH increases (more concentrated urine as water is being conserved)
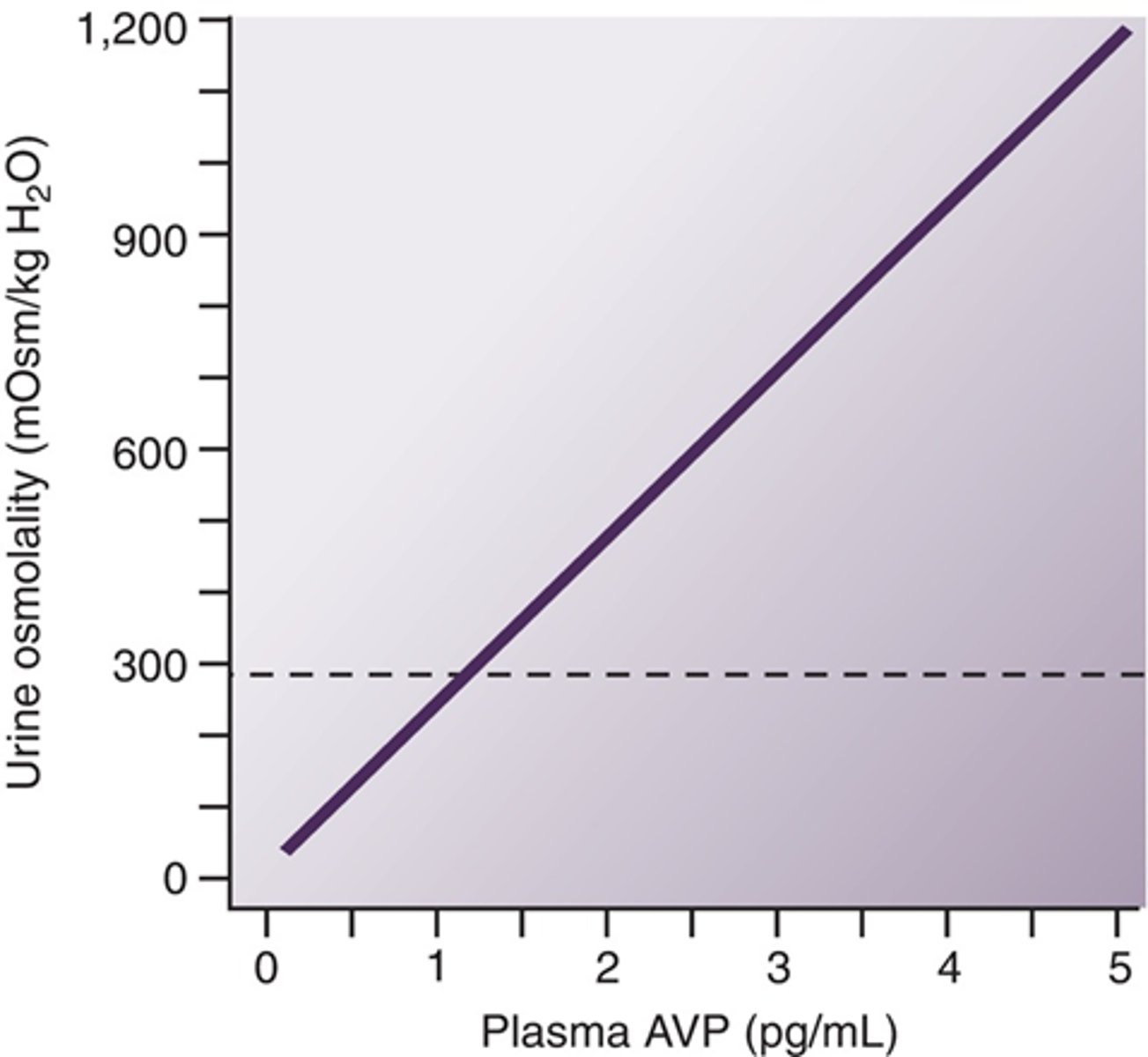
What are the two osmolalities that can be correlated with ADH?
plasma and urine (causing thirst and urine retention when high)
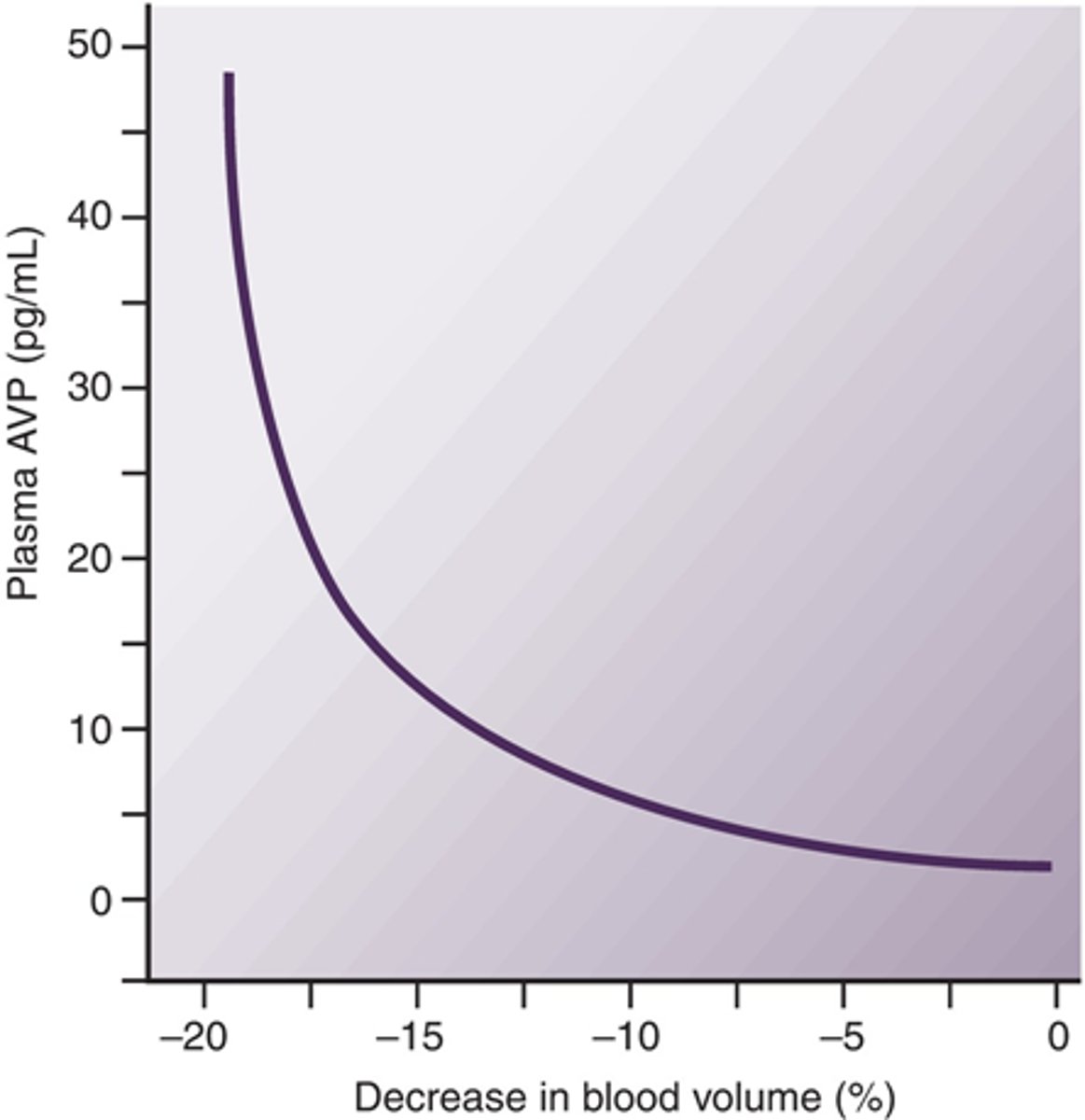
Describe the relationship between blood loss and ADH
ADH increases as blood cvolume decreases
Be able to draw the feedback mechanisms and receptors of water homeostasis