Micro-- Fungi
1/157
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
158 Terms
How do fungi get their nutrients?
Fungi are heterotrophs. They secrete enzymes to break down material and then absorb it, unlike autotrophs which make their own food using photosynthesis.
What kind of people are more at risk for fungal disease?
People who are immunocompromised or have other underlying conditions are more at risk.
Can fungi cause illness without infection?
Yes. Fungi can cause allergic or toxic reactions without direct infection.
What makes fungal infections dangerous?
Many are opportunistic, and if untreated, they can have a high fatality rate.
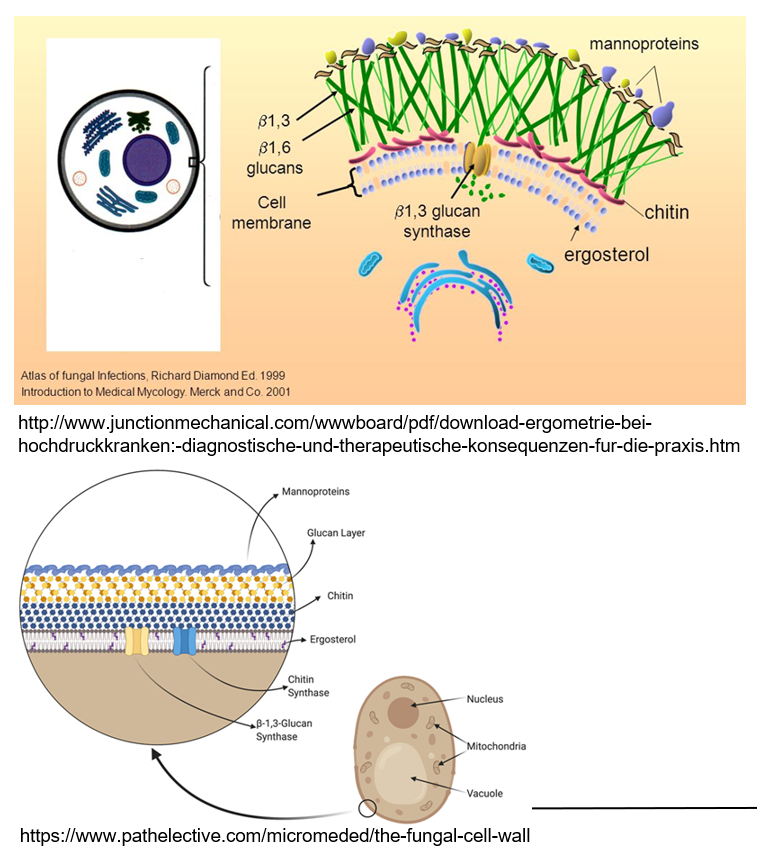
What makes fungal cells different from human cells?
Fungi are eukaryotes but belong to their own kingdom because they have special features like a rigid cell wall made of chitin and β-glucan, and they use ergosterol in their membranes instead of cholesterol.
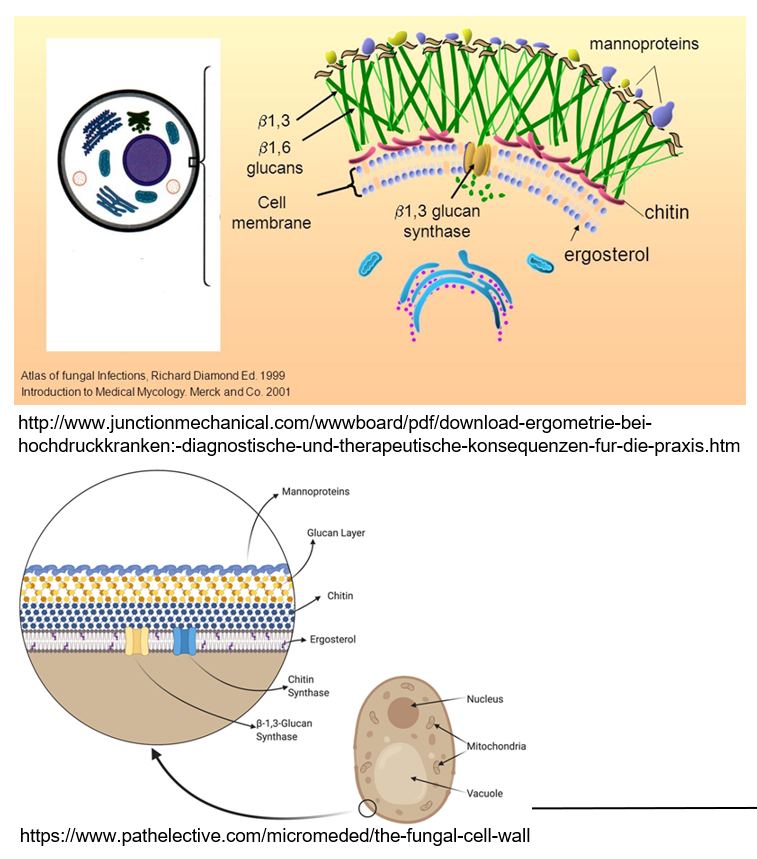
What are mannoproteins and what do they do?
Mannoproteins are found on the outside of fungal cell walls. They help make the wall stronger and protect the fungus.
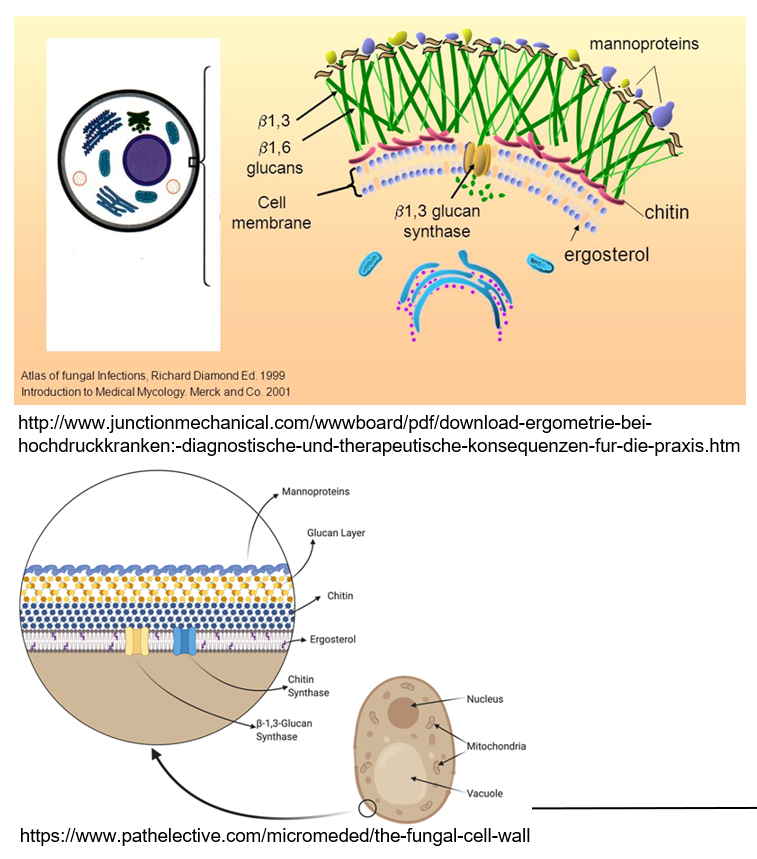
What is the role of ergosterol in fungi?
Ergosterol is a lipid in the fungal cell wall that helps maintain the structure, similar to how cholesterol works in human cells.
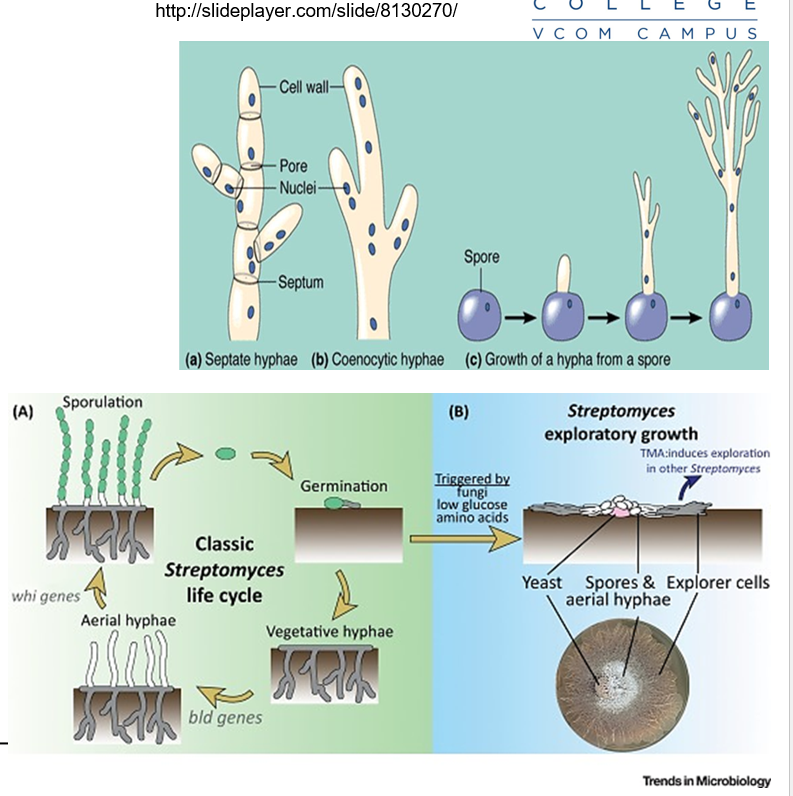
What are hyphae in fungi?
Long, thread-like structures that grow at their tips. They are the basic building blocks of many fungi and help them grow and spread.
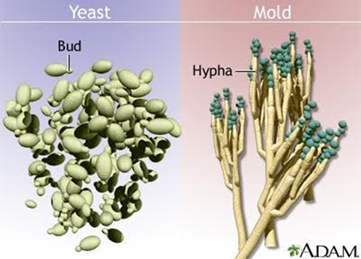
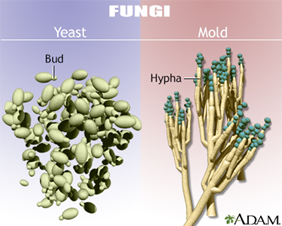
What are the differences between yeast and mold in fungi?
Yeasts are single-celled fungi that reproduce by budding; a small part of the cell pinches off to form a new one. These buds may form blastoconidia or stretch out to form pseudohyphae.
Molds are multicellular fungi that grow in long threads (hyphae). They reproduce by fission and hyphae extension, often looking fuzzy or hairy. They form conidia (spores) on structures called conidiophores.
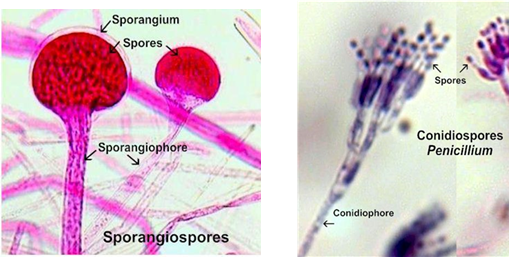
What are dimorphic fungi and what affects their form?
Dimorphic fungi can switch between two forms:
Yeast form at 37°C or in enriched environments
Mold form at 25–30°C
This change is temperature-dependent and can also be triggered by the nutritional environment.
How do fungi reproduce?
Through sporulation, which can be:
Sexual (via meiosis)
Asexual (via mitosis), producing:
Sporangiospores: asexual spores contained within a sac (sporangia)
Conidia: asexual spores that are not enclosed in a sac
What are dematiaceous fungi and where are they found?
Fungi that naturally produce pigments. They are typically found in humid, tropical environments and grow in soil and decaying vegetation.
How are dematiaceous fungi transmitted?
Through wounds in the skin after contact with contaminated soil or plant material.

How do fungi reproduce asexually?
Asexually by mitosis, where a single cell divides to make new cells. Examples include budding, forming hyphae, and producing spores.
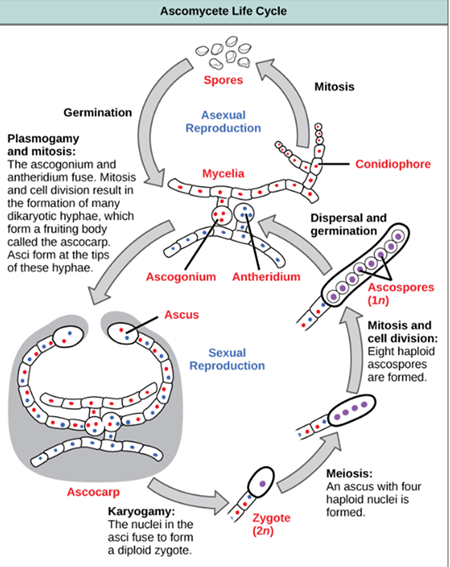
How do fungi reproduce sexually?
Fungi exchange genetic material and undergo meiosis. This includes processes like conjugation and the formation of ascospores.
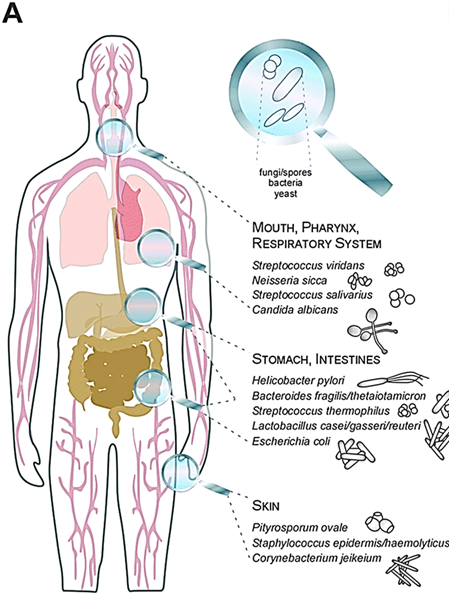
What are the major routes through which fungi can enter the body?
inhalation (breathing in spores)
dermal exposure (contact or trauma to skin)
iatrogenic means (medical procedures like injections)
How does immune status influence the development of fungal disease?
When the immune system is weakened (by drugs, infections like HIV, or genetics) or if skin barriers are breached, fungi can enter deeper tissues, leading to disease, even if the person was only colonized initially.
How do azole derivatives like miconazole work against fungi?
They interfere with sterol synthesis, disrupting the integrity of the fungal cell membrane.
What is the mechanism of action of polyene macrolides like amphotericin B, and what are their side effects?
Polyene macrolides bind to ergosterol in fungal membranes, forming pores that cause ion leakage.
Amphotericin B is effective but can cause cellular toxicity, especially nephrotoxicity, because it may also affect sterols in human cells.
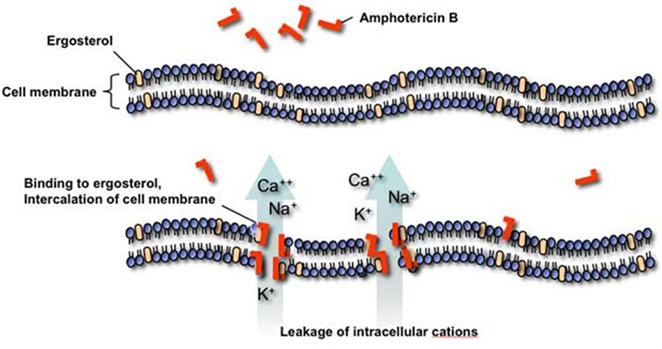
Why do anti-fungal drugs cause side effects in humans?
Fungi are eukaryotes, just like humans, so anti-fungal drugs can accidentally affect human cells due to cross-reactivity.
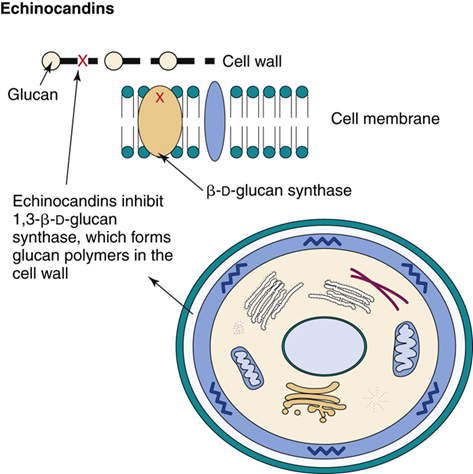
How do echinocandins work against fungal infections?
They stop fungal growth by blocking β-glucan synthesis, which is essential for building the fungal cell wall.
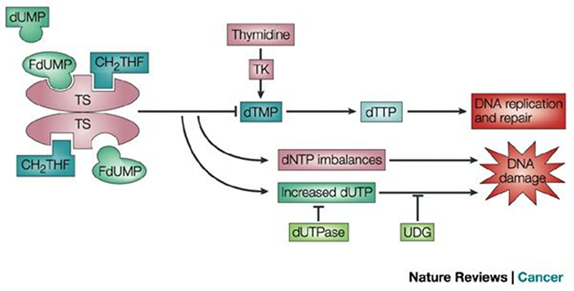
What is the role of 5-fluorocytosine (5-FU) in antifungal treatment?
It’s an antimetabolite that blocks DNA and RNA synthesis by inhibiting the enzyme thymidylate synthase, making it toxic to fungal cells.
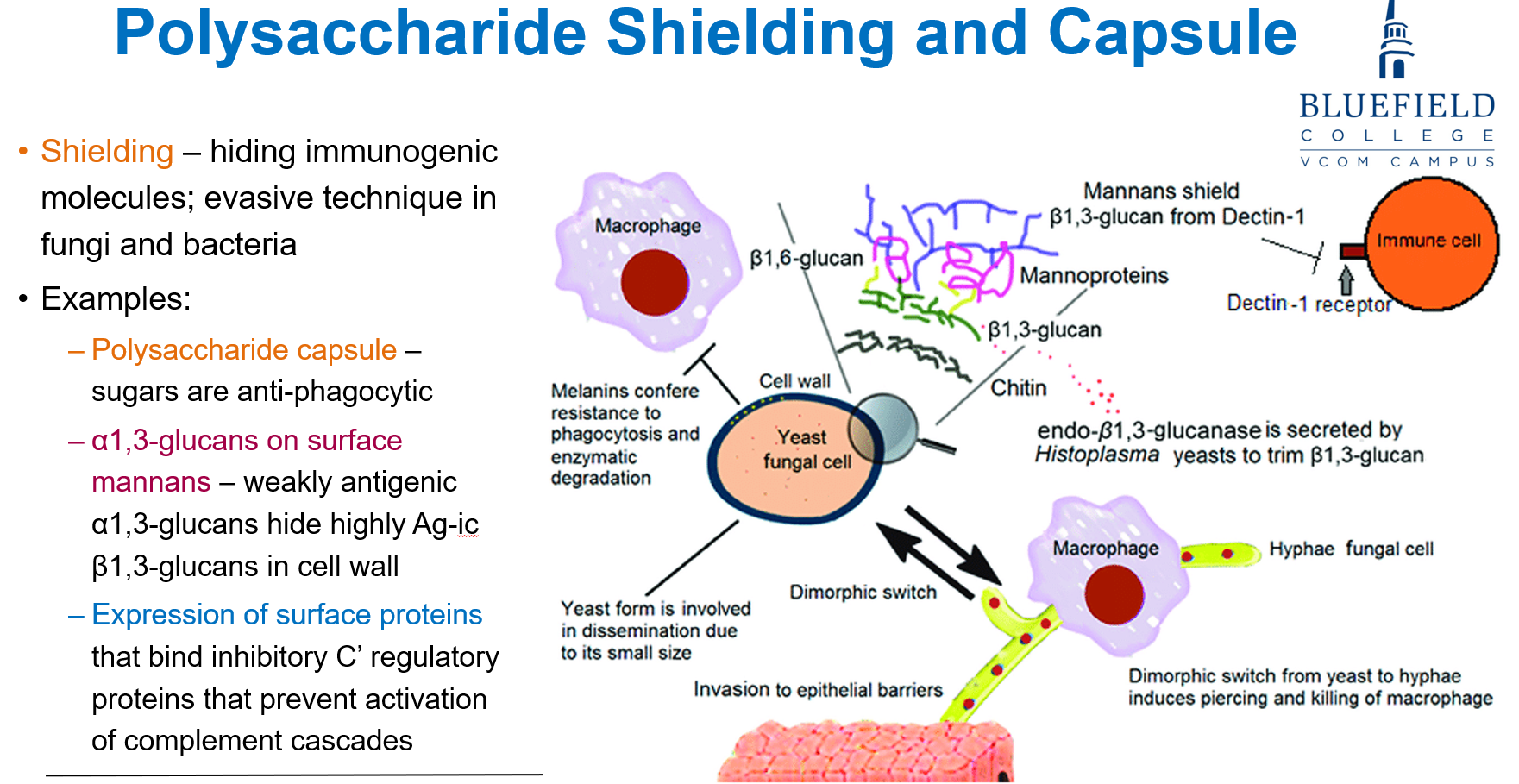
What is polysaccharide shielding in fungi and why is it important?
It’s a method fungi use to hide immunogenic molecules, helping them evade immune detection by host cells like macrophages.
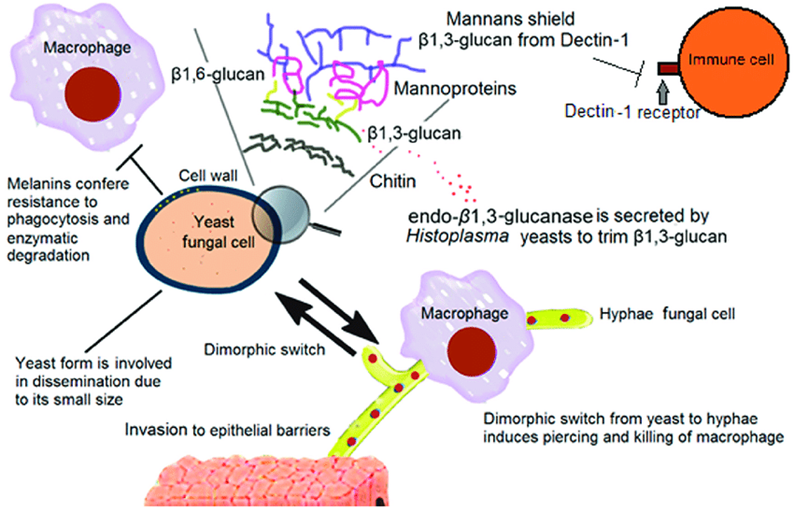
What are some key fungal components involved in immune evasion/polysaccharide shielding?
Polysaccharide capsule: contains sugars that are anti-phagocytic
α1,3-glucans on mannans: hide antigenic β1,3-glucans in the cell wall
Surface proteins: bind complement inhibitors to block immune responses
How do fungi adhere to host tissues and what are the consequences?
Fungi use adherence to bind host proteins like fibronectin and C-type lectins to anchor themselves at mucosal surfaces.
This helps them enter the body and may alter host immunity.
What are fungal biofilms?
Fungi can form biofilms, structured communities of yeast, hyphae, pseudohyphae, and proteins.
Biofilms help fungi resist immune attack and anti-fungal drugs.
What is phenotypic switching in fungi?
When fungi spontaneously change appearance and behavior, like switching between yeast and mold forms (i.e., dimorphic fungi)
It helps them adapt quickly to the environment and evade the immune system.
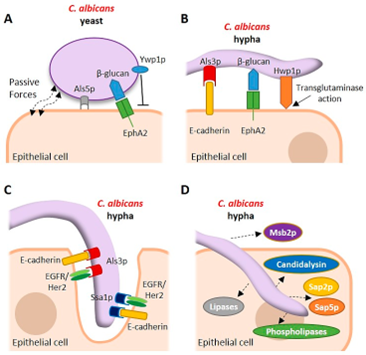
How does phenotypic switching virulence factors?
It alters surface proteins, changing antigens and functions like attachment. This boosts fungal survival, invasion, and immune evasion
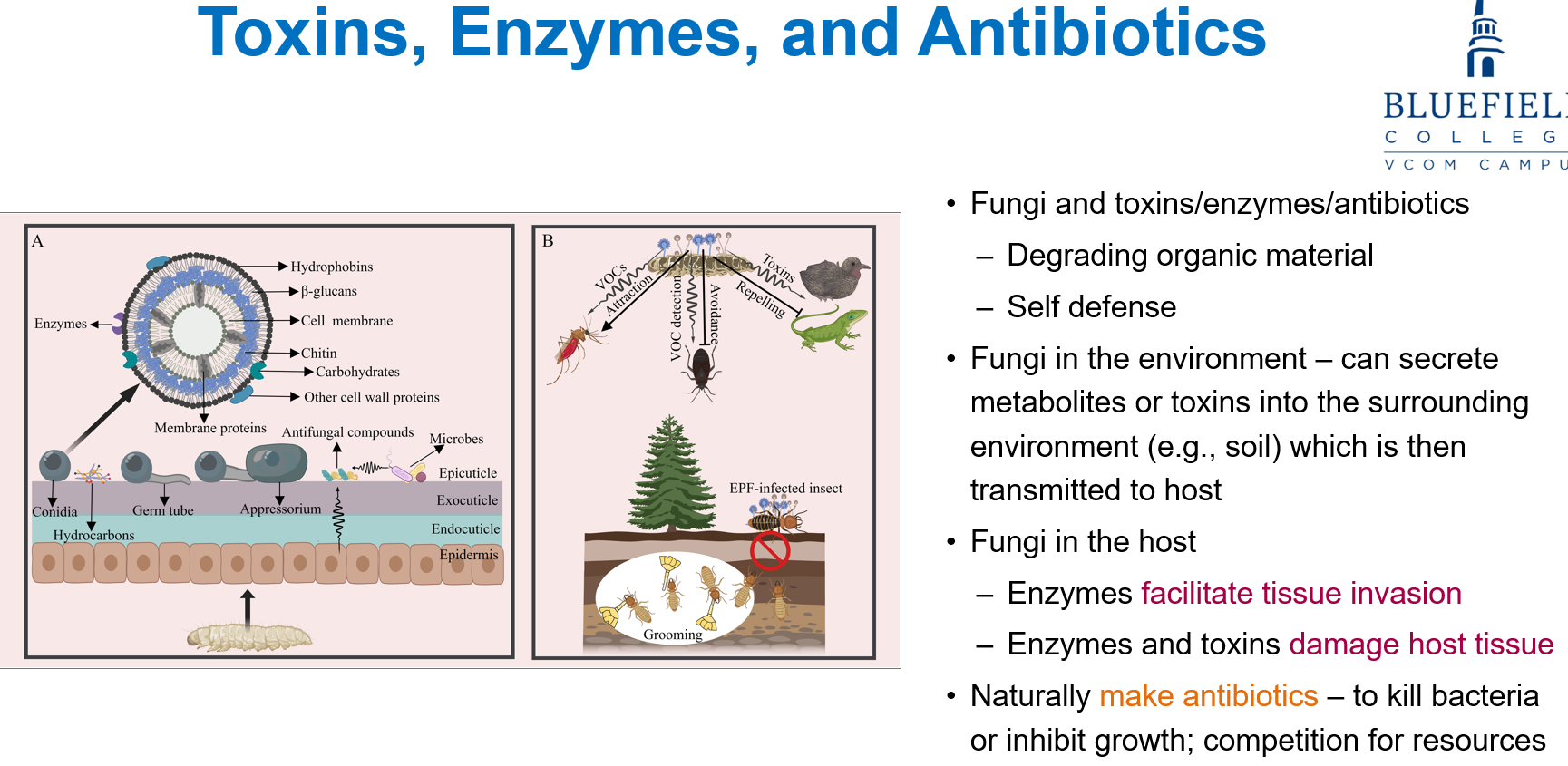
How do fungi affect host tissue and bacteria?
In hosts, fungal enzymes help tissue invasion and both enzymes and toxins damage host tissue.
Fungi also naturally make antibiotics to kill or block bacterial growth, helping them compete for resources.
How does the host immune system respond to fungi?
The immune system fights fungi using
Antimicrobial peptides from epithelial cells
PMNs (phagocytes that release ROS and antifungal agents)
Macrophages (cytokine release),
CD4 T helper cells.
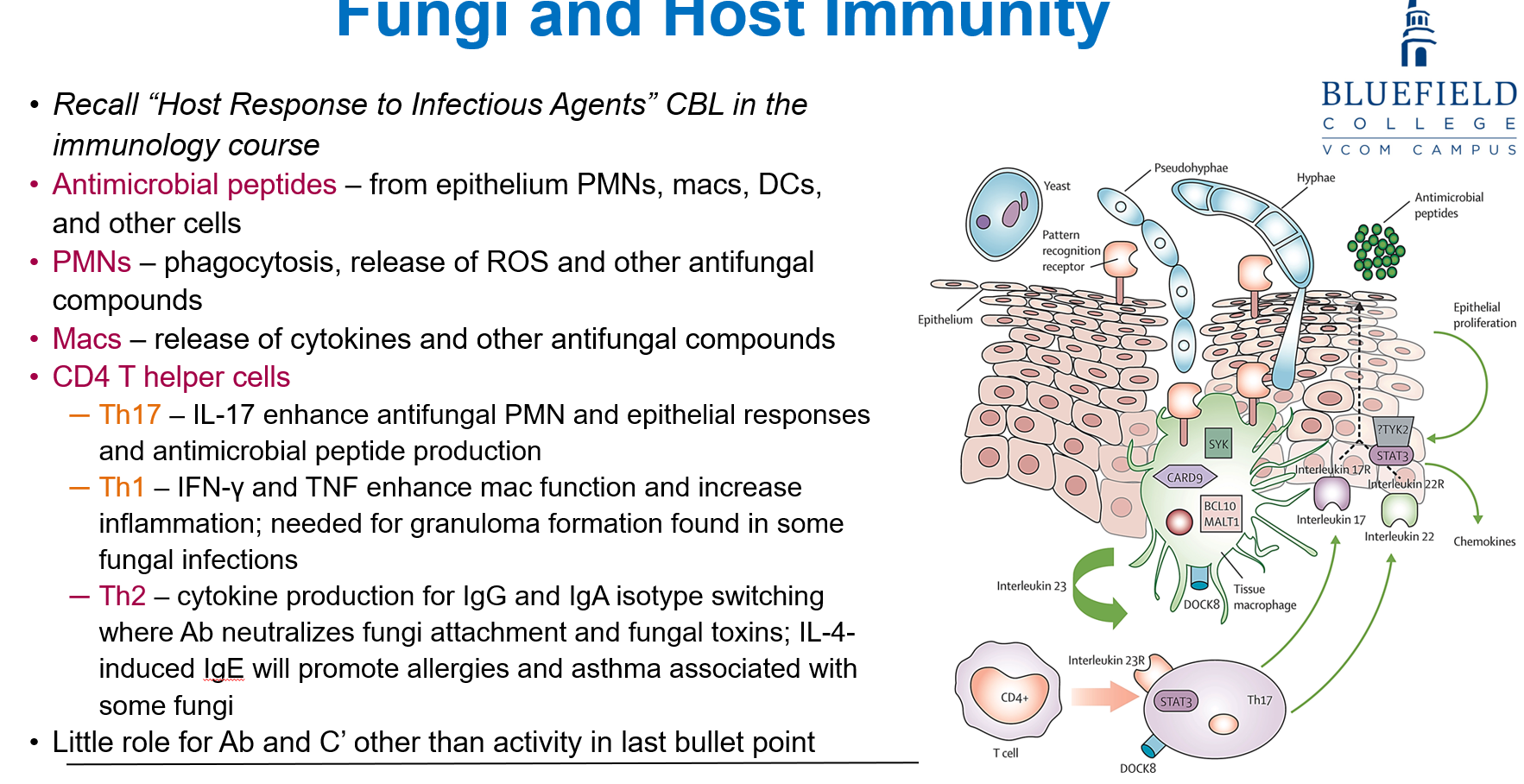
What roles do Th17, Th1, and Th2 cells play in fungal immunity?
Th17 boosts PMN and epithelial responses via IL-17.
Th1 produces IFN-γ and TNF, helping macrophages and forming granulomas.
Th2 helps antibody switching (IgG/IgA), and IL-4-induced IgE can worsen allergies/asthma related to fungi.
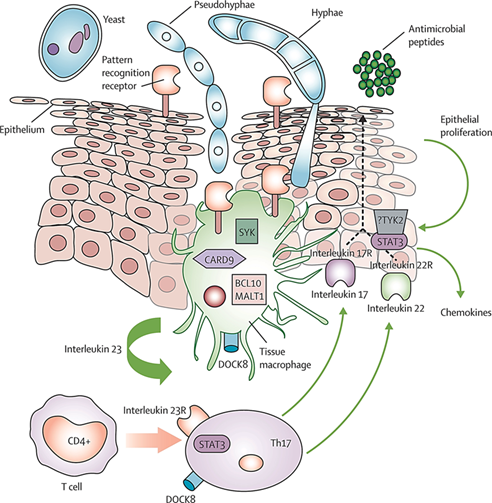
How do fungi evade the host immune system?
Blocking immune cell activation: They produce proteins that stop immune cells from turning on.
Changing surface antigens via phenotypic switching, forcing the immune system to keep adjusting.
Using molecular mimicry, where fungi look like host cells to avoid detection.
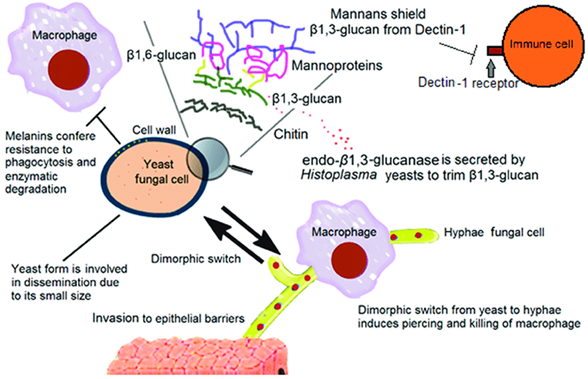
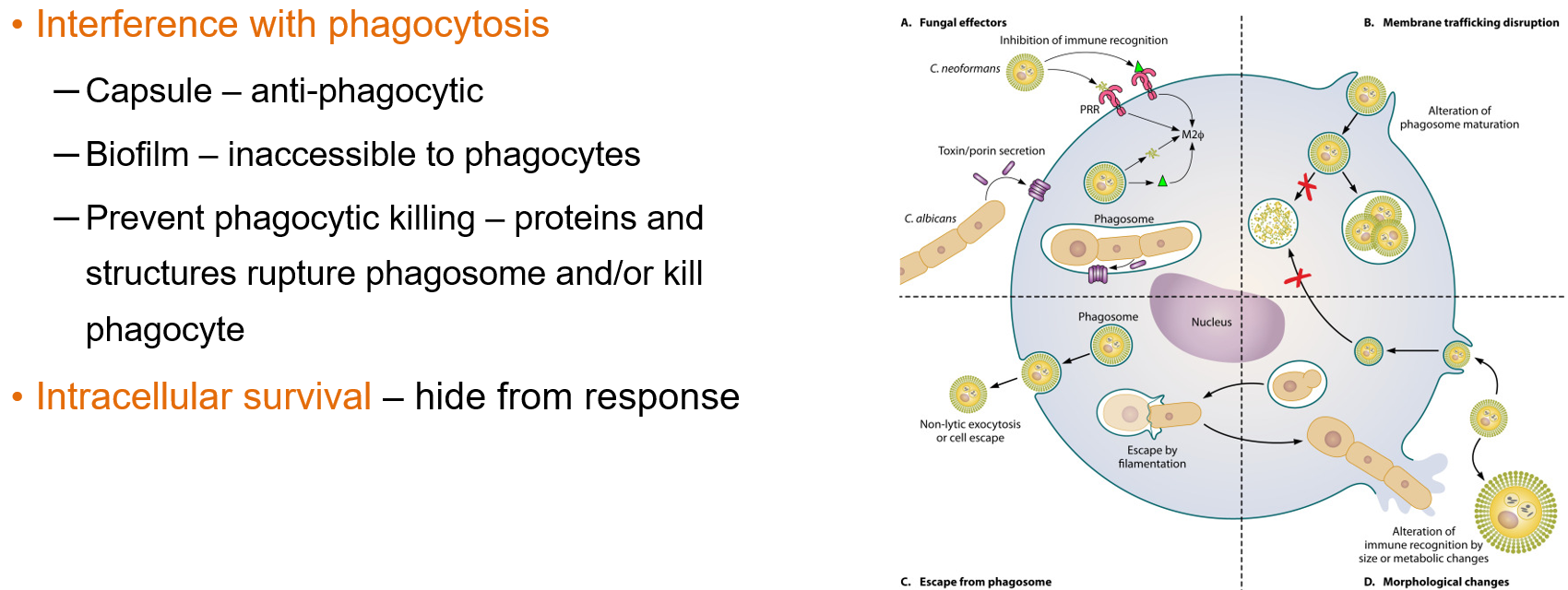
How do fungi evade phagocytosis and survive inside host cells?
Using a capsule or forming biofilms that prevent phagocytes from engulfing them
Releasing proteins that rupture the phagosome or kill the phagocyte
Surviving inside cells (intracellular survival), which allows them to hide from the immune response
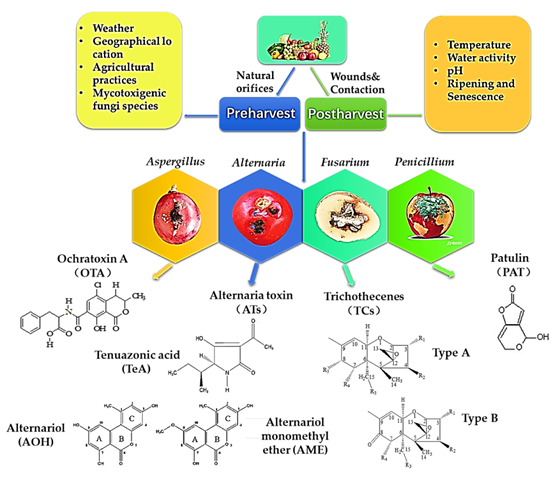
What are fungal mycotoxins and how do they affect humans or animals?
Mycotoxins are toxic secondary metabolites made by environmental fungi like Aspergillus, Penicillium, and Fusarium.
They contaminate crops during growth, harvest, or storage, and can cause serious disease when ingested (e.g., aflatoxin from Aspergillus flavus in peanuts and corn).
How is mycotoxicosis different from a fungal infection?
Mycotoxicosis is not caused by infection, but by eating or inhaling toxins made by fungi (especially in contaminated food).
These toxins are made best at 20–30°C, not body temperature, so little is made in vivo.
It’s not spread person-to-person, unlike fungal infections.
How are mycotoxins most commonly transmitted?
Mainly by eating contaminated food (e.g., grains, peanuts).
Other routes include inhaling spores or particles and skin exposure, but not through direct contact with others.

What are the pros and cons of diagnosing fungal infections with microscopy?
Pros:
Distinct morphology helps quickly identify fungi
Fast (~1 hour) and cost-effective
Can help guide culture choices
Cons:
Often only identifies the genus, not the species
What stains are commonly used for fungal microscopy?
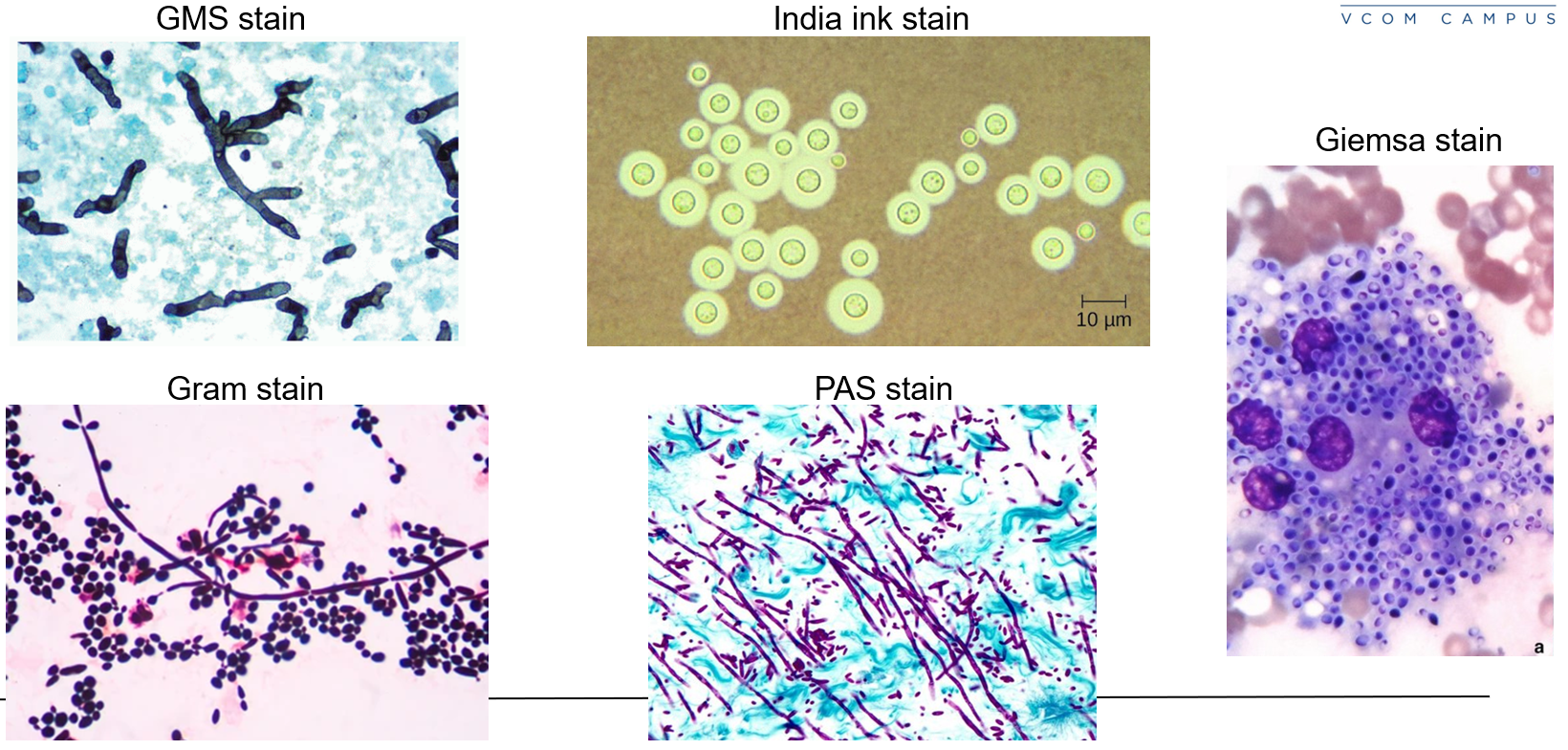
Calcofluor white, Gram, H&E, Giemsa, and immunofluorescence
Gomori methenamine silver (GMS) and India ink for more detail
Potassium hydroxide (KOH) prep for skin scrapings
Giemsa, GMS, India Ink
What are the advantages of using culture for diagnosing fungal infections?
More sensitive than microscopy
Allows species-level identification
Enables antifungal sensitivity testing
What are the main challenges with fungal culture diagnosis?
Slow fungal growth can delay diagnosis (days to weeks)
Bacterial overgrowth can outcompete fungi unless antibiotics are added to the media
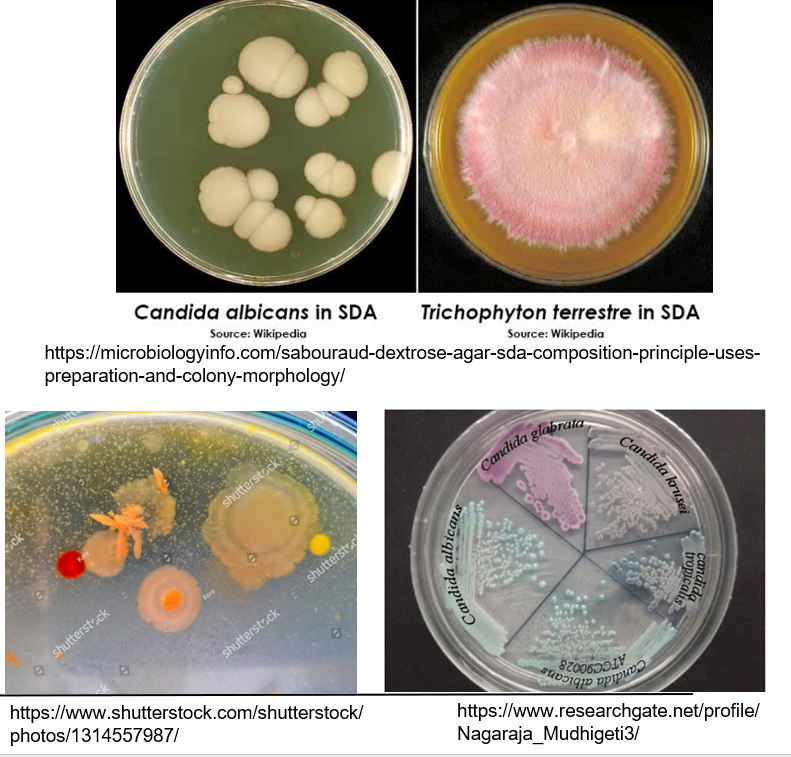
What media are used in fungal culture?
General media for bacteria (fungi just grow slower)
Sabouraud dextrose agar*
CHROMagar (e.g., for identifying Candida)
Media often includes antibiotics to suppress bacterial contamination*

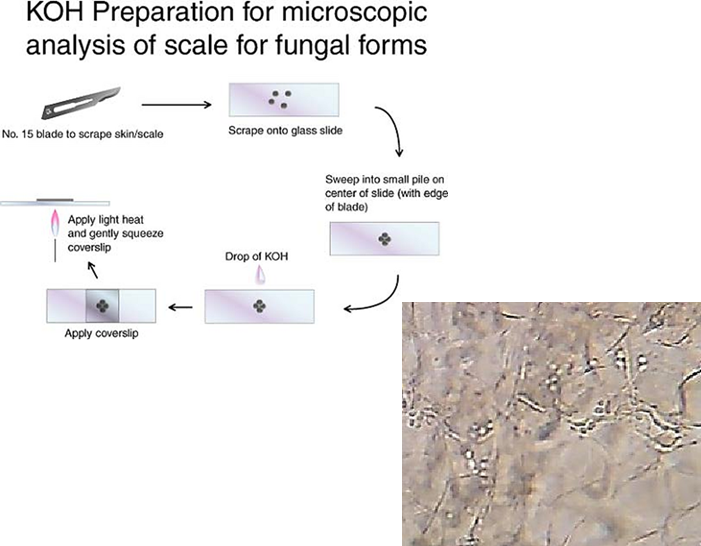
How is a KOH wet mount used to diagnose cutaneous fungal infections?
A skin scraping is taken, usually from the inner edge of a lesion.
The sample is placed on a glass slide.
A drop of potassium hydroxide is added to break down keratin and lyse other cells.
This process leaves only fungal elements visible, making it easier to identify fungi under a microscope.
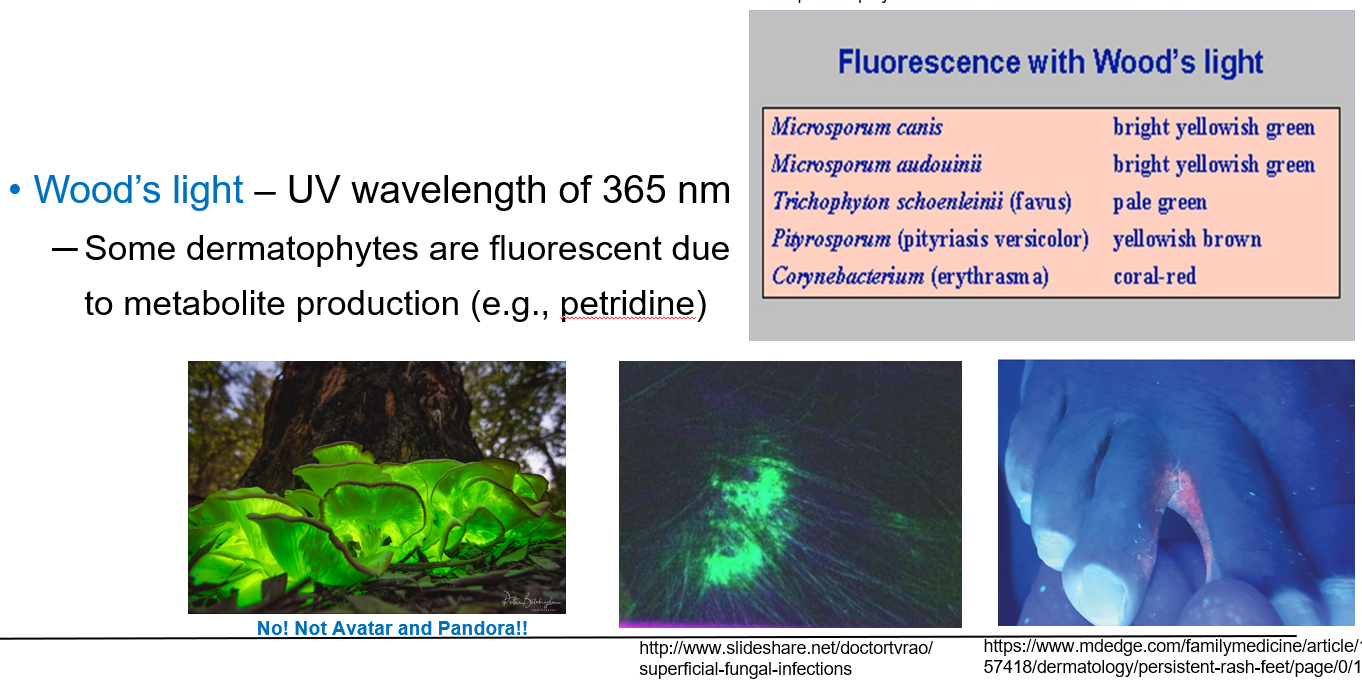
How is Wood’s light used in diagnosing cutaneous fungal infections?
Wood’s light is a UV light with a wavelength of 365 nm.
It is used to detect certain fluorescent dermatophytes on the skin.
Some fungi glow under UV light due to metabolites like petridine.
Fungi like Microsporum canis, Microsporum audouinii, and Trichophyton schoenleinii show fluorescence and help with quick diagnosis.
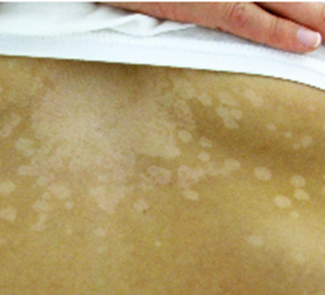
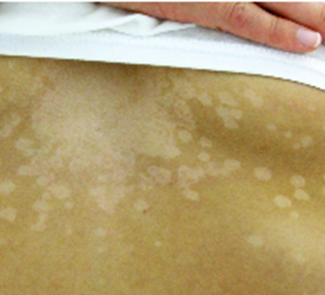
A 14-year-old girl presents to the pediatrician with a 1-week of history of pale spots on her skin. She says that she first noticed the spots after returning from summer field hockey camp and is concerned because they do not seem to be the same shade as the rest of her skin. She enjoys sunbathing at the beach and swimming in the local lake. She denies any symptoms such as fever, pain, itching, redness, or swelling. Physical exam reveals the finding shown in the attached image. Which of the following is the most appropriate test for diagnosing this patient’s disease?
Culture of skin scraping on Sabouraud dextrose
Immunohistochemical staining of punch biopsy
Polymerase chain reaction of punch biopsy
Potassium hydroxide prep of skin scraping
Serology of blood for fungus-specific IgG
D – KOH preparation of skin scraping.
The infection is superficial with no systemic symptoms, so distractors B, C, and E are not appropriate. Culture of the skin scraping could be done, but this would take much longer and is less sensitive.
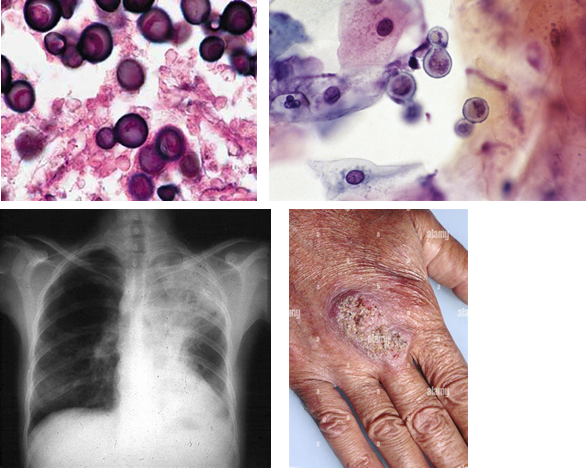
A 40-year-old male with no past medical history presents to the emergency department with a 3-day history of fevers, chills, and cough. He states that he just returned from Ohio, where he was visiting family. He is admitted to the hospital and started on intravenous antibiotics, but after 2 days he has no improvement. A bronchoscopy is performed, and cultures reveal an organism with broad-based budding. Which of the following is the most likely causative organism?
Aspergillus flavus
Blastomyces dermatitidis
Coccidioides immitis
Cryptococcus neoformans
Histoplasma capsulatum
B. dermatitidis. A hallmark morphologic feature of Blastomyces species is the broad base at the point of connection as a daughter cell is budding off the mother cell. The geographic reference to Ohio is also supportive of infection with B. dermatitidis.
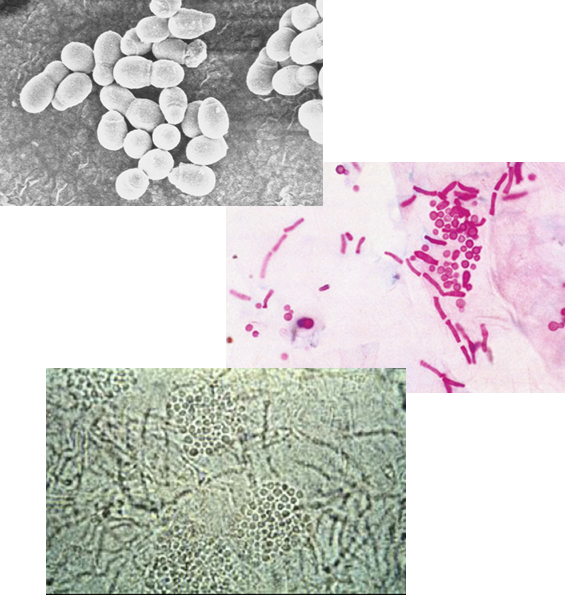
What is the morphology of Malassezia furfur under the microscope?
A budding yeast with short, branching hyphae that align end-to-end and are not a dermatophyte.
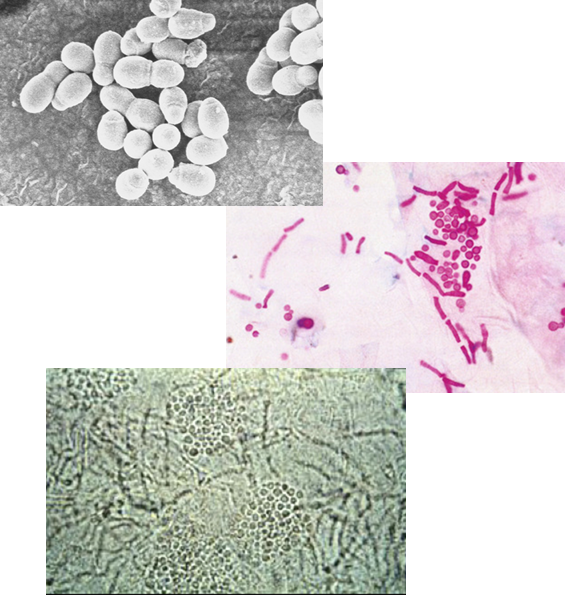
What is the hallmark appearance of Malassezia furfur in microscopy?
The presence of both hyphae and blastoconidia at the same time creates a distinctive “spaghetti and meatballs” appearance.
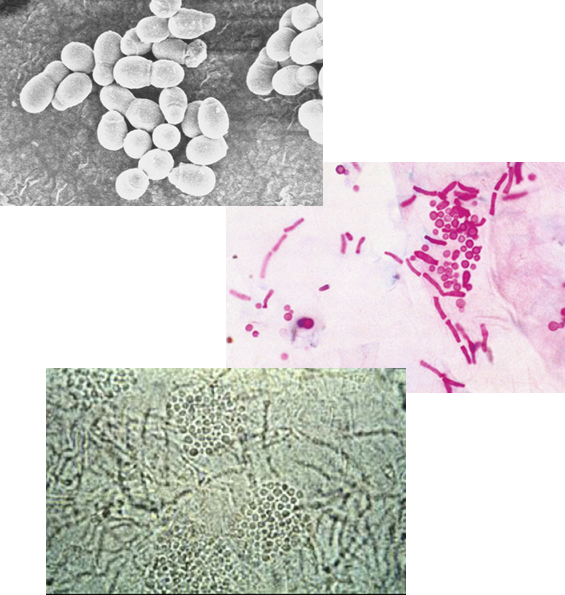
How is Malassezia furfur transmitted?
Via human-to-human transfer of keratinized tissue.
What disease does Malassezia furfur cause?
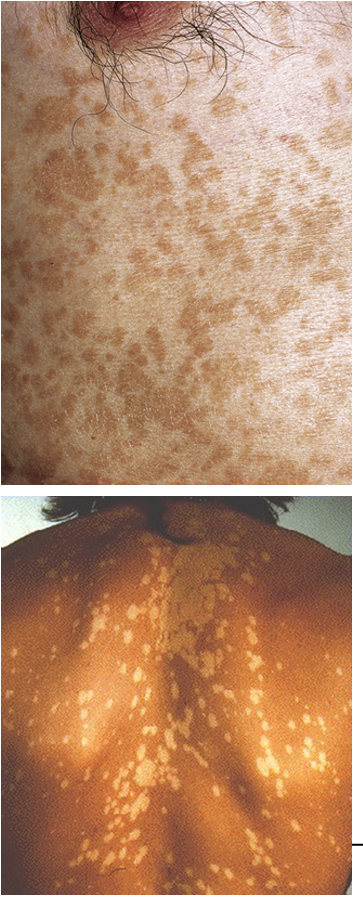
It causes tinea versicolor (also called pityriasis versicolor).
What are the symptoms of tinea versicolor?
Presents as small macules that may be hypo- or hyper-pigmented, often irregularly shaped with well-defined borders, typically found on the trunk, arms, shoulders, face, or neck. There is little to no inflammation.
What causes the pigmentation change in tinea versicolor?
The fungus requires high levels of exogenous lipids to grow.
By-products of lipid metabolism interfere with tyrosinase in melanocytes, thereby altering melanin production and skin color.
How is tinea versicolor diagnosed?
Diagnosis is based on clinical symptoms and visualization of fungi on KOH prep of skin scrapings.
Fungal elements can also be seen using histologic stains (e.g., H&E, calcofluor white) and show yellow-ish fluorescence under Wood’s lamp.

What is the cause and transmission route of tinea nigra?
Caused by Hortaea werneckii, a dematiaceous (pigmented) fungus that appears black due to melanin in its cell wall.
Where is Hortaea werneckii found?
It is found in soil or wood in warm, humid climates, mainly in Central and South America.
How is Hortaea werneckii transmitted?
Transmission occurs through injection or inoculation of fungal material into a break in the skin.
How does tinea nigra (Hortaea werneckii) present (symptoms)?
Presents as pigmented (gray to black), irregular patches, commonly on the palms of the hands or soles of the feet. It is superficial, with no inflammation, pain, or discomfort. There is no scaling or hair follicle invasion.
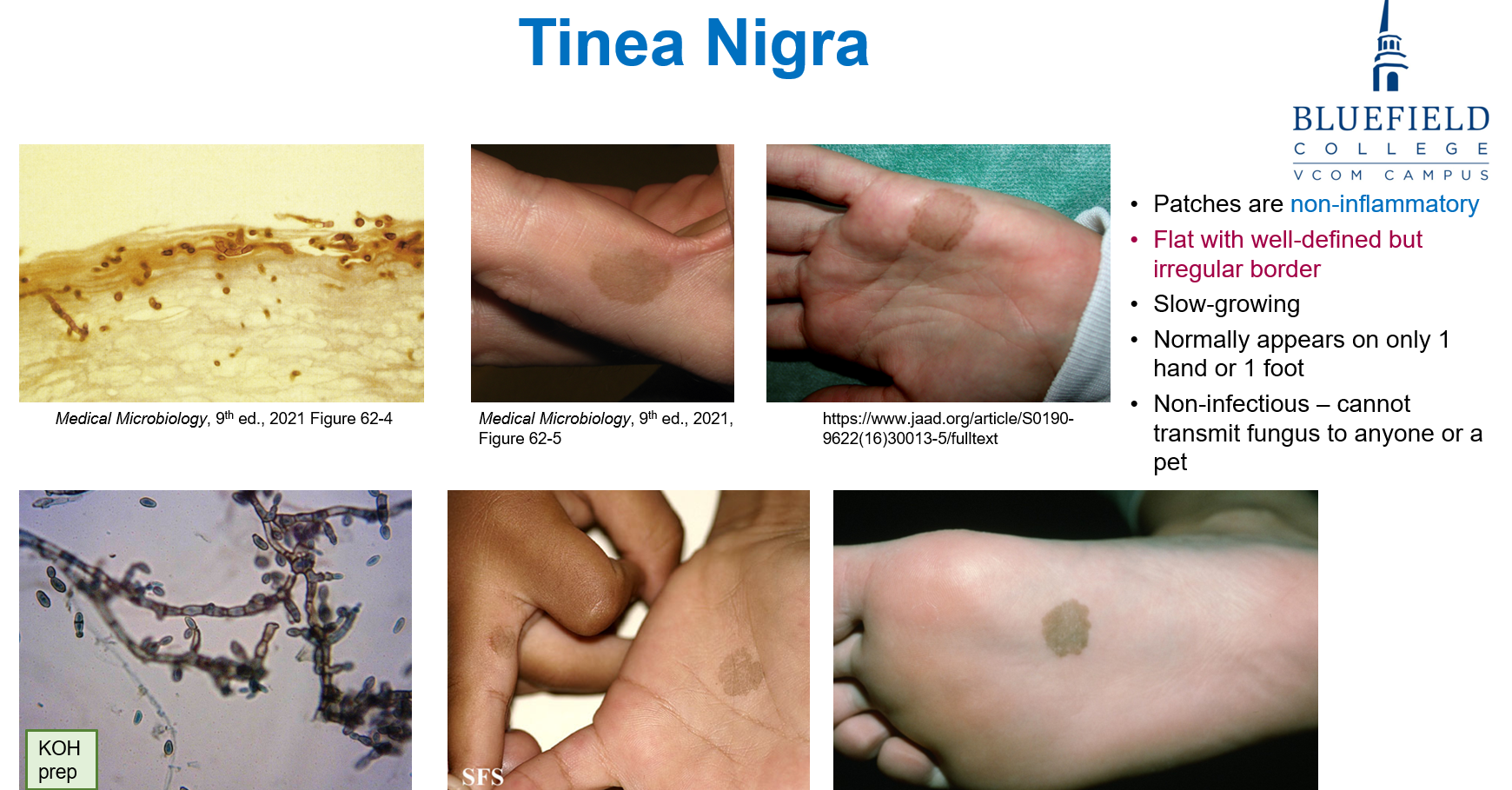
How is Hortaea werneckii diagnosed?
Diagnosis is confirmed via skin scrapings and KOH prep, followed by fungal culture.
What organisms are considered dermatophytes?
Include species of Microsporum, Epidermophyton, and Trichophyton.
These filamentous fungi form branching septate hyphae and conidia
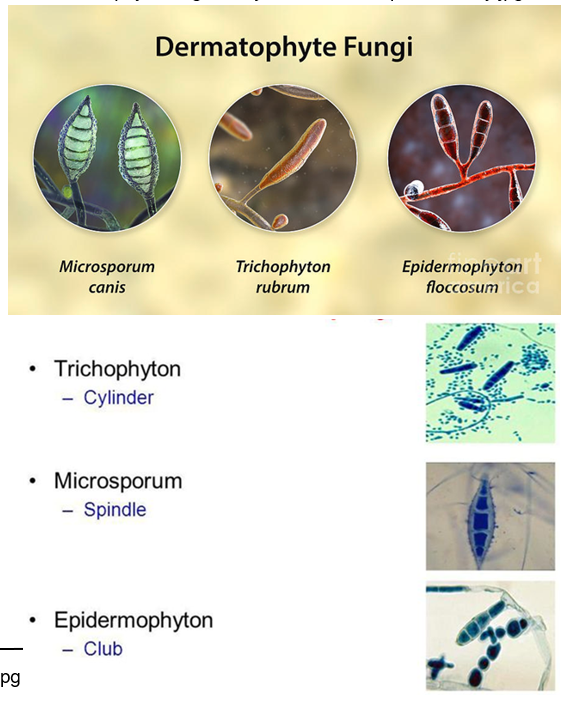
What is the transmission source of dermatophytes?
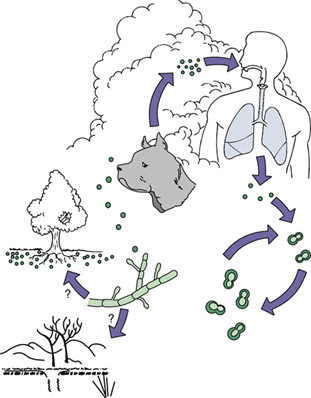
Transmitted from other humans, animals (e.g., dogs), or the environment, with all people susceptible.
What are the at risk populations for dermatophytes?
Individuals with weakened immune systems, those who frequently use public showers or locker rooms, and people who wear tight shoes or clothing.
How are dermatophytes transmitted?
Through direct contact with hyphae, conidia, or keratinized tissue containing fungal cells.
This can happen by sharing personal items (like grooming tools, clothes, hats), through animal contact (e.g., pets, livestock), or via the environment.
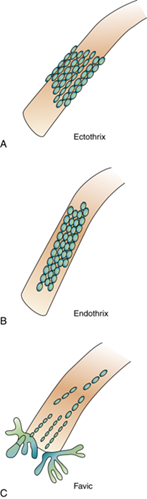
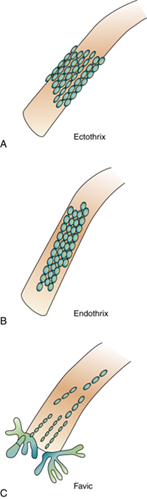
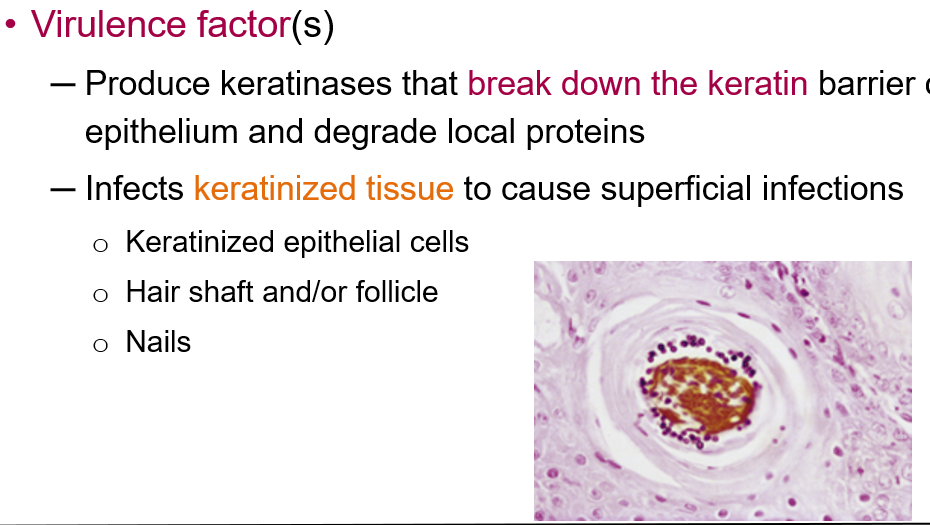
What are the virulence factors of dermatophytes?
Dermatophytes produce keratinases that break down the keratin barrier of the epithelium and degrade local proteins. They infect keratinized tissue, leading to superficial infections of:
Keratinized epithelial cells
Hair shafts and follicles
Nails
What is the disease caused by dermatophytes commonly called?
ringworm.
What are the key clinical features of tinea?
Spreads outward from the point of inoculation in concentric circles
Causes a pruritic, scaly, annular rash with well-demarcated borders
On non-hairy skin: erythema and scaly border
On hairy areas: central clearing due to hair loss
Can be itchy, leading to auto-inoculation to other sites
Infected nails may appear thickened, discolored, cracked, or misshapen
What is tinea capitis and how does it present?
Tinea capitis affects the scalp and may present as:
Black dot tinea capitis: areas of alopecia with black dots
Kerion: a boggy, pustular lesion due to an intense immune response to the fungus

What are the different forms of tinea based on location?
Tinea barbae: beard area
Tinea corporis: body (ringworm)
Tinea manuum: hand
Tinea cruris: groin ("jock itch")
Tinea pedis: feet ("athlete’s foot"), often causes maceration and fissures, especially between the toes
Tinea unguium: nails
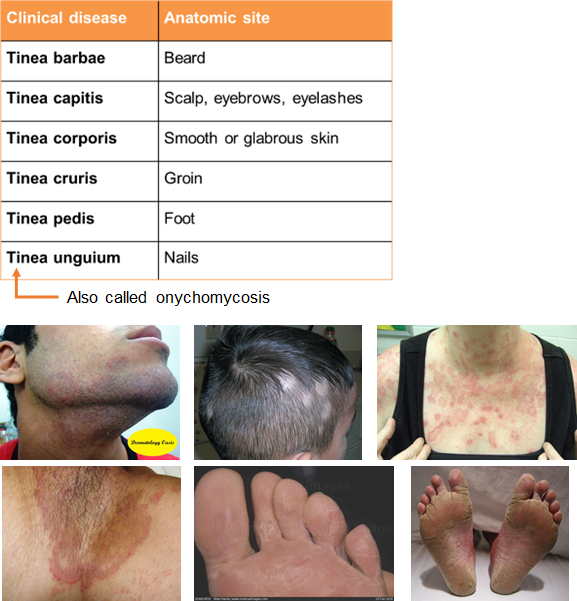
What is onychomycosis (tinea unguium) and what causes it?
A fungal infection of the fingernails or toenails, caused by:
Dermatophytes (leading to tinea unguium)
Non-dermatophyte molds or yeasts (e.g., Candida spp.)
Ony = Onyx meaning nail

What are the risk factors for onychomycosis (tinea unguium)?
Tinea pedis or other tinea infections elsewhere
Nail trauma
Immunocompromise (e.g., HIV, diabetes)
Smoking
Peripheral vascular disease

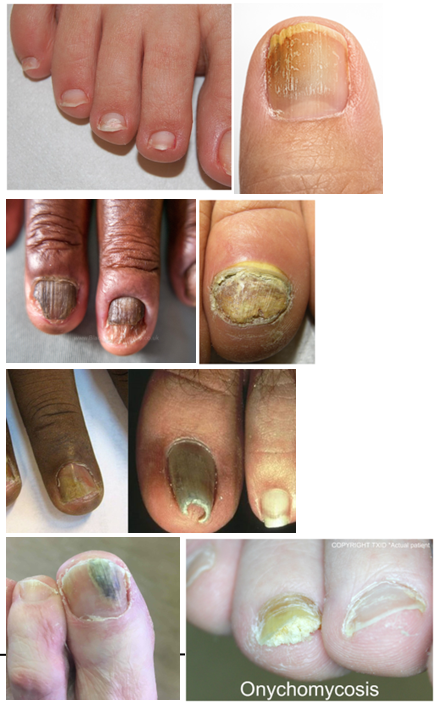
What are common presentations of onychomycosis (tinea unguium)?
Discoloration, thickening, and shape changes of the nail
Onycholysis (nail separation from nail bed)
Brittleness (onychorrhexis)

How is onychomycosis diagnosed?
By treating nail clippings or scrapings with KOH (potassium hydroxide)
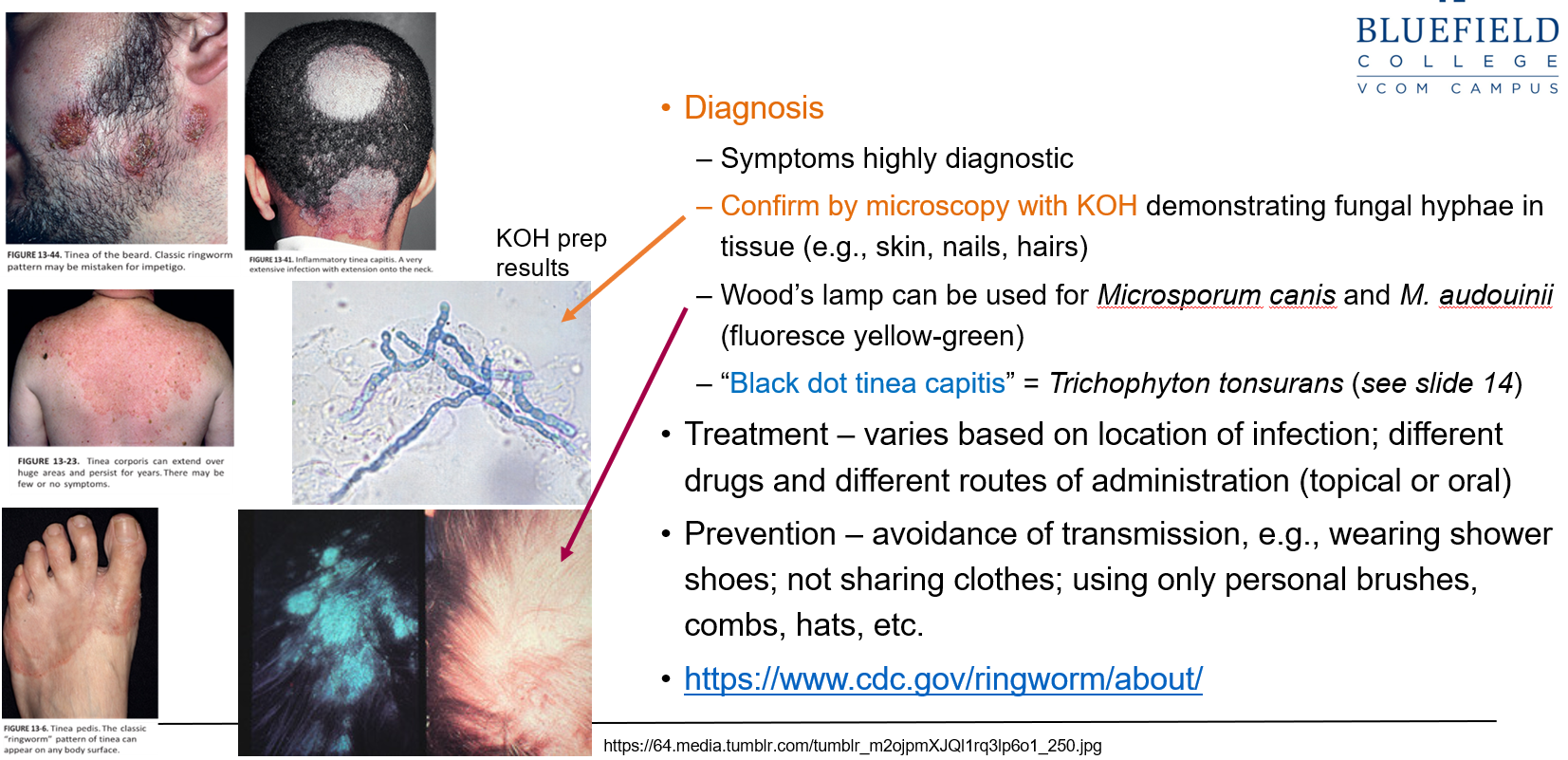
What are the primary methods used to diagnose tinea?
Symptoms are often highly diagnostic
Diagnosis is confirmed by KOH microscopy, which shows fungal hyphae in skin, hair, or nail samples
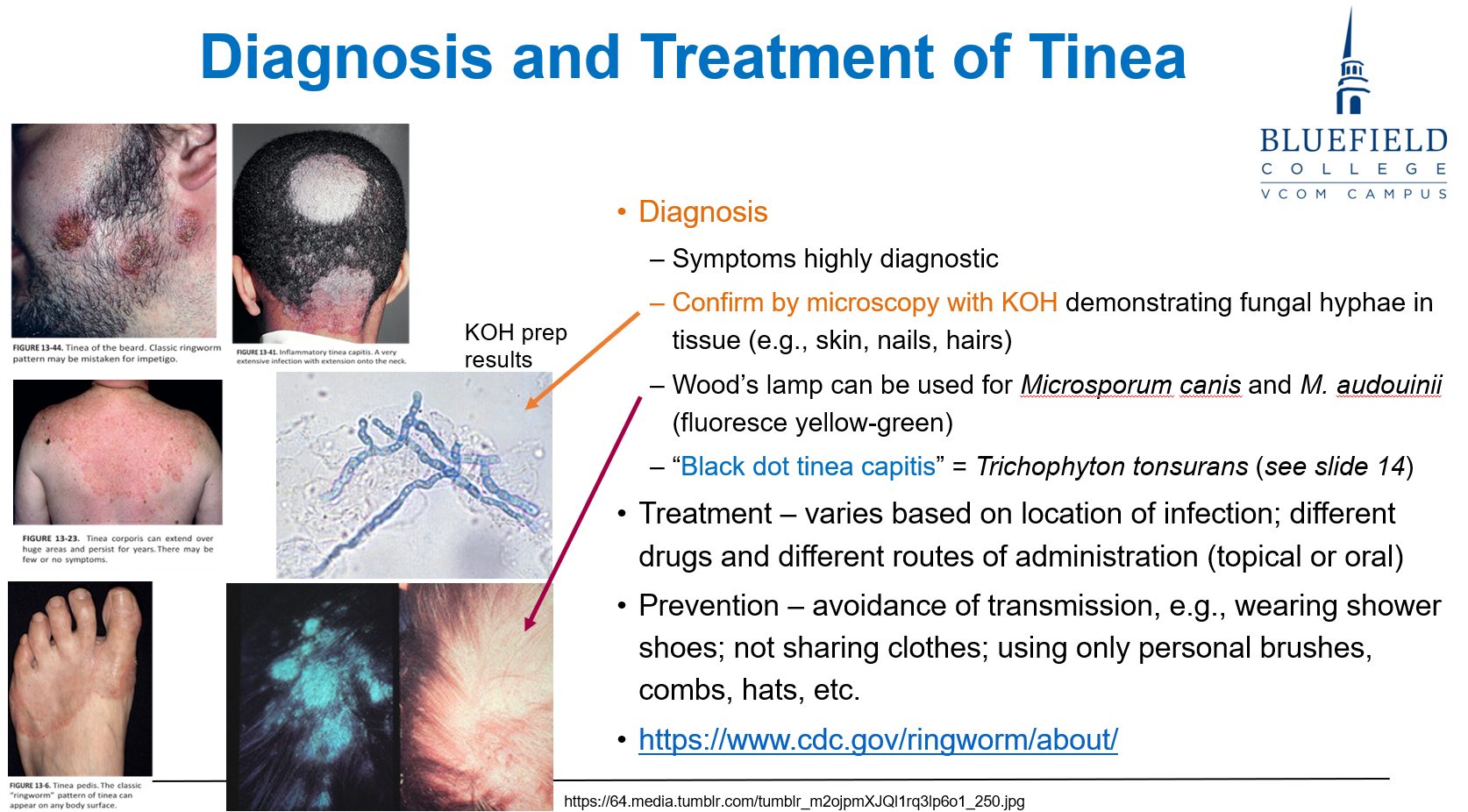
How can a Wood’s lamp assist in tinea diagnosis?
It can be used to detect Microsporum canis and M. audouinii, which fluoresce yellow-green under UV light
Where is Sporothrix schenckii commonly found in the environment?
It’s found worldwide in soil and decaying plant matter, especially sphagnum moss, rose bushes, and hay.

What occupations or activities increase risk of exposure to Sporothrix schenckii?
Exposure is common in warm climates during gardening, forest work, and mining, where skin contact with contaminated organic material occurs.

What are the two main modes of transmission for Sporothrix schenckii?
Direct inoculation (e.g., thorn injury) with contaminated soil or plant material
Zoonotic transmission via bites/scratches from animals like armadillos or wild cats (especially S. brasiliensis)

How does Sporothrix schenckii infection progress after entering the skin?
The yeast infects subcutaneous tissue, forming a lesion that ulcerates and may disseminate along lymphatic vessels.
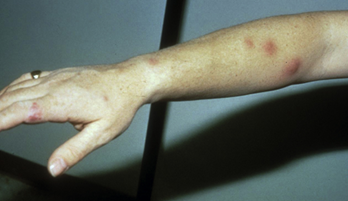
What are the two main symptom types of Lymphocutaneous Sporotrichosis (Sporothrix schenckii)?
Cutaneous: A small, painless red or purple bump that becomes an ulcer with pus, slow to heal, and may form secondary sores.
Lymphangitic: Painless subcutaneous nodules forming a linear chain along the lymphatic vessel draining the original site. Appears ~2 weeks after the first lesion.
*Lymphocutaneous Sporotrichosis is the most common clinical form of Sporothrix schenckii
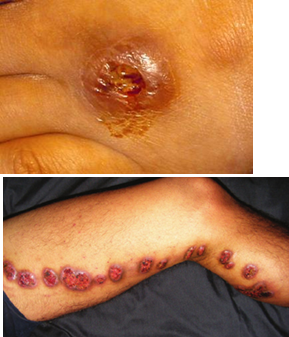
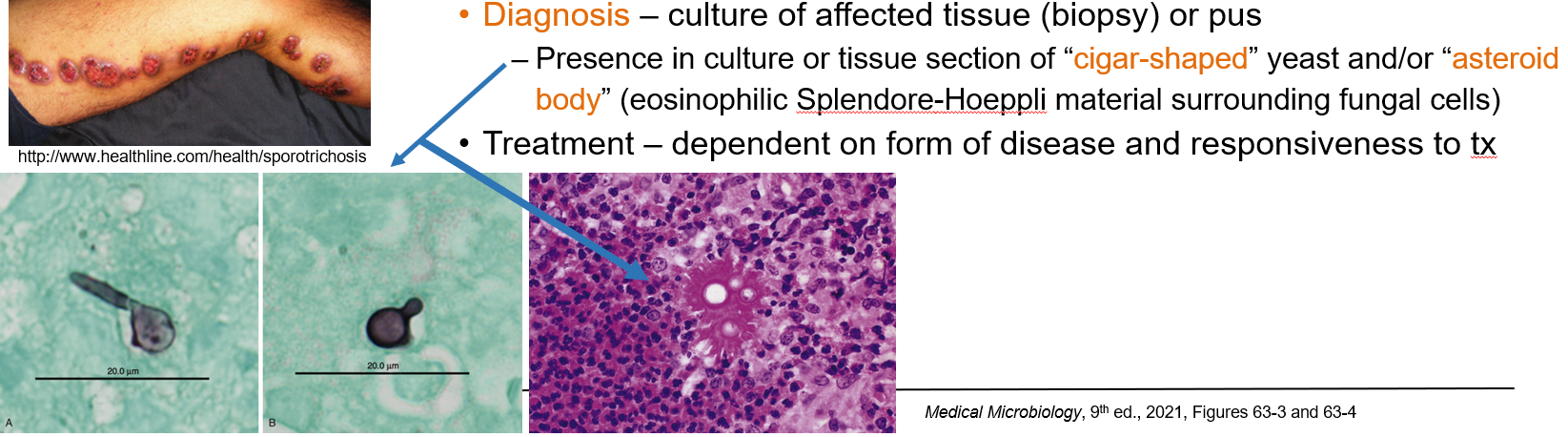
How is lymphocutaneous sporotrichosis (Sporothrix schenckii) diagnosed?
By culture of affected tissue (biopsy or pus). In tissue, look for:
“Cigar-shaped” yeast
“Asteroid body” (yeast surrounded by eosinophilic Splendore-Hoeppli material)
What is chromoblastomycosis and what causes it?
A rare infection caused by several genera of septate molds, mainly Fonsecaea.

How does chromoblastomycosis present?
Presents as a slow-growing, wart-like lesion on the legs or feet, often after trauma. It may ulcerate, crust, or show black dots. Caused by pigmented fungi; Medlar bodies seen on biopsy.
“cauliflower-like” and “warty”

Where and how is chromoblastomycosis typically acquired?
Most common in tropical, humid climates.
Transmission occurs via direct inoculation through skin, often due to lack of protective clothing/footwear in soil-rich environments.
Where are fungal cells found in chromoblastomycosis?
Fungal cells are usually found inside macrophages or giant cells, though they can also be extracellular in tissue.
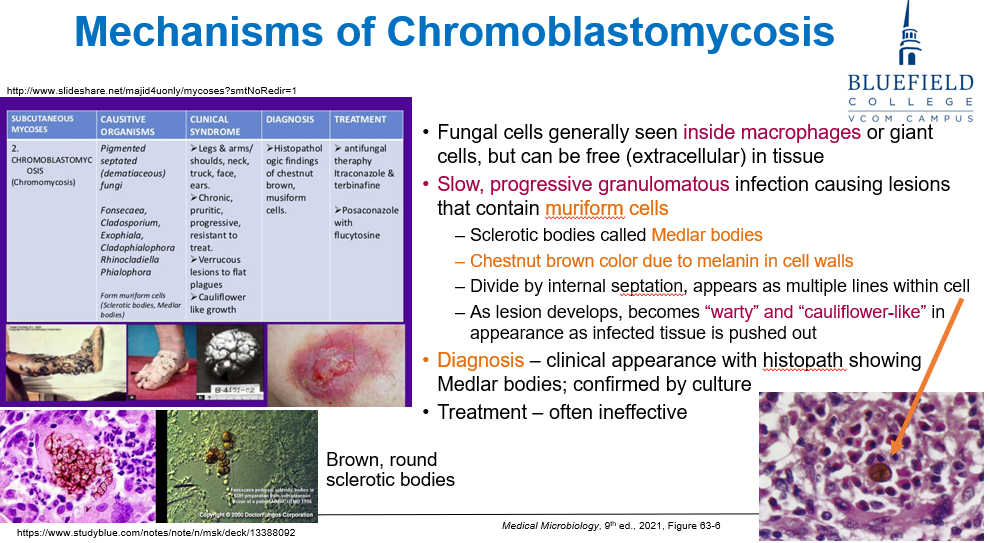
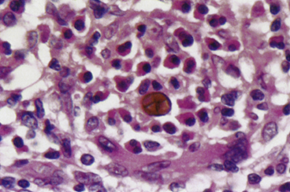
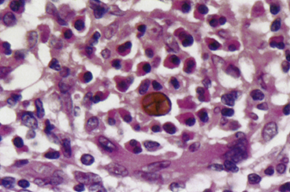
What kind of infection is chromoblastomycosis and what characteristic cells are seen?
A slow, progressive granulomatous infection that produces muriform cells (also called Medlar bodies or sclerotic bodies).
What are Medlar bodies and how do they appear?
Medlar bodies are muriform (brick arrangement) fungal cells with:
Chestnut brown pigmentation from melanin in the cell wall
Internal septation (horizontal and vertical walls), giving a cross-sectioned appearance
They are used to diagnose chromblastomycosis
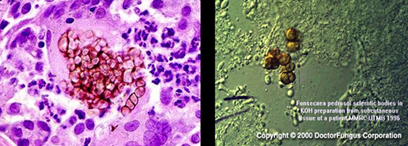
How is chromoblastomycosis diagnosed?
Histopathology showing Medlar bodies
Confirmation by culture
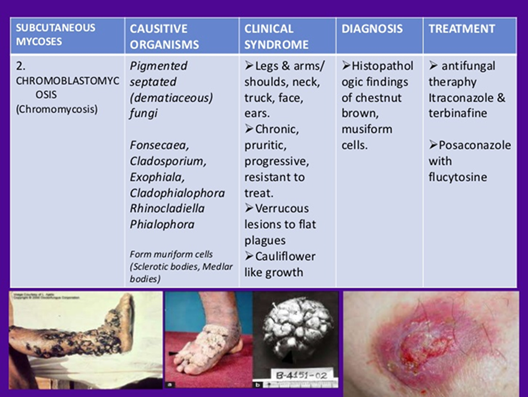
What type of fungus is Histoplasma and where does it survive in the host?
A small, dimorphic yeast that survives intracellularly in pulmonary macrophages, where it can be seen inside macrophages.
What are key virulence factors of Histoplasma?
Intracellular survival in macrophages, enabling dissemination to lymphoid and other tissues
Induces granuloma formation in lung tissue
Where in the U.S. is Histoplasma endemic?
The Midwestern U.S., especially around the Ohio and Mississippi River Valleys.
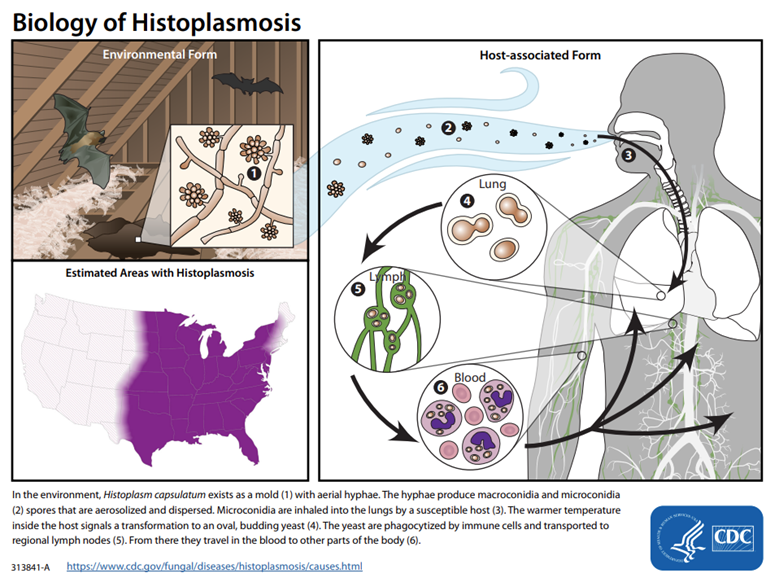
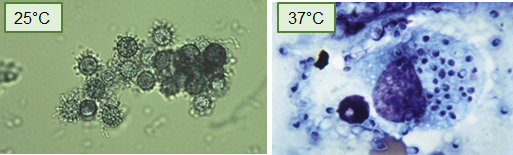
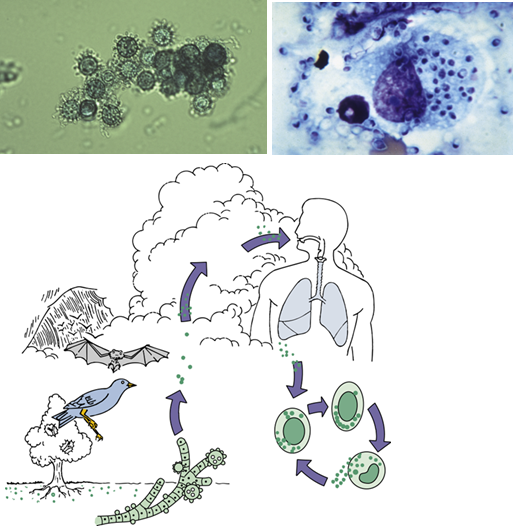
Where is Histoplasma commonly found in the environment?
In nitrogen-rich soil, particularly in areas contaminated with bird or bat droppings.
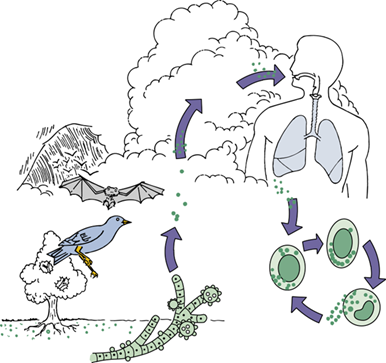
What environmental exposures are associated with Histoplasma transmission?
Exposure to bird roosts, caves, decaying buildings, or urban excavation/demolition increases risk.
How is Histoplasma transmitted?
Via inhalation of airborne spores from contaminated soil or environments.
Who is more likely to become symptomatic from Histoplasma infection?
Immunocompromised individuals and children are more likely to develop symptoms. Also, exposure to a large inoculum makes nearly everyone symptomatic.
What are the common symptoms of Histoplasmosis?
Generally a self-limiting pulmonary disease with flu-like symptoms: chills, fever, chest pain
Can progress to acute respiratory distress with high exposure
May disseminate in immunocompromised patients or children under 1 year

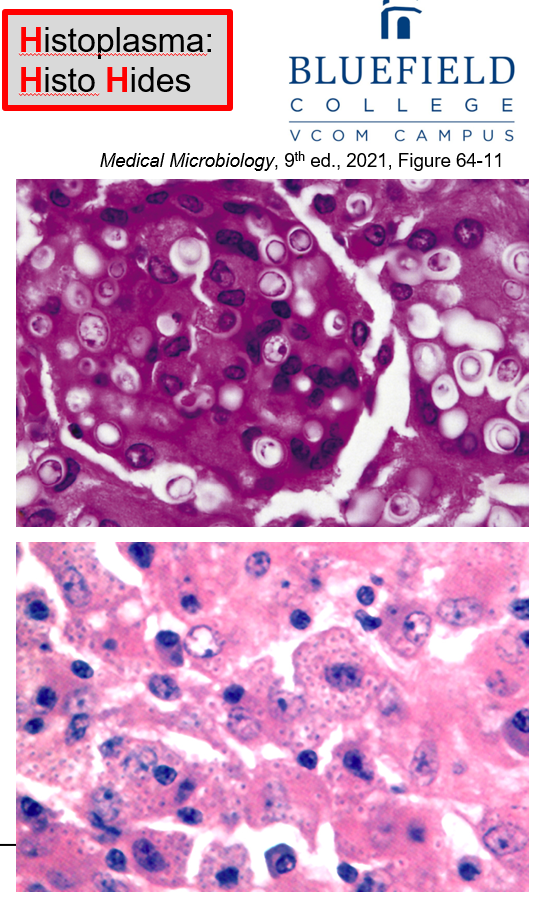
How is Histoplasmosis diagnosed?
By visualizing fungal particles, especially inside macrophage cytoplasm, but also outside macrophages
Serology can also help
Samples include sputum, BAL (bronchoalveolar lavage), peripheral blood, bone marrow, or tissue biopsy

What type of fungus is Blastomyces and what is its morphology?
A dimorphic fungus that forms septate hyphae at 25°C and broad-based budding yeast at 37°C.
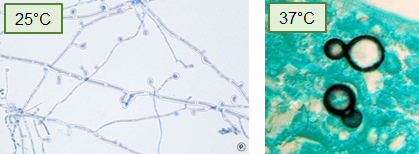
What is the geographic distribution of Blastomyces in the United States?
They’re associated with the Ohio and Mississippi River Valleys, Great Lakes region, and southeast/south central US.
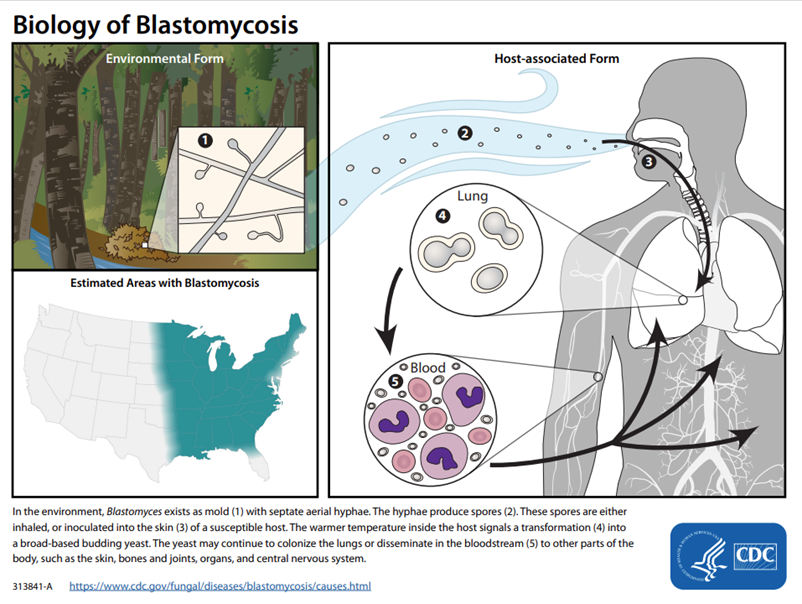
How is Blastomyces typically transmitted?
Via inhalation of spores from soil and decaying organic matter, especially in damp environments linked to occupational or recreational activities.
What is the most common presentation of Blastomycosis in infected individuals?
Over 50% of infected individuals are asymptomatic, but symptomatic cases often present with respiratory illness.

What are the respiratory symptoms of Blastomycosis?
Symptoms range from mild flu-like illness to bacterial pneumonia-like disease, with high fever and cough.

How does Blastomycosis manifest in the skin?
Cutaneous disease occurs via hematogenous spread from the lungs. Skin lesions can be pustular, papular, or nodular, usually painless and on exposed areas like the face, scalp, neck, or hands.