Unit 8 and 9 Thermochemistry and Kinetics
1/263
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
264 Terms
Energy
The capacity to do work or produce heat.
Heat
The transfer of thermal energy between systems.
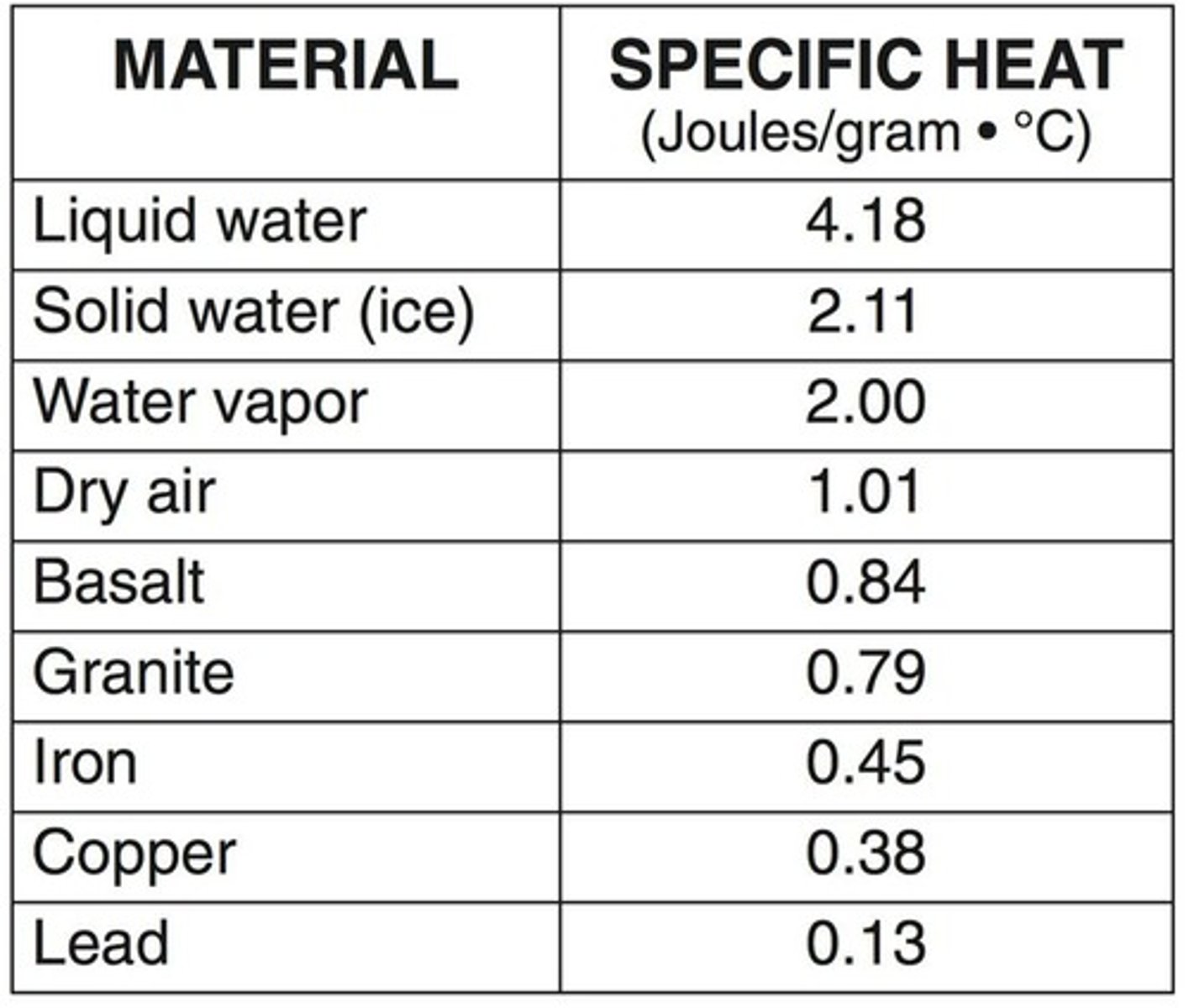
Specific Heat Capacity
The amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
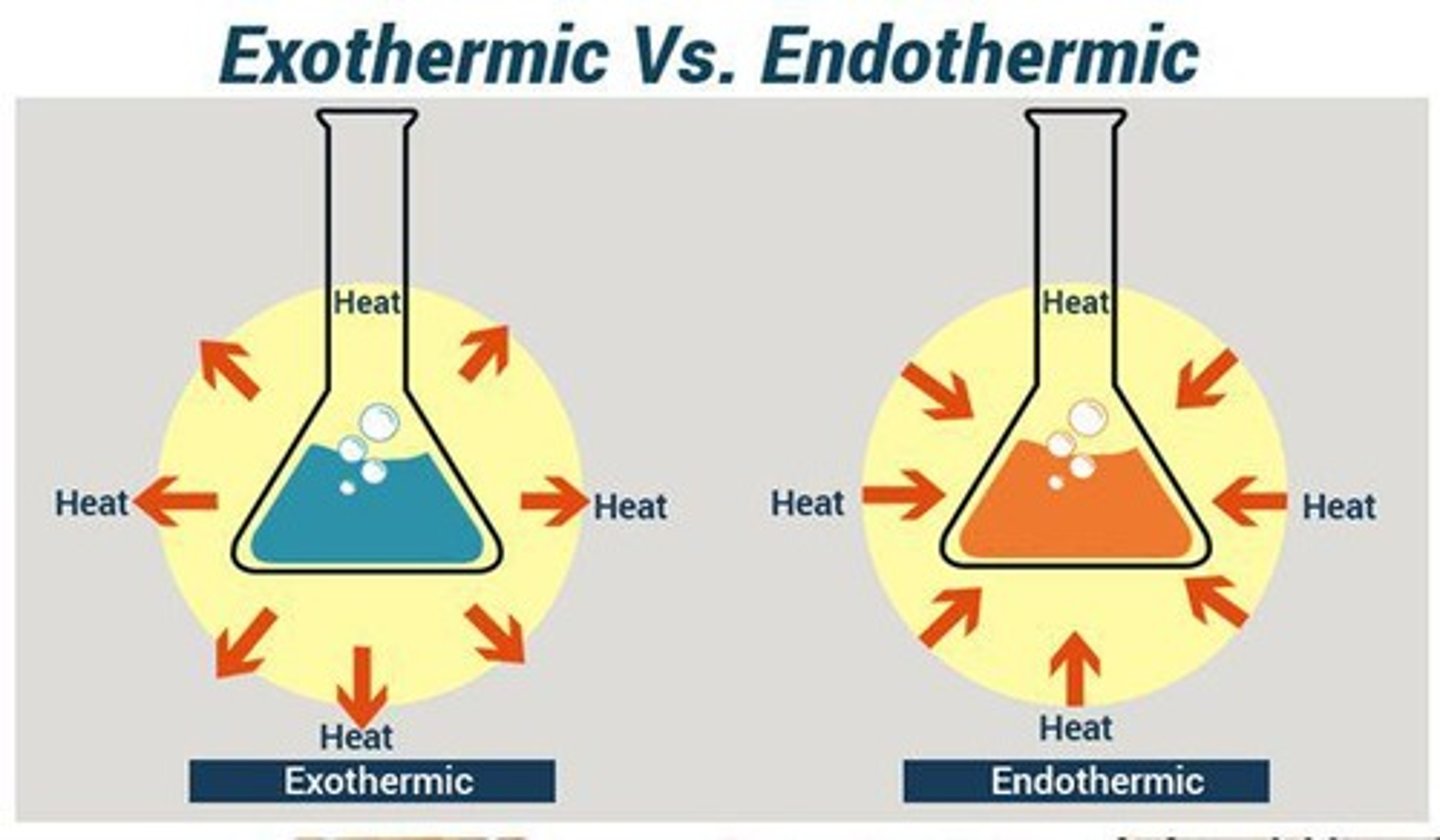
Endothermic vs Exothermic
Endothermic processes absorb heat, while exothermic processes release heat.
Enthalpy
A measure of the total heat content in a system.
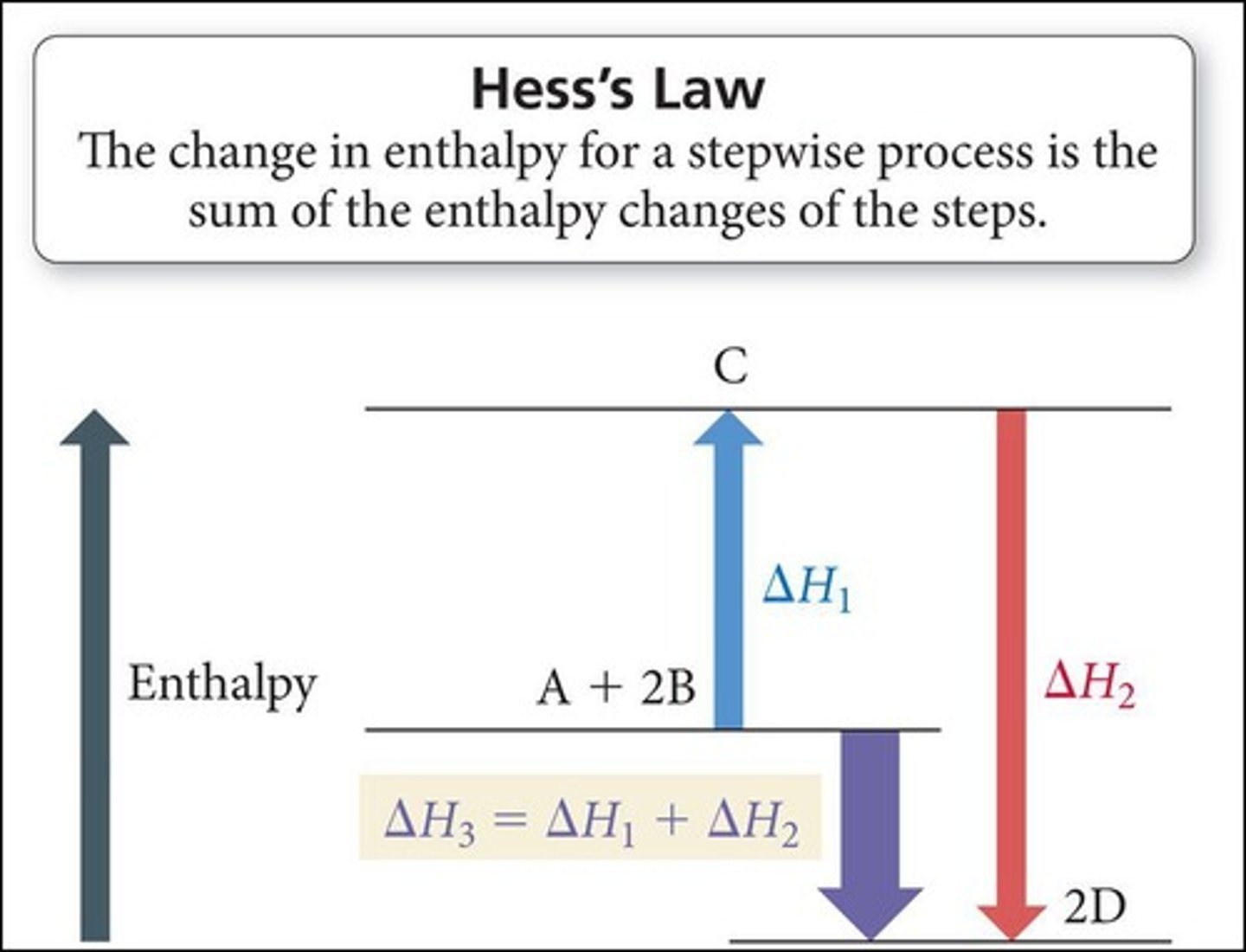
Hess's Law
The total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps.
Entropy
A measure of the disorder or randomness in a system.
Convection/Conduction
Convection is the transfer of heat by the movement of fluids, while conduction is the transfer of heat through direct contact.
Heat of Fusion vs Heat of Vaporization
Heat of fusion is the energy required to change a substance from solid to liquid, while heat of vaporization is the energy required to change a substance from liquid to gas.
When to use q=mCΔT vs ΔHvap = mHv or ΔHfus = mHf
Use q=mCΔT for temperature changes, and ΔHvap = mHv or ΔHfus = mHf for phase changes.
Spontaneity
The tendency of a process to occur without outside intervention.
When ΔS and ΔH are + or - (chart)
A chart that indicates the signs of entropy change (ΔS) and enthalpy change (ΔH) for various processes.
Collision Theory
A theory that states that for a reaction to occur, reactant particles must collide with sufficient energy and proper orientation.
Factors that influence rxn rate
Concentration, temperature, surface area, and catalysts are factors that can affect the rate of a reaction.
Rate Determining Step
The slowest step in a reaction mechanism that determines the overall reaction rate.
Equilibrium expressions
Mathematical representations of the concentrations of reactants and products at equilibrium.
Activation Energy/Heat of Reaction
Activation energy is the minimum energy required for a reaction to occur, while heat of reaction is the change in enthalpy during a reaction.
Heat of Formation
The change in enthalpy when one mole of a compound is formed from its elements in their standard states.
Nomenclature
The system of naming chemical compounds.
Q=mCΔT
An equation used to calculate heat transfer, where Q is heat, m is mass, C is specific heat, and ΔT is the change in temperature.
ΔHvaporization = mHv
An equation for calculating the enthalpy of vaporization, where ΔHvaporization is the heat required to vaporize a substance, m is mass, and Hv is the specific heat of vaporization.
ΔHfusion = mHf
An equation for calculating the enthalpy of fusion, where ΔHfusion is the heat required to melt a substance, m is mass, and Hf is the specific heat of fusion.
ΔHrxn = Σ∆H0f (products) - Σ∆H0f (reactants)
An equation for calculating the change in enthalpy of a reaction using the standard enthalpies of formation.
ΔG = ΔH - TΔS
An equation for calculating Gibbs free energy, where ΔG is Gibbs free energy, ΔH is enthalpy, T is temperature, and ΔS is entropy.
Rate = k[A]n and (R2/R1) = (M2/M1)m
Equations used to describe reaction rates, where k is the rate constant, [A] is concentration, and m is the experimental exponent.
Keq = [C]c[D]d / [A]a[B]b
An equation for calculating the equilibrium constant, where [C], [D], [A], and [B] are the concentrations of the products and reactants.
Thermochemistry
Concerned with heat changes that occur during chemical reactions or phase changes.
Chemical potential energy
Energy within chemical substances.
Conduction
Heat transferred by particles colliding into one another, primarily in solids.
Convection
Heat transferred by the circulation of a fluid (or gas), such as in a heating system.
Radiation
Heat transferred by the flow of electromagnetic radiation; can happen in a vacuum.
System
The specific part of the universe that contains the reaction or process you wish to study.
Surroundings
Everything else outside the system.
Exothermic process
Heat flowing out of a system into its surroundings; system loses heat.
Endothermic process
Heat flowing into a system from its surroundings; system gains heat.
Law of Conservation of Energy
In any chemical or physical process, energy is neither created nor destroyed.
Heat of Fusion
Energy required to change from solid to liquid; some attractive forces are broken.
Heat of Vaporization
Energy required to change from liquid to gas; typically higher than heat of fusion.
Thermal Energy
Total energy of particles, including kinetic and potential energy.
Kinetic Energy
Energy of motion of particles; increases as particles move faster.
Potential Energy
Energy of arrangement of particles; increases as particles move farther apart.
Temperature
Measure of the average kinetic energy of all the particles in a sample.
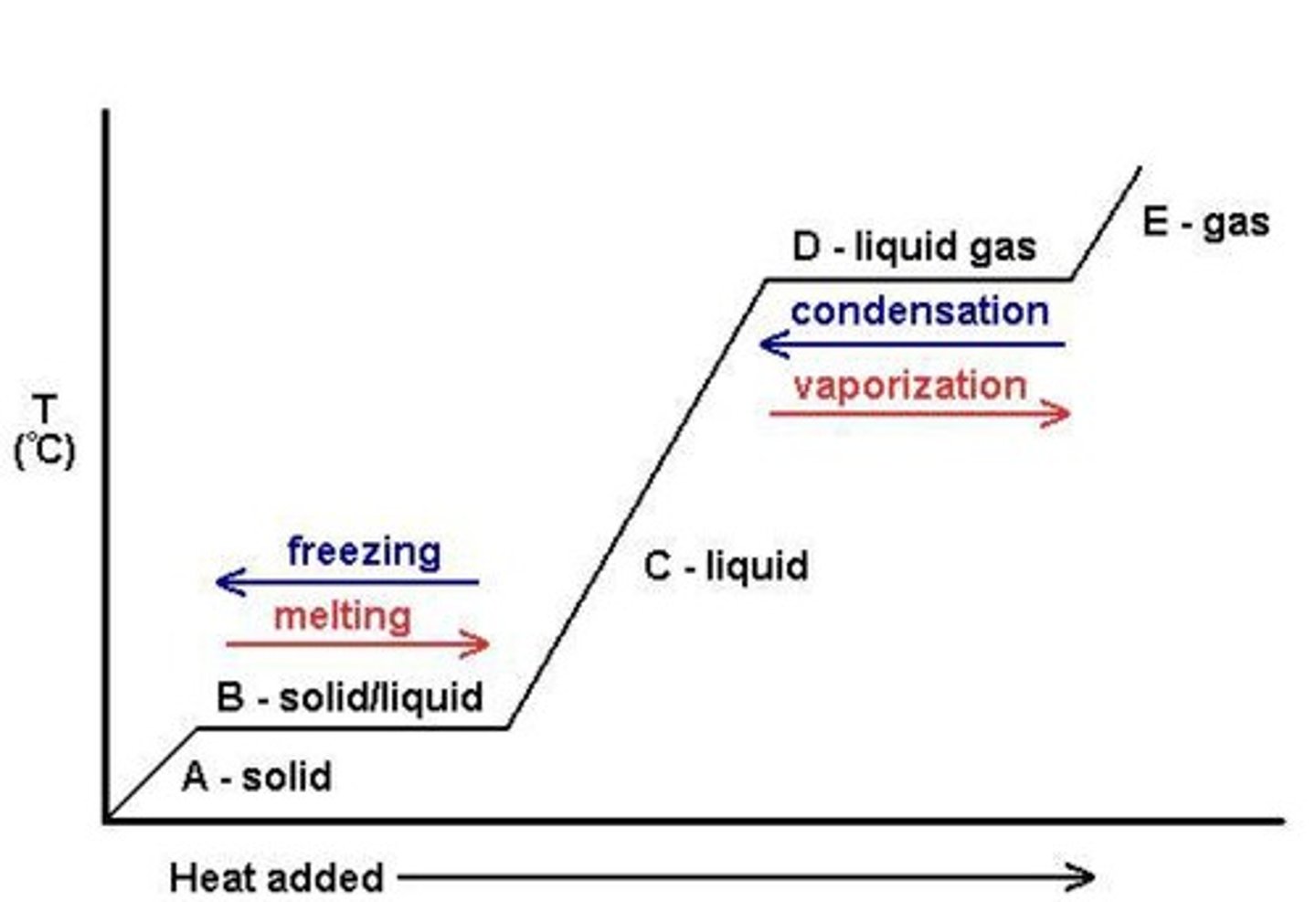
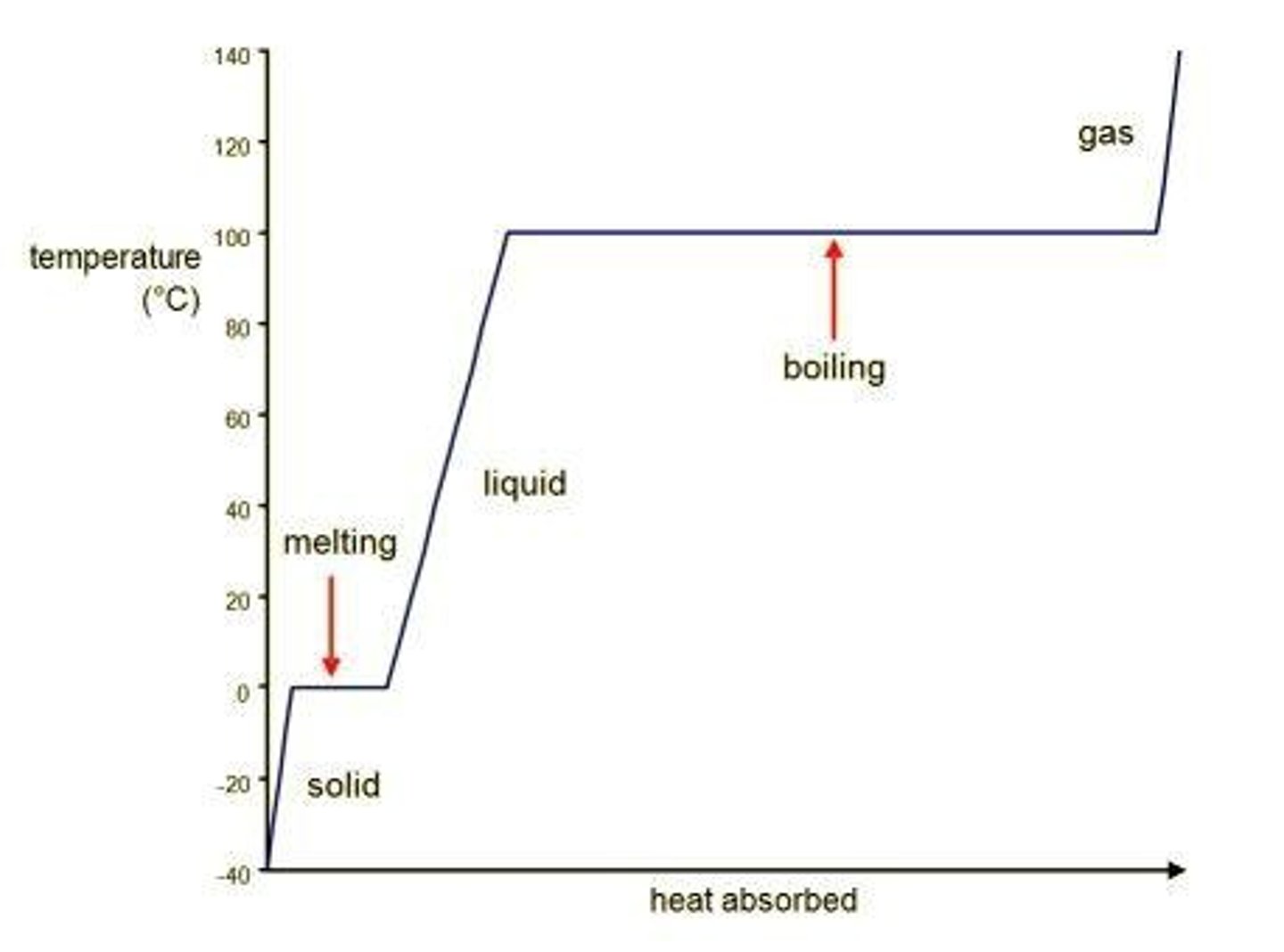
Heating Curve
Graph showing energy (heat) being added as time passes.
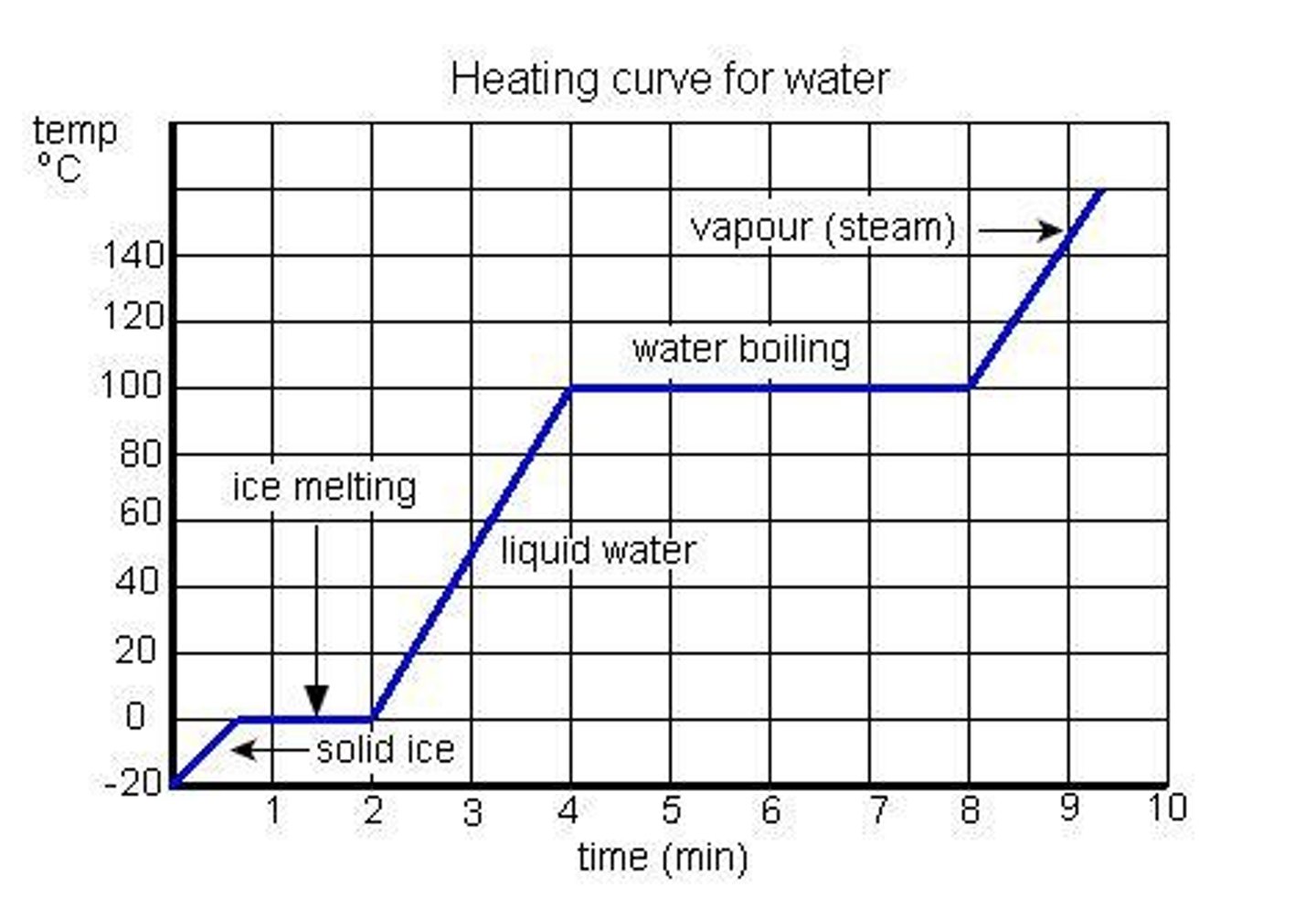
Calorie
Quantity of heat needed to raise the temperature of 1 g of pure water by 1 °C.
Calorie (capital C)
Refers to the energy in food; 1 Calorie = 1 kilocalorie = 1000 cal.
Phase changes
Can be exothermic or endothermic; going from solid to liquid to gas requires heat input.
Exothermic reactions
Release energy, usually in the form of heat.
Endothermic reactions
Absorb energy.
Energy storage
Energy is stored in bonds between atoms.
Heat Capacity
The amount of heat needed to increase the temperature of an object exactly 1 °C.
Specific Heat of Water
For water, C = 4.18 J/(g °C) in Joules, and C = 1.00 cal/(g °C) in calories.
Heat Absorbed or Released
Calculated using the formula q = mCpΔT, where q is the heat absorbed or released.
q
The heat absorbed or released during a change in temperature.
Cp
The specific heat of the substance.
m
The mass of the substance in grams.
ΔT
The change in temperature in °C.
Specific Heat Example 1
For 34.4 g of ethanol increasing from 25.0 °C to 78.8 °C, q = 4.52 x 10^3 J.
Specific Heat Example 2
For a 155 g sample of ethanol heated from 25.0 °C to 40.0 °C, Cp = 2.45 J/g·°C.
Specific Heat Example 3
For a 95.4 g piece of copper increasing from 25.0 °C to 48.0 °C, the specific heat is 0.387 J/g°C.
Specific Heat Example 4
A piece of copper absorbs 249.5 J of heat, changing temperature from 21.3 °C to 26.8 °C, mass = 117 g.
Specific Heat Example 5
A 75.8 g piece of titanium absorbs 642.1 J of heat, starting at 21.2 °C, ending at 37.4 °C.
Calorimetry
The measurement of the heat into or out of a system for chemical and physical processes.
Calorimeter
The device used to measure the absorption or release of heat in chemical or physical processes.
Enthalpy (H)
The heat content of a system at constant pressure.
Calorimetry Example 1
A 35.0 g piece of metal heated to 100 °C in 50.0 g of water at 21.4 °C, water temp rises to 25.8 °C.
Calorimetry Example 2
A 37.6 g sample of metal heated to 100.0 °C in 75.0 g of water at 22.3 °C, water temp rises to 29.4 °C, Cmetal = 0.839 J/g°C.
Calorimetry Example 3
A 15.7 g sample of metal heated to 95.0 °C in 35.0 g of water at 21.2 °C, water temp rises to 25.9 °C, Cmetal = 0.634 J/g°C.
Exothermic Reaction
A reaction that produces heat.
Change in Enthalpy (ΔH)
The difference between the enthalpy of the substances at the end and the start of a reaction.
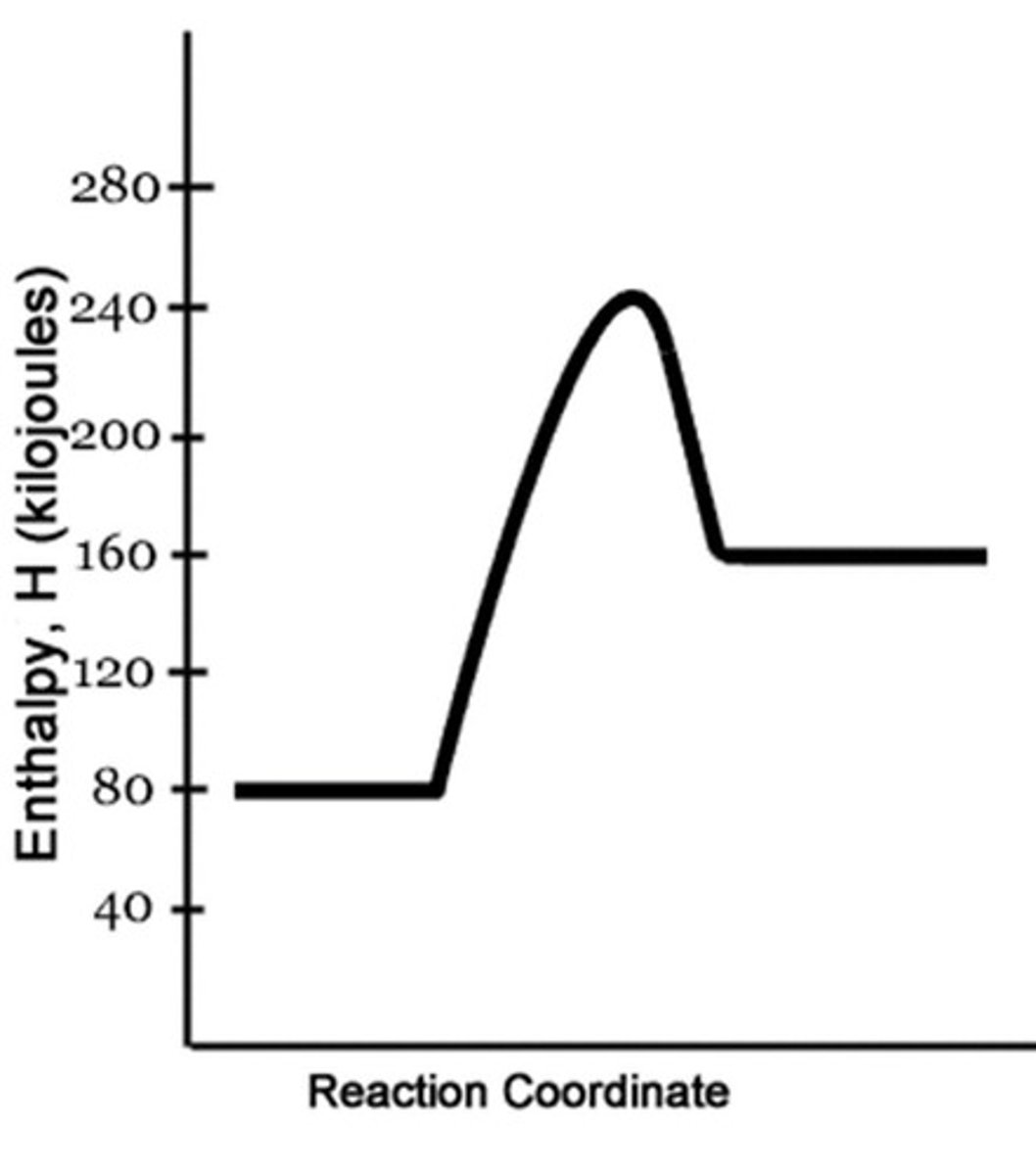
Enthalpy of Reaction (ΔHrxn)
The change in enthalpy for a reaction.
Thermochemical Equation
A balanced equation that includes states of matter and the energy change.
Enthalpy of Combustion (ΔHcomb)
The enthalpy change for the complete burning of one mole of a substance.
Enthalpy of Fusion (Hf)
The heat required to melt one gram of a solid substance.
Enthalpy of Vaporization (Hv)
The heat required to vaporize one gram of a liquid substance.
Heat (q)
The energy transferred due to temperature difference.
Formula for Heat (q)
q = mCpΔT
Formula for Enthalpy of Fusion
ΔH = mHf
Formula for Enthalpy of Vaporization
ΔH = mHv
Molar Enthalpy of Fusion
ΔH = nHf
Energy Released in Reaction
ΔHrxn = Hproducts - Hreactants must result in a negative number.
Combustion of Methane
To liberate 12,880 kJ of heat, 231 g of CH4 must be burned.
Heat from Ammonia Condensation
q = 275 g x 1371.2 J/g = 377080 J or 377 kJ.
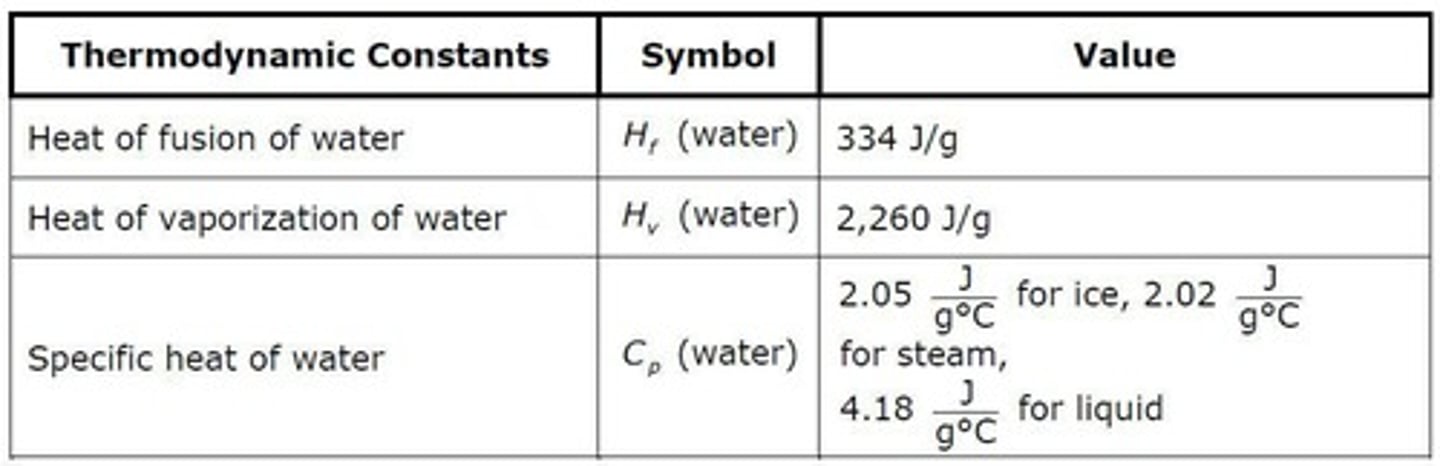
Heat to Melt Ice
q = 24.5 g x 334 J/g = 8183 J.
Heat to Vaporize Water
q = 24.5 g x 2260 J/g = 55370 J.
Heat to Melt Ice at -20.0 °C
Total q = 2050 J (heating) + 16700 J (melting) = 18750 J.
Heating Ice to Steam
Energy needed to heat 55.0 grams of ice from -15.0 °C to steam at 150.0 °C.
Step 1 Heating Ice
q = (55 grams)(2.05 J/g°C)(150) = 1,691 J.
Step 2 Phase Change
ΔH = (55 g)(334 J/g) = 18370 J.
Step 3 Heating Liquid
q = (55 grams)(4.18 J/g°C)(100) = 22,990 J.
ΔH
The change in enthalpy for a reaction.
ΔHrxn
The enthalpy of reaction, defined as Hfinal - Hinitial.
Endothermic Reaction
A reaction that absorbs heat, resulting in products having more energy than reactants.
mCpΔT
The formula used to calculate heat, where m is mass, Cp is specific heat capacity, and ΔT is the change in temperature.
Specific Heat Capacity (C)
The amount of heat required to raise the temperature of 1 gram of a substance by 1 °C.
Standard Enthalpy of Formation
The enthalpy change for the formation of one mole of a compound from its elements in their standard states.
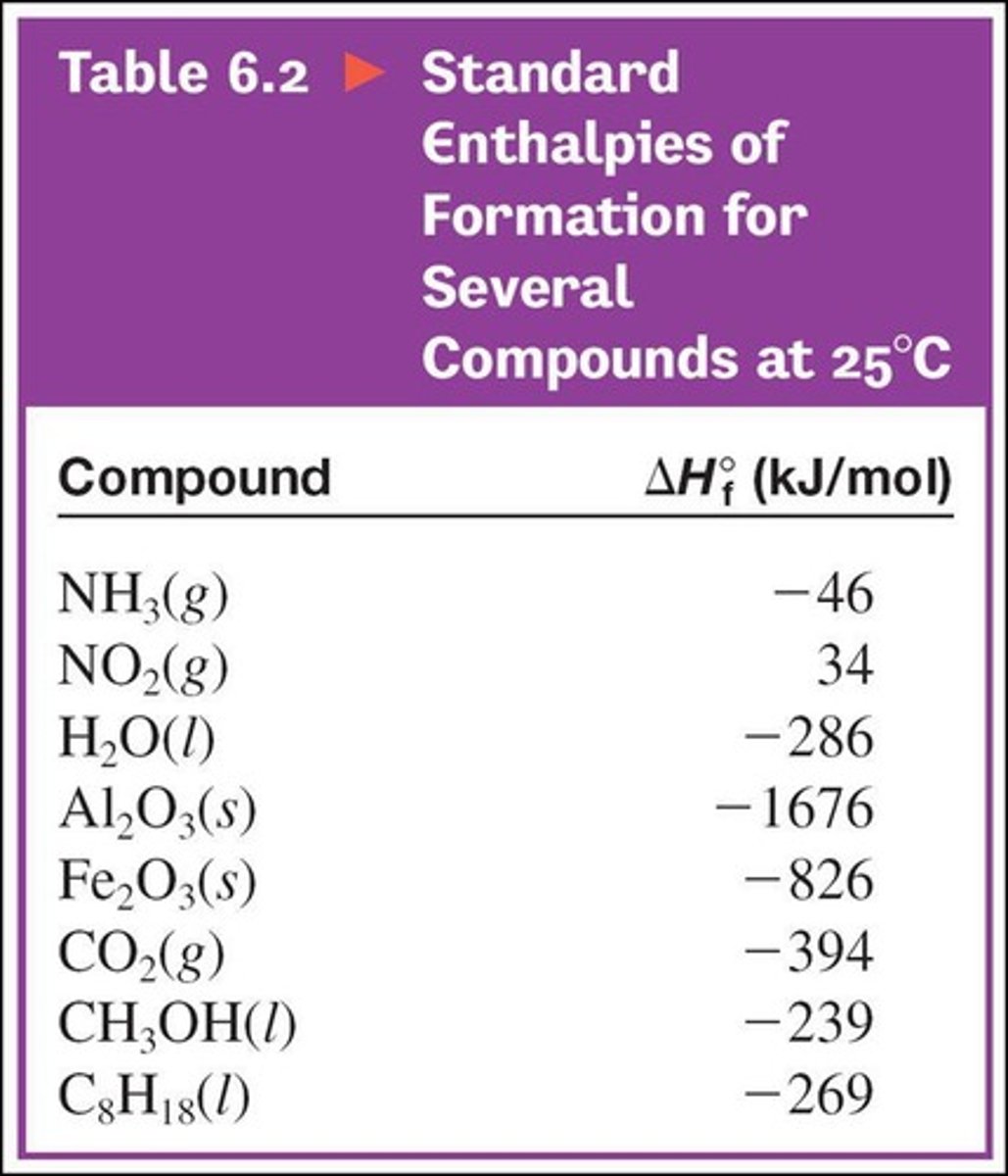
ΔHtotal
The total enthalpy change calculated by summing individual enthalpy changes.
Heat Released
The energy released to the environment during a cooling process.
Combustion of Propane
The reaction of propane with oxygen producing carbon dioxide and water, with an associated ΔHrxn of -2044 kJ.
Mass of Water
The amount of water involved in a reaction, affecting the heat transfer calculations.
Temperature Change
The difference in temperature before and after a reaction, used to calculate heat transfer.