Topic 7 - Equilibrium
0.0(0)
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
Last updated 5:35 PM on 4/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
List four essential facts about systems in equilibrium.
* Its a closed system
* The forward and reverse reactions occur at the same rate
* There is not an equal amount reactants and products
* The concentrations of reactants and products do not change
* The forward and reverse reactions occur at the same rate
* There is not an equal amount reactants and products
* The concentrations of reactants and products do not change
2
New cards
Does adding a catalyst effect the position of equilibrium? Shift the equilibrium?
No.
3
New cards
What does it mean when the equilibrium constant is
* larger than 1
* smaller than 1
* equal to 1
* larger than 1
* smaller than 1
* equal to 1
* K >> 1 : products are favoured
* K << 1 : reactants are favoured
* K = 1 : neither are favoured
* K << 1 : reactants are favoured
* K = 1 : neither are favoured
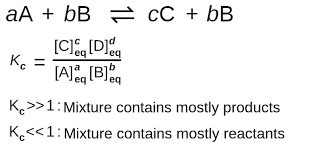
4
New cards
What is the only factor that changes the equilibrium constant K?
Temperature.
5
New cards
List three qualities of a closed system in dynamic equilibrium.
* Concentrations of reactants and products remain constant
* The rate of the forward and reverse reactions are equal
* The macroscopic properties remain the same
* The rate of the forward and reverse reactions are equal
* The macroscopic properties remain the same