Chemistry Quiz 1
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Nuclear Magnetic Resonance
Changes magnetic orientation.
Infra-red spectroscopy
Molecular vibration. This allows use to see single and double bonds and metal-ligand interactions in transition metals.
UV-visible
Promoted to a higher energy state
x-ray crystallography
How all atoms in a molecule are connected in a three-dimensional arrangement.
How to figure out energy
E=hv
E
energy in Js
v
frequency
λ
Wavelength
λ measurements
nm so have to multiple by 10-9 to get back into meters to calculate
How to calculate wavelength
c=λv
c
speed of light - 2.998×108m/s
h
Planck’s constant - 6.626×10-34
Using a IR Spectrum
Find the peaks and compare with the most unique of the 3. THE C-H BONDS DONT REALLY MATTER.
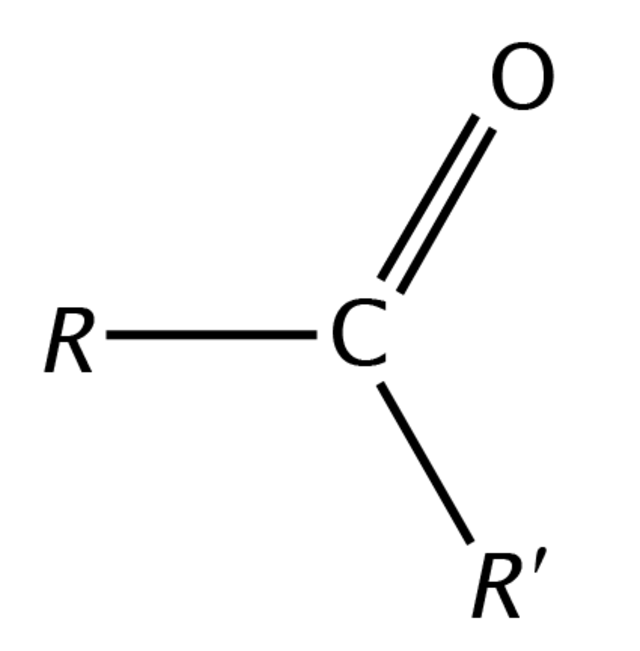
Ketone
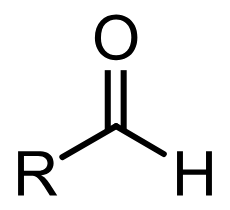
Aldehydes
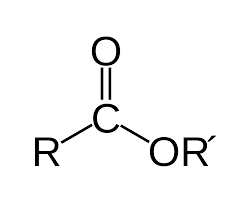
Esters
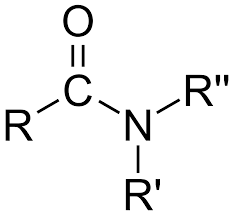
Amides
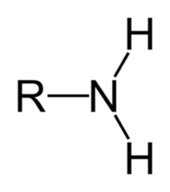
Amines
Can be with an H group or 3 R groups.
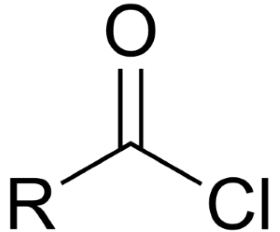
Acid chlorides
Photoelectron Spectroscopy
Allows scientists to know the ionization energy of all electrons in the atom.
What electron energy has the highest ionization energy?
1s because it is the closest the the nucleus and has the least amount of energy so this would have less of a shielding affect.
Equation to calculate Ionization Energy
E=IE+KI
Quantum Number
n,l,ml,ms
n
Principle quantum number (THE ENERGY LEVEL)
l
Orbital angular movement
l numbers for 2nd quantum number
s=0,p=1,d=2,f=3
ml
the negative l to l (ie d goes from -2 to 2)
ms
This is for spin. up=1/2, down= -1/2
Steps to solve a redox acid reaction
Balance out any of the molecules THAT ARE NOT WATER OR HYDROGEN
Add water to balance out the oxygens
Add H+ ion to balance out
Add electrons to both half reactions
multiple by a co-efficient to make the 2 half-reactions balance
Steps to solve a redox basic reaction
Balance out any of the molecules THAT ARE NOT WATER OR HYDROGEN
Add water to balance out the oxygens
Add H+ ion to balance out
ADD OH- IONS TO BALANCE OUT THE H+ ADD THEM TO BOTH SIDES
Add the OH- and H+ ions together to make water
Add electrons to both half reactions
multiple by a co-efficient to make the 2 half-reactions balance
Photons
As electrons after being excited relaxes it releases a photon particle of light.
Action of a photon
Photons act both as a particle and a wave.
Beer-Lambert Law
A = εbc
A
Absorbency
ε
Molar Absorbance Coefficient (NO UNITS)
b
Pathway of curette (usually 1.0cm) (Units:cm)
c
Concentration (moles/L)
Electromagnetic Spectrum
The spectrum that all light is measured in as LIGHT IS ENERGY
Higher energy light
Shorter wavelength (Violet and UV)
Lower energy light
Longer wavelengths (Red and Infrared)
How to read a PES
The number of humps is the number of orbital shells, and the ones with the least amount of ionization energy should be a specific level as you could then tell the element.
Why would there be a shift left for all humps but the last one which shifts right for a PES?
There is 1 more proton so there is more attraction but at the end there end up also being one more electron so there is more of a shielding affect which allows for it to release with less energy.