bio will ace
1/126
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
127 Terms
metabolism
all of the chemical reactions in an organism
metabolic pathways
series of chemical reactions that either build complex molecules or breakdown complex molecules
a series of steps to produce a product- catalyzed by a specific enzyme
ex. substrate>enzyme1.> intermediate reaction>enzyme2.> intermediate reaction>product
two types of metabolic pathways
catabolic (cat-astrophe, breakdown, release)
anabolic (A na, A te, build up, consume)
catabolic pathways
pathways that release energy by breaking down complex molecules into simpler compound
ex. cellular respiration-breaking down glucose when O2 is present to make ATP
anabolic pathways
pathways that consume energy to build complicated molecules from simpler compounds
ex. synthesizing proteins from put together amino acids, putting monosaccharides together to build a disaccharide
energy
the ability to do work
necessary to survive and function
must be transferred from one form to another to live
a loss of energy results in
death
kinetic energy
associated with motion
thermal energy
associate with the movement of atoms or molecules
heat
type of kinetic (at the molecule scale)
heat
thermal energy that transfers from one object to another)
potential energy
stored energy
because of location or structure
chemical energy
type of potential energy (structure)
released in a chemical energy
thermodynamics
study of energy transformation in matter
its laws apply to all the universe
3 laws
1) energy cannot be created or destroyed, it can be transferred or transformed (principle of conservation of energy)
2) energy transformation increases entropy (disorder; [S]) of the universe, during transfers/transformations, some energy is unusable and is lost as heat
3) ?
entropy examples
heat
multiple pieces
solid(least) to liquid to gas (most disorder)
diffused particles/spaced apart
isolated system
unable to exchange energy or matter with its surroundings
open system
ex organisms
energy os transferred between the system and its surroundings
organisms pay for their complexity and organization by
creating disorder themselves
free energy
determines the likelihood of reactions in organisms or in the reactions are energetically favorable
delta G= delta H-TdeltaS
energy that can do wor is usable
delta G
change in free energy
high G
more free energy
less stable
greater work capacity
in spontaneous change
free energy of the system decreases (delta G<0)
system become more stable
released energy is harnessed to do work
low G
less free energy
more stable
less work capacity
delta H
change in total energy
T
absolute temp in kelvin
delta S
change in entropy
free energy change of reactions determine
whether reactions occur spontaneously (no outside input of energy is required)
based on this, reactions are classified as exergonic (expel) or endergonic (engorge??)
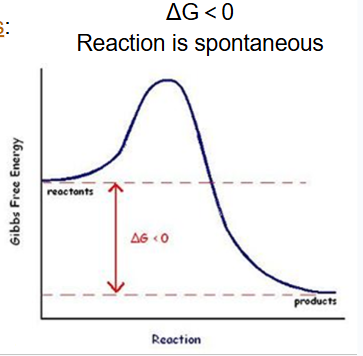
exergonic reactions
reactions that release energy (ex. cellular respiration)
delta G is less than 0
spontaneous
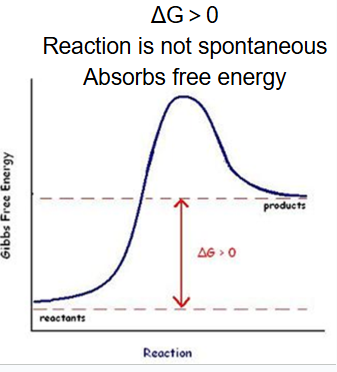
endergonic reactions
reactions that absord energy (ex. photosynthesis
delta G is greater than 0
not spontaneous (decrease entropy; require energy)
cells perform three kinds of work
mechanical
transport
chemical
mechanical work
movement
ex.beating cilia, movement of chromosomes, contraction of muscle cells
transport work
pumping substances across membranes against spontaeous movement
chemical work
synthesis of molecules
ex building polymers from monomers
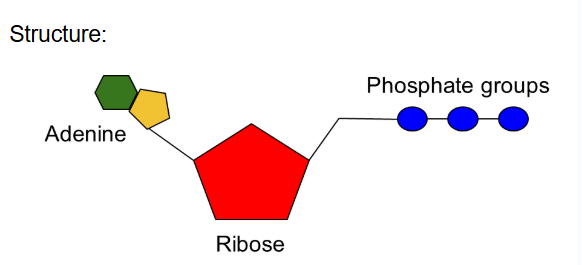
ATP
adenosine triphosphate- molecule that organisms use for energy
couples exergonic to endergonic reactions to power cellular work
-the exergonic process drives the enderognic process
also used to make RNA
ribose, adenine (nitrogenous base) and three phosphate groups
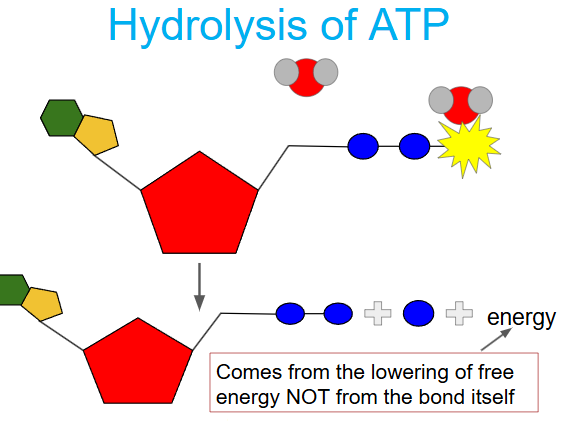
ATP>ADP (hydrolysis)
organisms obtain energy by breaking the bond between the 2nd and 3rd phosphate in a hydrolysis reaction (addition of water)
energy come from lowering (-delta G) of free energy, not phosphate bonds (more pieces, greater entropy, -delta G)
phosphorylation
the released phosphate moves to another molecule to give energy
regeneration of ATP
ATP cycle
ADP +Pi uses energy from exergonic process to become ATP and water and energy is released in hydrolysic for cellular work to become ADP +Pi
spontaneous reactions are not necessarily fast
can be sped up with enzymes
enzymes
type of protein that catalyze or speed up reactions by lowering activation energy
ends in -ase
not consumed by reaction
enzyme acts on
active site of a reactant called a substrate
ways enzymes lower activation energy
substrates may be oriented to facilitate reaction
substrate stretched to make bonds easier to break
active site may provide a microenvironment that favors the reaction
amino acids in active site may participate in reactions
enzyme function
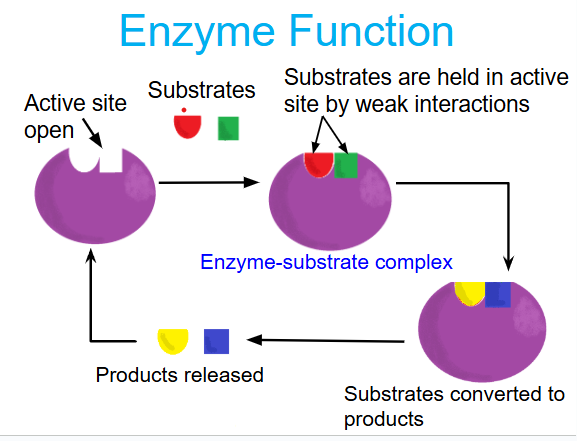
induced fit
enzymes will change the shape of their active site to allow the substrate to bind better
enzyme catabolism
enzyme break down complex molecule
enzyme anabolism
enzyme build complex molecules
shape of enzyme affected by
temperature
pH
chemicals
change in shape is a change in function
optimal conditions
best conditions (tmep and ph) for enzymes to function
cofactors
non-protein molecules that assist enzyme function
can consist of metals
can be tightly or loosely bound
holoenzyme: when enzyme has cofactor attached
coenzymes: organic cofactors (ex vitamins)
enzyme inhibitors
reduce enzyme activity
can be permanent (covalent bonds) or reversible (weak interactions)
competitive inhibitors
reduce enzyme activity by binding to active site before substrate
can be reversed with increased substrate concentration
noncompetitive inhibitors
bind to an allosteric site (not active site) and changes active sites shape to prevent binding
allosteric enzymes have 2 binding sites
active and allosteric (regulatory)
allosteric regulation
molecules bind noncovalently to allosteric site to change shape of active site
can help (stimulation of enzyme activity) or hinder (inhibition)
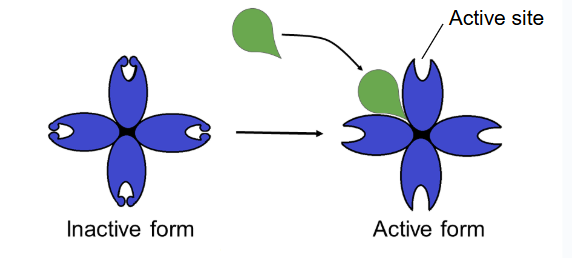
allosteric activator
substrate bonds to allosteric site and stabilizes shape of enzyme so that the active site remains open
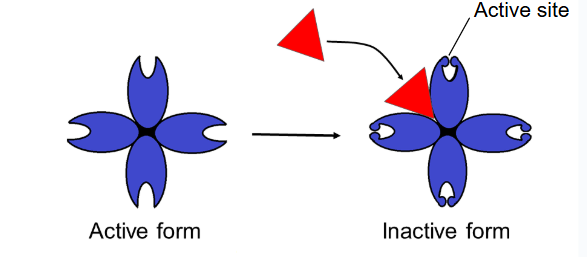
allosteric inhibitor
substrate binds to allosteric site and stabilizes the enzyme shape so that the active sites are closed/inactive
cooperativity
substrate binds with one active site (enzyme that has multiple) which stabilizes active form
considered allosteric regulation since binding at one site changes shape of others
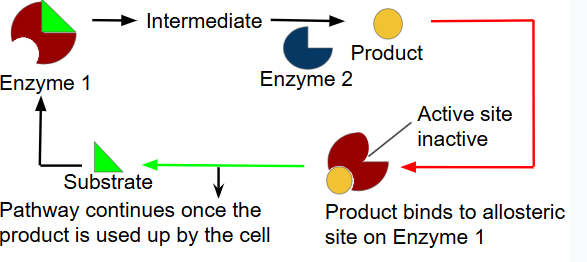
Sometimes, the end product of a metabolic pathway can act as an inhibitor to an early enzyme in the same pathway
prevents excess products
starch is the major fuel for animals
starch breaks down into glucose
cellular respiration is exergonic
oxidation of glucose transfers e- to a lower energy state, releasing energy to be used in ATP synthesis
downhill exergonic path:
glucose>nadh>etc>oxygen
Dehydrogenases
Oxidizing agent for glucose
take 2 e- and 2 protons from glucose
transfer 2e- and 1 proton to the coenzyme NAD+. Reduces to NADH (stores the energy, carries e- to the electron transport chain) last proton is release into surrounding solution
stages of cellular respiration
glycolysis
pyruvate oxidization and the citric acid cycle
oxidative phosphorylation (ETC and chemiosmosis)
oxidized
+
reduced
H
energy investment: glucose (6C) starts glycolysis uses two atp
2 adp + 2p
energy payoff: 4 adp +4p and 2 NAD+ + 4e-+ 4+
4 atp and 2 nadh + 2 H+ (reduction)
glycolysis results in
2 pyruvate and 2 H20
glycolysis summary
ei: in: 2 ATP
out: 2 ADP+p
ep: in: 4 Adp +p, 2nad++4e+4h+
out: 4 atp, 2 nadh+2h+
net: 2 pyruvate and 2h2o
2 atp
2nadh+2h+
pyruvate oxidation
if O is present
pyruvate undergoes oxidation
acetyl coA, 2NADH is made, 2co2 is released,
citric acid cycle
krebs cycle occurs in mitochondrial matrix
2 acetyl coa
first turns into citrate then turns into
2 atp
6 nadh
4 co2 (all co2 is now released)
and 2 fadh2
pyruvate oxidation summary
in: 2 pyruvate, 2 nad+
out: 2 nadh, 2 co2, acetyl coA
citric acid cycle summary
in: 2 acetyle coA
out: 2atp
6nadh
4co2
2fadh2
oxidative phosphorylation
electron transport chain
chemiosmosis
etc
occurs in inner membrane of mitochondria
proteins reduced by uphill neighbor and oxidized by downhill
“fall”
cristae increase surface area for chain
do no create ATP directly byt manages release of energy through small steps
final e- acceptor aerobic
oxygen
each ) pairs w 2H+ and 2e-
makes h20 releases energy
etc creates proton gradient across membrane
as electron travel down H+ is pumped
Use the exergonic flow of electrons from NADH and FADH2
powers chemiosmosis (uses proton to power cellular work)
atp syntase
make s atp ADP + P uses energy from H+ gradient
chemiosmosis
h+power
atp synthase acts like a rotor where H+ bind asd it spins activating catalytic sites to make ADP into ATP
produces 26-28 atp
cellular respiration summary
gylcolysis: in: 1 glucose
out: 2 pyruvate,2 ATP,2 NADH
pyruvate oxidation: in: 2 pyruvate
out:2 acetyl CoA,2 CO2,2 NADH
krebs: in: 2 acetyl coa
out:4 CO2,2 ATP,6 NADH,2 FADH2
oxidative phosphorylation: in: 10 nadh, 2 fadh2
out: 26-28 atp
total atp 30-32
anaerobic respiration
generates ATP using an ETC in the absence of oxygen
the final electron acceptors: sulfates or nitrates
fermentation
generates ATP without an ETC
Extension of glycolysis
Recycles NAD+
Occurs in the cytosol
NO oxygen
Two types:
Alcohol fermentation
Lactic acid fermentation
alcohol fermentation
pyruvate is converted into ethanol
in: 2 pyruvate
out: 2co2
in: 2nadh+2h+
out: 2nad+, 2 ethanol
2atp
nadh v nadph
nadh-cellular respiration
nadph-photosynthesis
lacticc acid fermentation
pyruvate is reduced directly by NADH to form lactate
in 2 pyruvat3, 2 nadh+2h+
out: 2 lactate
NO RELEAE OF CO2
2atp
breakdown of lactate
lactate goes in blood, broken down back to glucose in liver
lowers ph in blood and if is not broken down can cause lactic acidosis(Excessively low blood pH
photosynthesis
light energy to chemical
photosynthesis first developed in prokaryotic organisms
cyanobacteria: early prokaryotes capable of photosynthesis
Oxygenated the atmosphere of early Earth
foundation of eukaryotic photsynthesis
primary location of photosynthesis in most plants
leaves (mesophyll, the primary location of photosynthesis in most plants)
chloroplast
found in mesophyll’
surrounded by a double membrane
have stroma and thylakoid
stomata
pores in leaves that allow CO2 in and O2 out
stroma
aqueous internal fluid
Thylakoids
form stacks known as grana
Chlorophyll
green pigment in thylakoid membranes
simplified formula
6 CO2 + 6 H2O + light energy C6H12O6 + 6 O2
Redox reaction:
reaction involving complete or partial transfer of one or more electrons from one reactant to another
redox in photo synthesis
reduction co2 to glucose
water to oxygen
photo light reactions
in thylakoid
synthesis calvin cycle
in stroma