Chapter 1 : Stratosphere
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Sunscreens made of ZnO and TiO2 nanoparticles ___ or scatter light
reflect
sunscreens that block UV-B but not UV-A may lead to an increase in ____ skin cancer
melanoma
ozone is only formed in the middle of the stratosphere because the air is ___ which means the ___ molecule concentration is relatively ___ compared to the top of the ___.
also, UV- __ light is relatively less compared to the top of the stratosphere
so __ + O = O3 + heat
denser, oxygen, high, stratosphere
C
O2
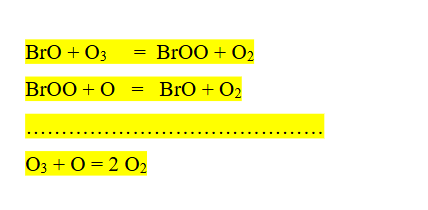
Write down mechanism I of ozone destruction by BrO as a pollutant
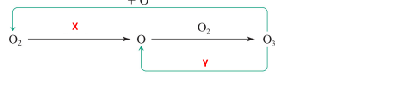
In the Chapman Mechanism, Which UV lights are required in ‘X’ and ‘Y’ respectively
UV-C, UV-B
why should chemical derivatives be avoided?
it uses additional ___ and ____ waste
reagents, generates
UV light effect on fish include: decreased ____ capacity and impaired ___ development
reproduction, larval
mechanism II does not require atomic ___ and involves __ different starting materials X and X’
oxygen, two
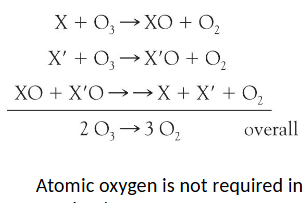
peptide based sunscreen is
1)?
2)?
3)?
non-toxic, biodegradable, environment friendly
UV-B light on the eyes can cause loss of color ___ and eventually ____
vision, blindness
ZnO and Oxybenze sunscreen block which two forms of light?
UV-A and UV-B
Avobenzene only blocks?
UV-B
What are the four factors that affect reaction rates?
chemical nature of the reactants
temperature of the reactants
concentration of the reactants
the presence of a catalyst
the rate of a reaction ___ with temperature
increases
The rate of a reaction ___ with the concentration of reactants
increases
Collision theory: reactants must ___ in order to react with each other
collide
Postulates of Collision Theory:
1) The rate of the reaction is proportional to the rate of ___ collisions
2) Molecules must be ____ properly when they collide
reactant
oriented
Molecules must have adequate ____ energy to react.
kinetic
In collision theory, the kinetic energy supplied must be high enough to break the ____
chemical bonds
In Collision Theory, molecules with kinetic energies that are too small will ___ each other and ____ (will or won’t) react?
bounce off of each other
won’t