Chemistry II - IMF
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Dispersion Force
An instantaneous dipole on any one helium atom induces instantaneous dipoles on neighboring atoms, which then attract one another.
It is every molecule
dipole–dipole force
The positive end of one permanent dipole attracts the negative end of another; this attraction
Only happens in polar molecules
Hydrogen Bonding
When H bonds directly to F, O, or N, the bonding atoms acquire relatively large partial charges, giving rise to strong dipole–dipole attractions between neighboring molecules.
What should you not confuse about Hydrogen bonds and Chemical bonds?
Mistaking one for the other. Chemical bonds occur between individual atoms within a molecule, whereas hydrogen bonds—like dispersion forces and dipole–dipole forces—are intermolecular forces that occur between molecules.
ion–dipole force
occurs when an ionic compound is mixed with a polar compound; it is especially important in aqueous solutions of ionic compounds.
Have No Fear Of Ice Cold Beer
homonuclear diatomic elements: Hydrogen, Nitrogen, Fluorine, Oxygen, Iodine Chlorine, Bromine
What are the Factors that affect the strength of Dispersion Forces?
• Larger atoms or molecules are more polarizable than smaller atoms or molecules • The Strength of dispersion forces increases with the ease of distorting the e-cloud.
increased surface area = increased interactions between molecules (stronger the dispersion force) • Linear molecules (remember drawing) experience higher dispersion forces than branched molecules
Adhesive Forces
Interactions between unlike particles
ex: water in a glass tube. Water is more attracted to the glass than itself.
Cohesive Forces
Interactions between like particles
ex: hydrogen bonding in water. Hg(l) (mercury) more attracted to itself than the glass
Concave meniscus:
adhesive forces between H2O and glass [interactions that occur between unlike particles] (greater than or equal) cohesive forces of water with itself [interactions that occur between like particles]
Convex meniscus:
cohesive forces of Hg(l) > adhesive forces of Hg with glass
The ability of a liquid to flow against gravity up a narrow tube. • Involves adhesive forces with the structure and cohesive forces within the liquid. small diameter = large rise in liquid
Surface Tension
is the tendency of a liquid's surface to resist external force due to the cohesive nature of its molecules. It is caused by intermolecular forces (IMFs). Remember bulk water and surface water.
Viscosity
Measure of resistance of a fluid to flow units = Poise, but typically se cP (centipoise)
Factors Affecting Viscosity:
Molecular Shape – More surface area → stronger intermolecular forces → higher viscosity.
Molar Mass – Higher mass → stronger intermolecular forces → higher viscosity.
Temperature – Lower temperature → less kinetic energy → stronger intermolecular forces → higher viscosity.
Vapor Pressure
Pressure of a gas in dynamic equiibrum with liquid
Remember Pressure converions
1 atm = 1.013 × 10^5 Pa = 101.3 kPa = 760 mmHg + 760 Torr = 14.7 9(bs/in²) psi
Factors Affecting Evaporation Rates &Vapor Pressure: Temperature
The higher the temperature, the more molecules can break free from the bulk liquid and escape to the gas phase • More molecules escaping to the gas phase = higher vapor pressure
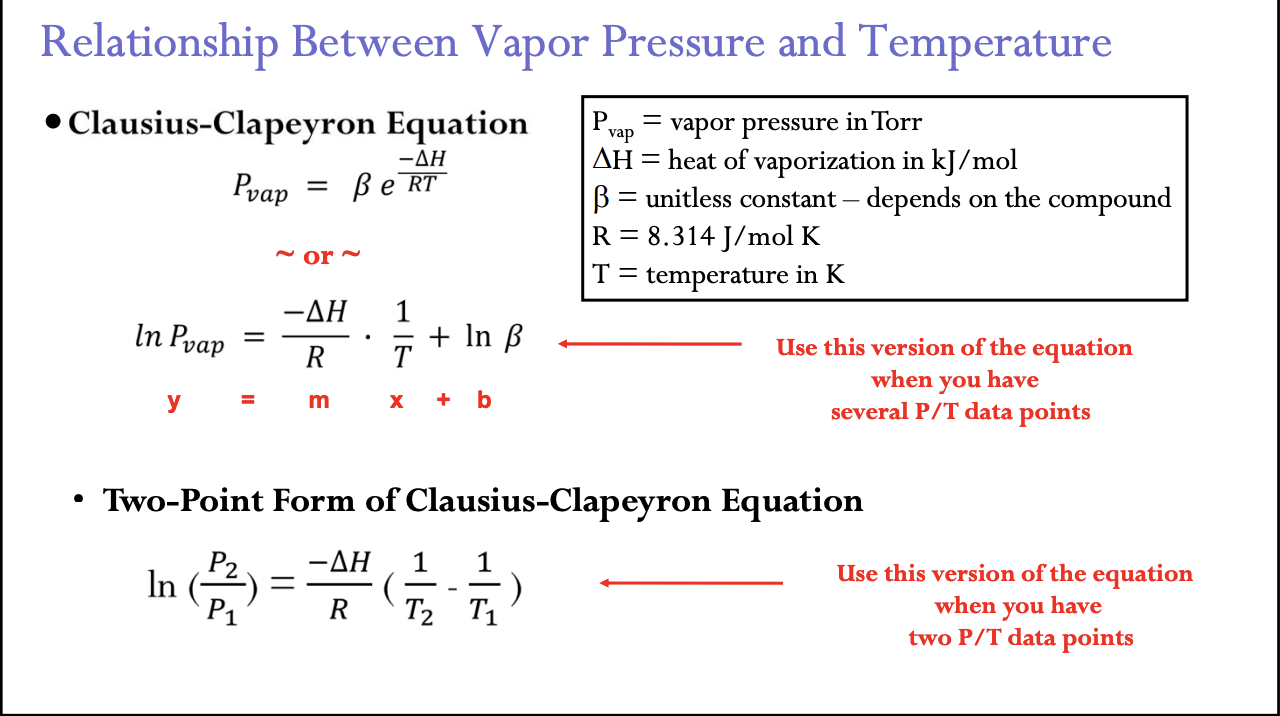
Clausius-Clapeyron Equation
P vap = vapor pressure in Torr
(delta) H = heat of vaporization in kJ/mol
B = unitless constant – depends on the gas
R = 8.314 J/mol K
T = temperature in K
Factors Affecting Evaporation Rates &Vapor Pressure: Surface Area
• More surface area, more molecules on the surface
• More molecules on the surface, the faster the evaporation
How can a molecule enter the gas phase?
• The stronger the IMF, the higher the amount of KE required to enter gas phase
If there is a presence of nonvolatile( liquids that don’t evaporate easily) Solute then…
• Decreases vapor pressure of solution compared to pure solvent • Ocean salt water evaporates more slowly than fresh lake water
Heat of vaporization ~ ∆H vap
The energy required to convert one mole of liquid at its boiling point into a vapor state
• Also called enthalpy of _________
Heat of fusion ~ ∆H fus
• Energy required to convert one mole of solid at its melting point into liquid state
• Also called enthalpy of fusion
Deposition-
• Compound goes from gas to a solid
Ex: Frost in your freezer
Sublimation -
Compound goes from solid to gas and does not go through the liquid state
Ex: Dry Ice & Freezer Burn
Fusion-
Better known as melting • Compound goes from solid to a liquid — Ex: Melting ice
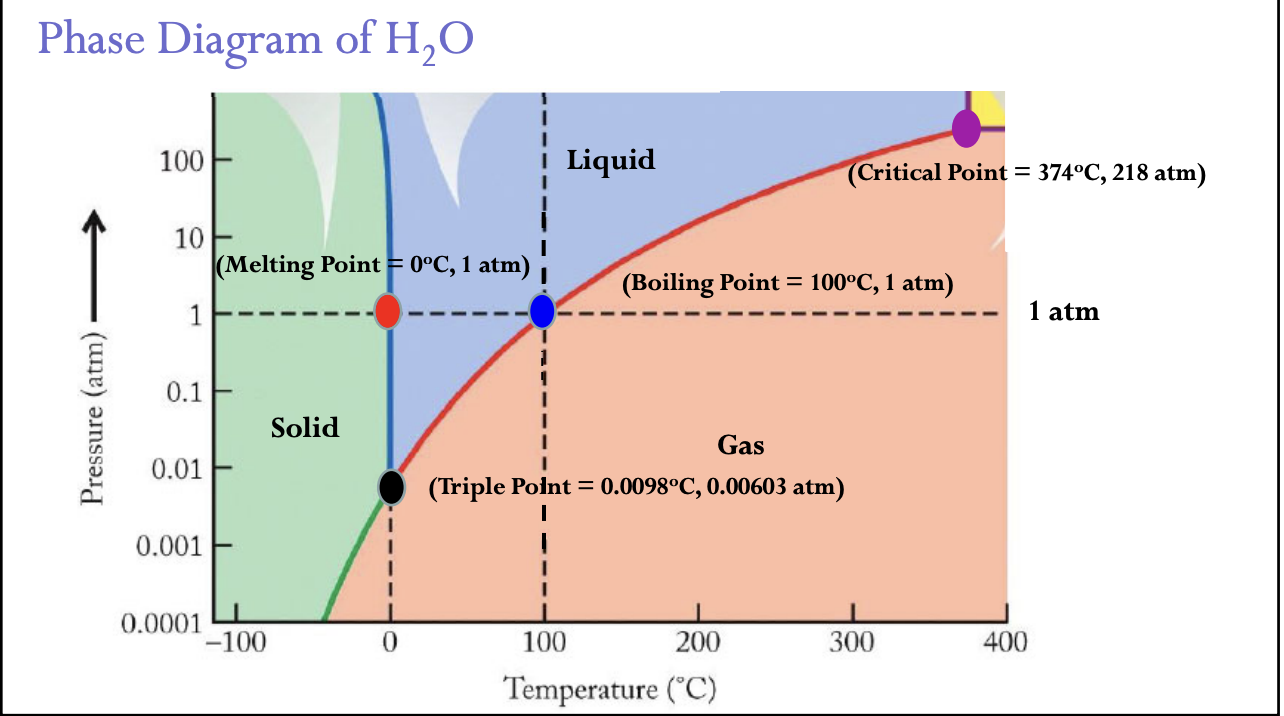
Phase Diagram