C1.2: Cell Respiration | IB Biology HL
1/48
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
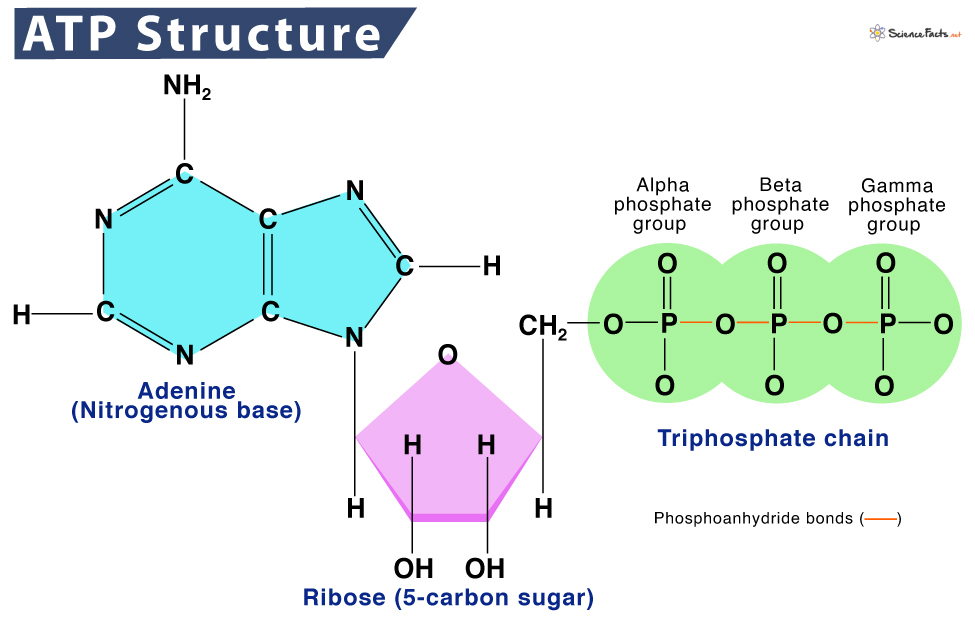
Describe the structure of ATP.
C1.2.1: ATP as the molecule that distributes energy within cells.
ATP (Adenosine triphosphate) is a nucleotide made of adenine, ribose, and 3 phosphates.
The adenine (nitrogenous base) is bonded to the ribose sugar. The 3 phosphates are connected by high-energy bonds to the ribose sugar as well. The bonds in the last 2 phosphates are unstable because their negative properties make them repel.
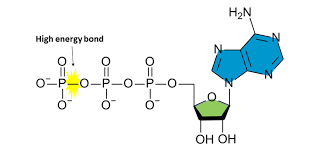
Outline properties of ATP that make it suitable for the use as an energy currency.
C1.2.1: ATP as the molecule that distributes energy within cells.
ATP has 3 phosphates that are connected by high-energy bonds. ATP is suitable for carrying energy because energy is stored in these bonds.
Because the last 2 phosphates have negative properties, they repel. As a result, the bond in the last 2 phosphates is unstable. This makes ATP suitable to be used as the energy currency because breaking this bond releases energy that is used for metabolic reactions.
Outline example cellular processes that require use of ATP.
C1.2.2: Life processes within cells that ATP supplies with energy.
ATP provides energy for chemical reactions within cells such as active transport across membranes, anabolism (the synthesis of macromolecules, or complex molecules), movement of the cell wall, or movement of components with the cell (such as chromosomes during mitosis or meiosis).
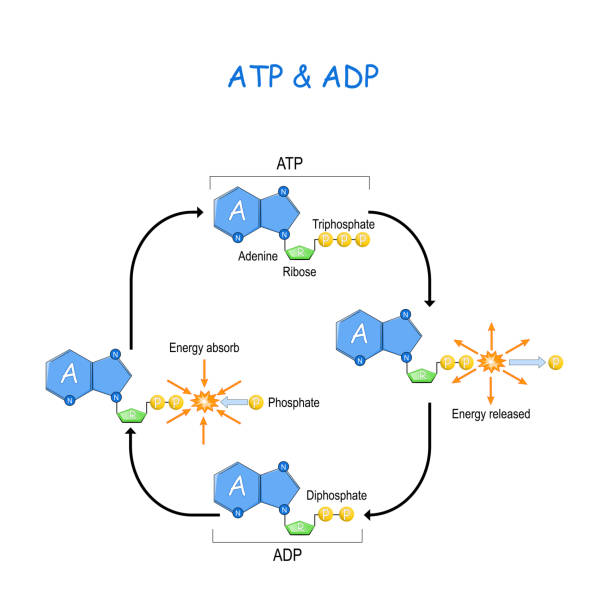
Describe the ATP-ADP cycle, including the roles of hydrolysis and phosphorylation.
C1.2.3: Energy transfers during interconversion between ATP and ADP.
In the ATP-ADP cycle, ATP is first converted into ADP and inorganic phosphate (Pi). This is known as ATP hydrolysis, where ATP is broken down (ATP —> ADP + Pi). ATP hydrolysis releases energy (in the form of heat) to use as activation energy for metabolic reactions. This is an exothermic reaction (catabolic reaction), and the energy is used for many tasks in the cell (C1.2.2).
Next, is the regeneration of ATP from ADP and Pi through phosphorylation (a type of condensation reaction). This reaction requires energy. The energy needed for ATP regeneration comes from cellular respiration.
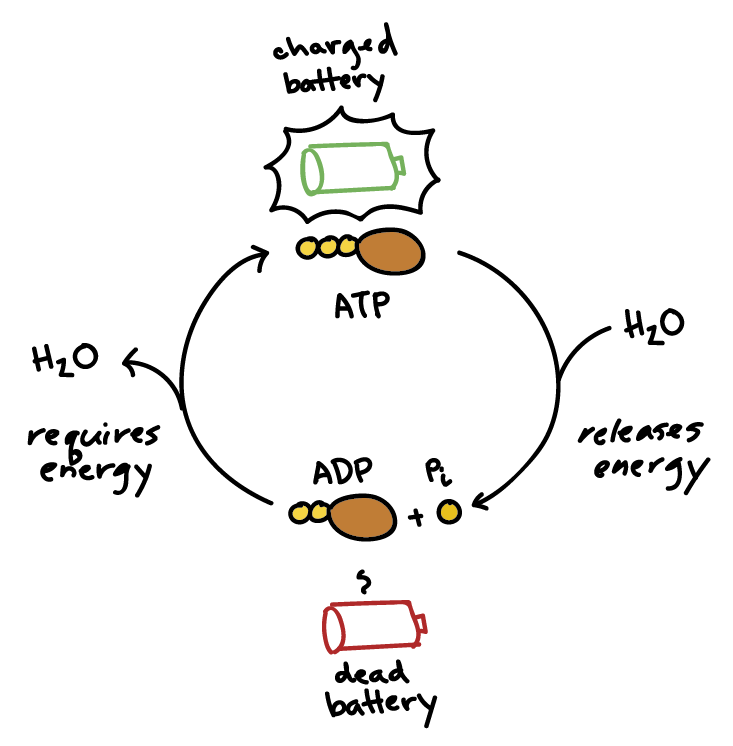
State why heat is generated during the ATP-ADP cycle.
C1.2.3: Energy transfers during interconversion between ATP and ADP.
Heat is generated during the ATP-ADP cycle because energy is released during ATP hydrolysis. Because the conversion process isn’t 100% efficient, energy is released in the form of heat.
Define cellular respiration.
C1.2.4: Cell respiration as a system for producing ATP within the cell using energy released from carbon compounds.
Cellular respiration is the controlled release of ATP energy from food in cells.
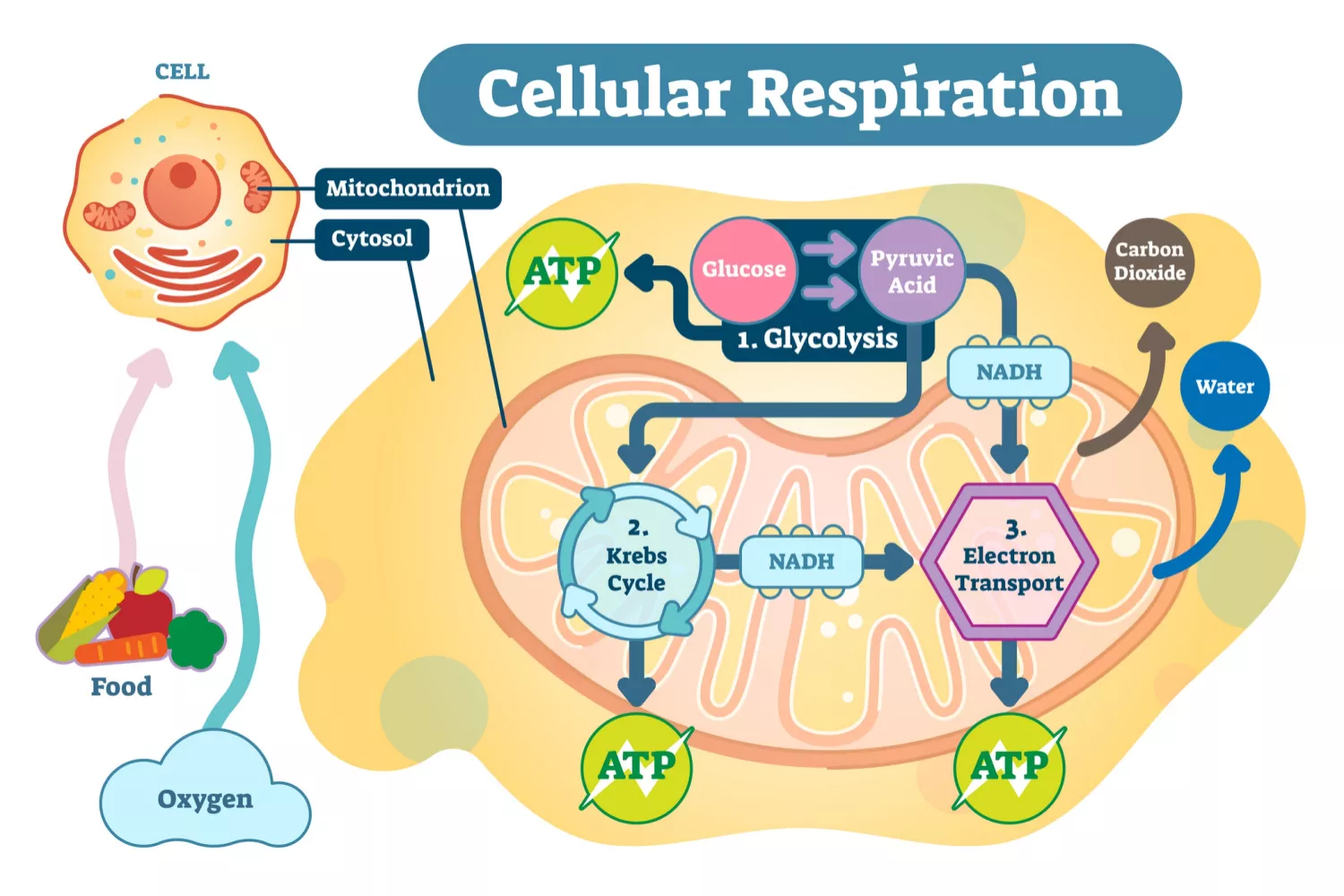
Distinguish between cellular respiration and gas exchange.
C1.2.4: Cell respiration as a system for producing ATP within the cell using energy released from carbon compounds.
Cellular respiration is the controlled release of ATP energy from food within cells. There are chemical changes.
Gas Exchange, however, is an exchange of carbon dioxide and oxygen in living cells and tissues through diffusion. It occurs in the alveoli of the lungs and respiring tissues (not between cells).
List reasons why cellular respiration must be continuously performed by all cells.
C1.2.4: Cell respiration as a system for producing ATP within the cell using energy released from carbon compounds.
Cellular respiration must be continuously performed by all cells because…
Cells require a constant supply of energy
ATP isn’t transferred from cell to cell
When energy in ATP is used, some of it generates heat, which keeps the organism warm (maintain internal temperatures)
List common substrates of cellular respiration.
C1.2.4: Cell respiration as a system for producing ATP within the cell using energy released from carbon compounds.
Glucose
Fatty acids
Other organic materials (molecules that contain carbon but not oxides or carbonates)
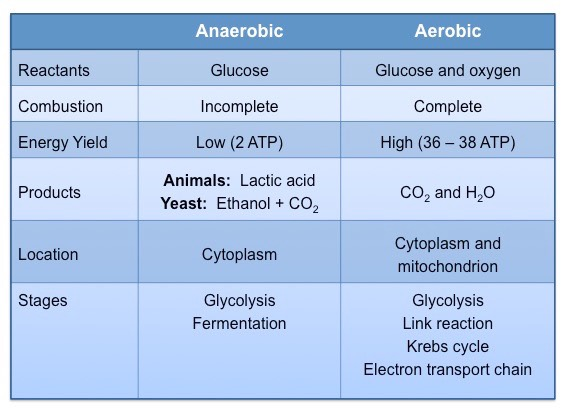
Compare and contrast anaerobic and aerobic respiration in humans.
C1.2.5: Differences between anaerobic and aerobic cell respiration in humans.
Both anaerobic fermentation and aerobic respiration occur in cells. They both use glucose as the initial substrate and produce ATP by using enzymes to catalyze reaction pathways.
Anaerobic fermentation does not require oxygen, while aerobic respiration does.
Both anaerobic fermentation and aerobic respiration produce ATP, but aerobic respiration has a higher ATP yield (net 36 ATP) than anaerobic fermentation/respiration (low yield of net 2 ATP).
Aerobic respiration produces carbon dioxide and water as waste products, while anaerobic respiration produces lactate as a waste product.
Aerobic respiration primarily occurs in the mitochondria, while anaerobic fermentation typically occurs in the cytoplasm.
Mitochondria are required for aerobic respiration, but not anaerobic respiration.
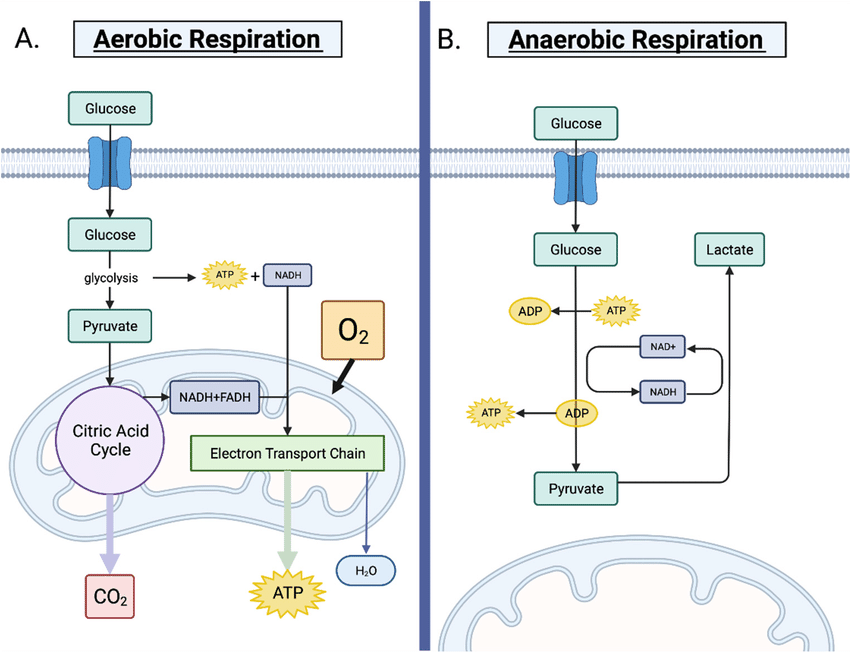
Write simple word equations for aerobic and anaerobic cell respiration.
C1.2.5: Differences between anaerobic and aerobic cell respiration in humans.
Aerobic respiration: Glucose + Oxygen —> Carbon Dioxide + Water
Anaerobic respiration: Glucose —> Lactate
List factors affecting the rate of respiration for insects/seeds or yeast.
C1.2.6: Variables affecting the rate of cell respiration.
Factors affecting the rate of respiration of insects or seeds:
Temperature
Factors that affect the rate of respiration of yeast:
Temperature
Mass of yeast
pH of suspension
Type of substrate (food source)
Substrate concentration
Chemical inhibitors of enzymes
List three approaches for determining the rate of cellular respiration.
C1.2.6: Variables affecting the rate of cell respiration.
Using a respirometer
Measure the decrease in oxygen (as it is used during respiration)
Increase in carbon dioxide (as it is produced by respiration)
List alternative methods to measure respiration (other than using respirometers).
C1.2.6: Variables affecting the rate of cell respiration.
Measure volume of gas produced by yeast (with several methods)
Measure the change in oxygen concentration (using oxygen probes)
Measure the change in carbon dioxide (using carbon dioxide probes)
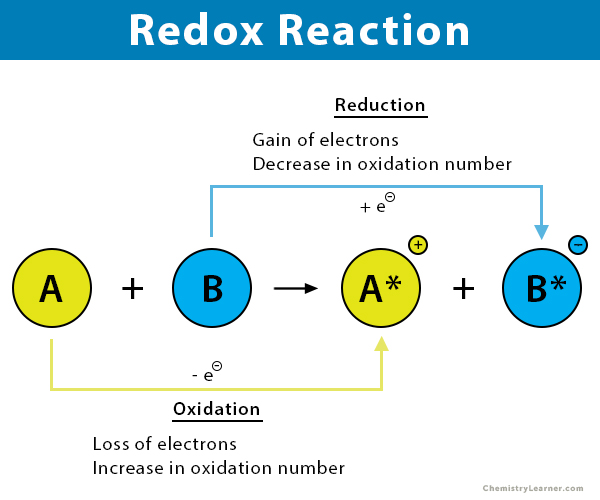
Outline oxidation and reduction reactions in terms of movement of hydrogen and electrons.
C1.2.7 (AHL): Role of NAD as a carrier or hydrogen and oxidation by removal of hydrogen during cell respiration.
Redox reactions are reactions that involve oxidation and reduction of substances.
Oxidation reactions are the loss of electrons and the removal of hydrogen (increased oxidation number).
Reduction is the gain of electrons and the addition of hydrogen (decreased oxidation number).
(“OILRIG” - Oxidation Is Loss, Reduction Is Gain of electrons and hydrogen)
Define “electron carrier.”
C1.2.7 (AHL): Role of NAD as a carrier or hydrogen and oxidation by removal of hydrogen during cell respiration.
A small organic molecule involved in the transfer or shuttling of electrons from one molecule to another.
State the name of the electron carrier molecule in cellular respiration.
C1.2.7 (AHL): Role of NAD as a carrier or hydrogen and oxidation by removal of hydrogen during cell respiration.
Nicotinamide adenine dinucleotide (NAD)
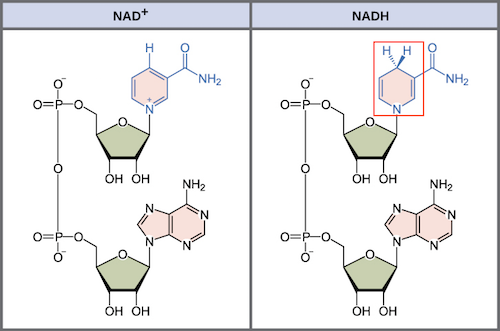
Outline the formation of reduced NAD (or NADH, NAD + H+) during glycolysis.
C1.2.7 (AHL): Role of NAD as a carrier or hydrogen and oxidation by removal of hydrogen during cell respiration.
During glycolysis, NAD is reduced when it accepts hydrogen and electrons from the 3-carbon compound.
The 3-carbon compound is oxidized because hydrogen with an electron is removed from the substrate (dehydrogenation).
State which types of reaction glycolysis occurs in.
C1.2.8 (AHL): Conversion of glucose to pyruvate by stepwise reactions in glycolysis with a net yield of ATP and reduced NAD.
Glycolysis occurs in both anaerobic and aerobic respiration.
State the location of the glycolysis reaction in a cell.
C1.2.8 (AHL): Conversion of glucose to pyruvate by stepwise reactions in glycolysis with a net yield of ATP and reduced NAD.
Glycolysis occurs in the cytoplasm of cells.
State that glycolysis is an example of a metabolic pathway catalyzed by enzymes.
C1.2.8 (AHL): Conversion of glucose to pyruvate by stepwise reactions in glycolysis with a net yield of ATP and reduced NAD.
All reactions in glycolysis are catalyzed by specific different enzymes.
Therefore, glycolysis is an example of a metabolic pathway catalyzed by enzymes.
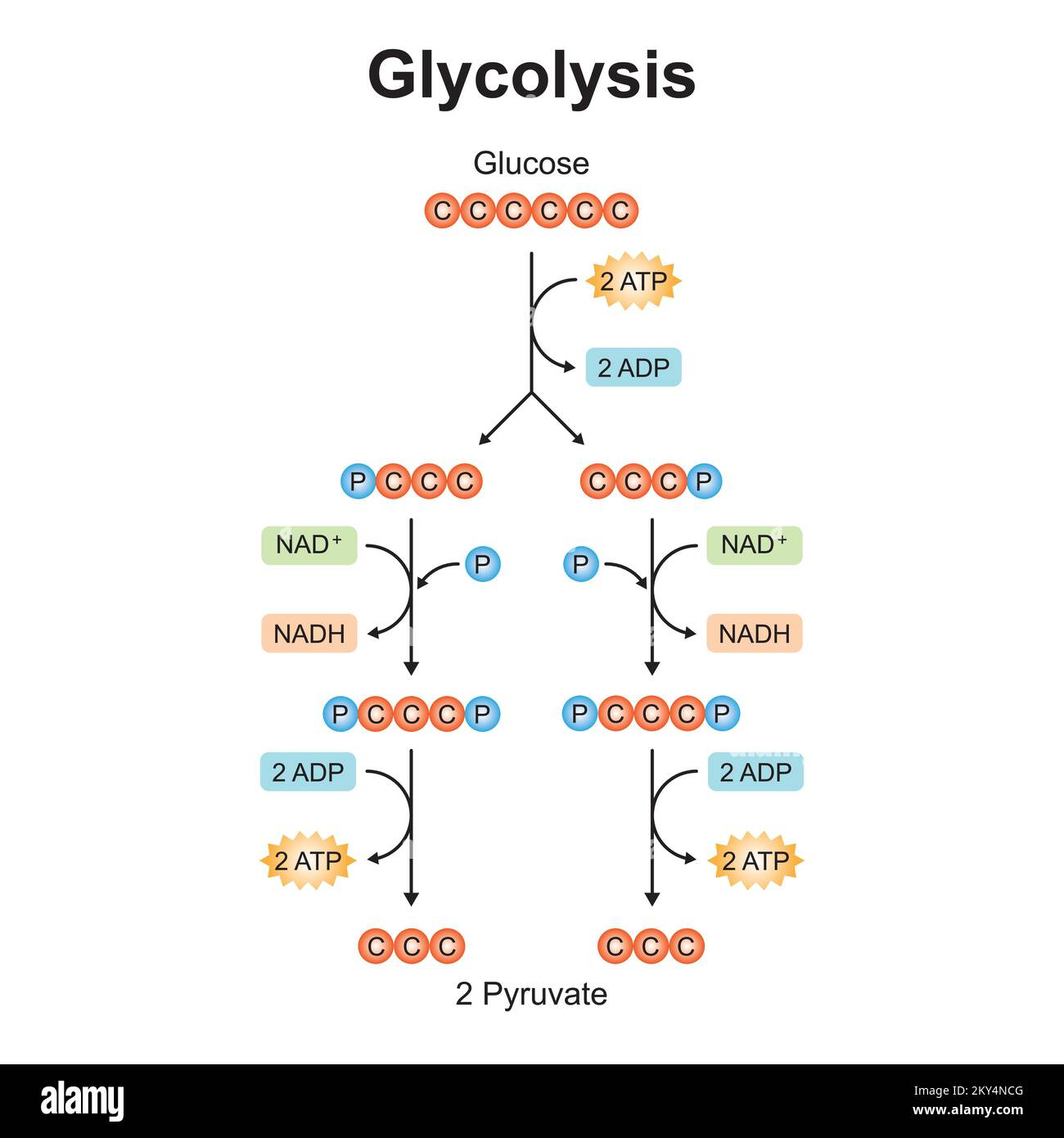
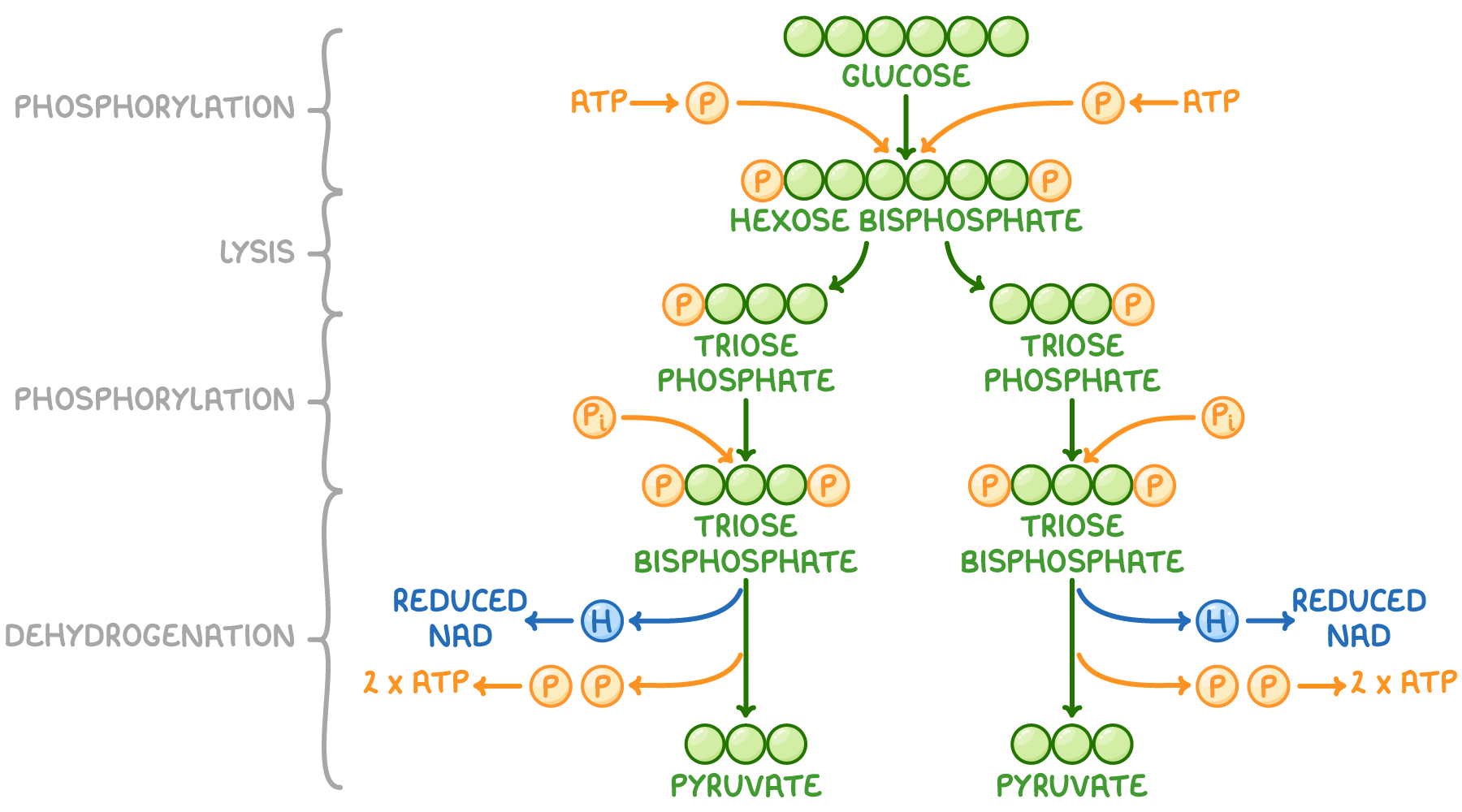
Outline the glycolysis reaction (including phosphorylation of glucose, lysis, oxidation, and ATP formation).
C1.2.8 (AHL): Conversion of glucose to pyruvate by stepwise reactions in glycolysis with a net yield of ATP and reduced NAD.
There are 4 main stages of glycolysis: phosphorylation, lysis, oxidation, and ATP formation.
First, in phosphorylation, 2 ATP molecules are hydrolyzed (broken down with water). Each contributes a phosphate group. The 2 phosphate groups from 2 ATPs attach to glucose. This creates an unstable 6-carbon compound known as hexose biphosphate.
Next, in lysis, the unstable 6-carbon compound is split into two 3-carbon compounds, or 2 triose phosphates.
Then, in oxidation, NAD is reduced to NADH by accepting electrons and hydrogen from the triose phosphate. Accordingly, the triose phosphate loses electrons and hydrogen, so the 3-carbon compounds are oxidized. In this stage, two molecules of NADH are produced (one from each triose phosphate). Note: An inorganic phosphate is also attached to the triose phosphate so that it has 3 carbons and 2 phosphates.
Lastly, in ATP formation, each 3-carbon compound is converted to pyruvate. Two ADP molecules take the two phosphates of each compound, so a total of 4 ATP molecules are produced. The 3-carbon compound is converted to pyruvate. A net of 2 ATP molecules are produced.
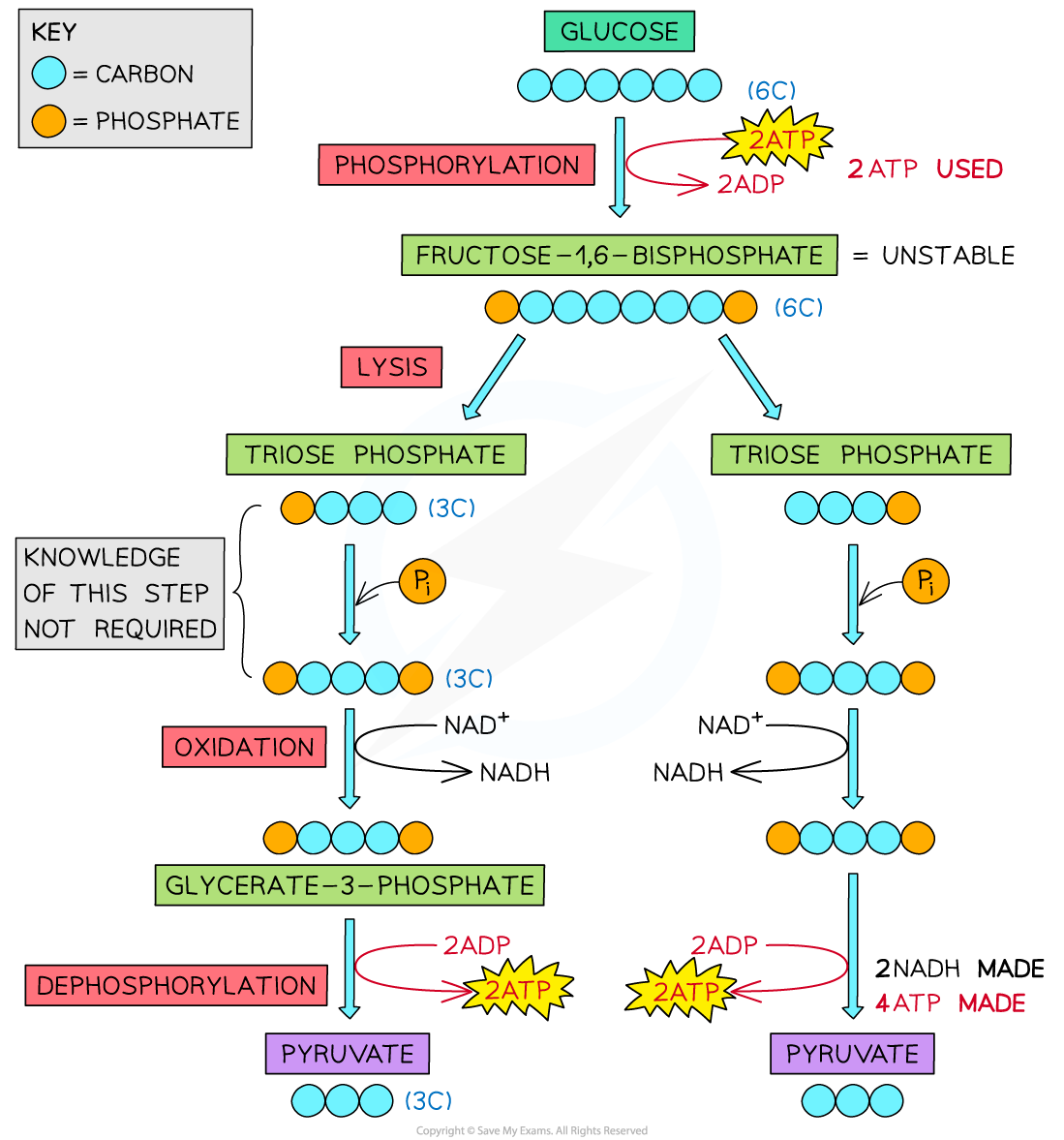
State the net yield of ATP and reduced NAD produced in glycolysis.
C1.2.8 (AHL): Conversion of glucose to pyruvate by stepwise reactions in glycolysis with a net yield of ATP and reduced NAD.
There is a net yield of 2 ATP molecules and 2 NADH (reduced NAD) molecules in glycolysis.
State why NAD must be regenerated in anaerobic respiration.
C1.2.9 (AHL): Conversion of pyruvate to lactate as a means of regenerating NAD in anaerobic cell respiration.
NAD must be regenerated in anaerobic respiration to allow glycolysis to continue. It allows ATP energy to be produced even without the presence of oxygen.
Outline the process of regenerating NAD and production of lactate in humans during anaerobic respiration.
C1.2.9 (AHL): Conversion of pyruvate to lactate as a means of regenerating NAD in anaerobic cell respiration.
After glycolysis, pyruvate is converted to lactate in humans. For this process to occur, NADH has to donate its electrons to convert pyruvate to lactate. As a result, this reaction oxidizes NADH (reduced NAD) to NAD+ while producing lactate as a waste product.
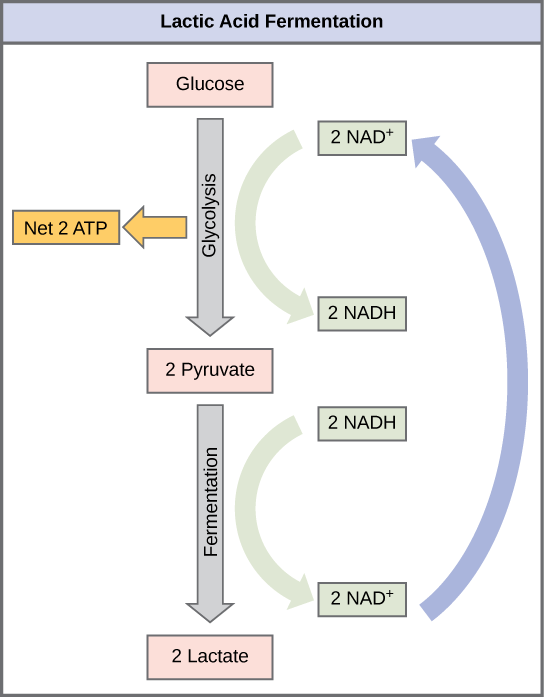
Outline the process of regenerating NAD and production of ethanol in yeast during anaerobic respiration.
C1.2.10 (AHL): Conversion of pyruvate to lactate as a means of regenerating NAD in anaerobic cell respiration.
Anaerobic respiration in yeast begins with glycolysis, which yields 2 ATP, 2 pyruvate, and 2 NADH molecules.
Pyruvate is converted to ethanol and carbon dioxide. To do this, NADH has to donate its electrons to convert pyruvate to ethanol, so NADH is oxidized into NAD while producing ethanol.
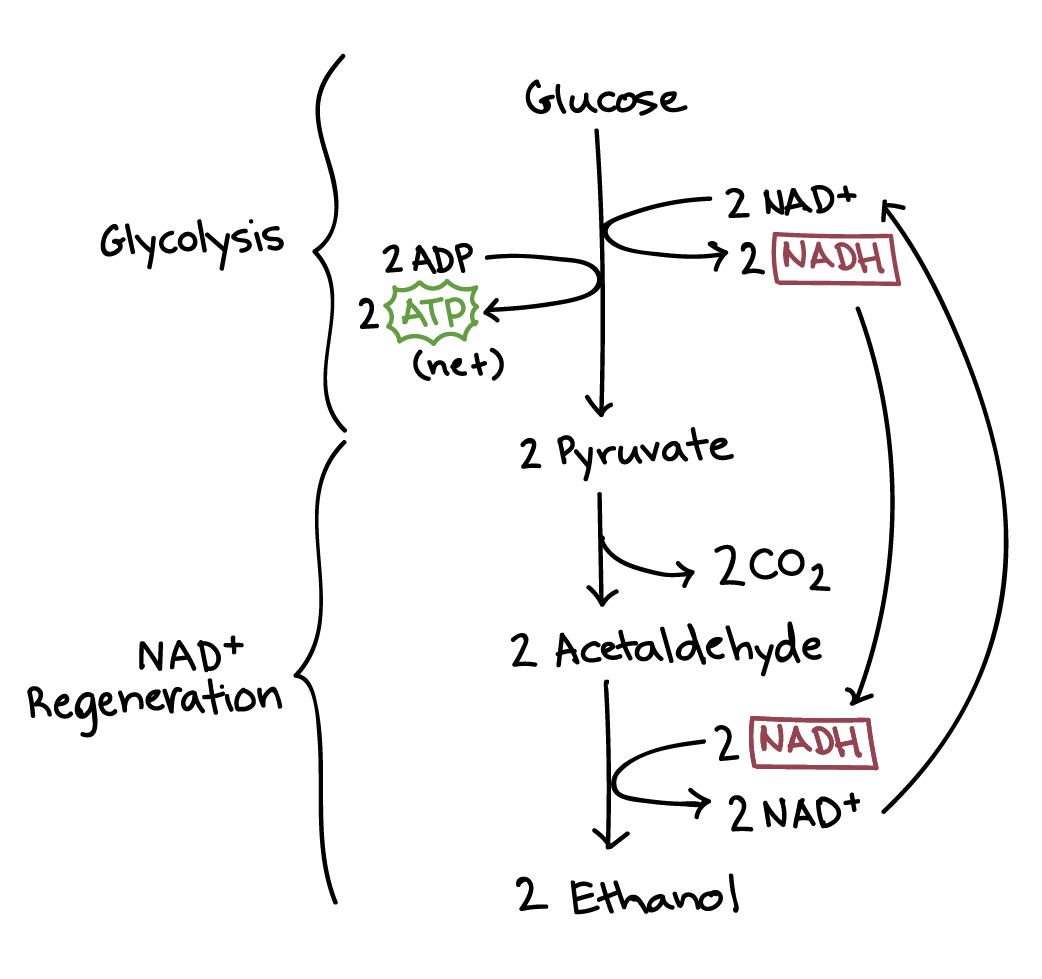
Compare anaerobic respiration in yeasts and humans.
C1.2.10 (AHL): Conversion of pyruvate to lactate as a means of regenerating NAD in anaerobic cell respiration.
The pathways of anaerobic respiration in yeasts and humans are the same for the most part.
In both processes, glycolysis is the first step that produces 2 pyruvate, net 2 ATP and 2 NADH. Both processes are important for NAD regeneration to allow glycolysis to cintinue.
However, the regeneration of NAD using pyruvate is different. In humans, NADH is oxidized to NAD when it donates its electrons to convert pyruvate to lactate. In yeast, NADH is oxidized to NAD when it donates its electrons to convert pyruvate to ethanol and carbon dioxide.
Accordingly, the products formed in anaerobic respiration are different. Anaerobic respiration in humans produces lactate. Anaerobic respiration in yeasts produces ethanol and carbon dioxide.
Summarize the reactants and products of the link reaction.
C1.2.11 (AHL): Oxidation and decarboxylation of pyruvate as a link reaction in aerobic cell respiration.
The reactants of the link reaction are pyruvate and acetyl coenzyme. Pyruvate comes from glycolysis. Coenzyme A is needed to form acetyl-CoA.
The products of the link reaction is Acetyl coenzyme A (Acetyl-CoA), carbon dioxide, NADH + H+.
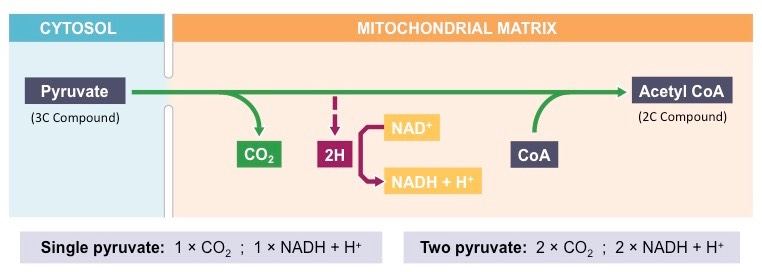
State the location of the link reaction.
C1.2.11 (AHL): Oxidation and decarboxylation of pyruvate as a link reaction in aerobic cell respiration.
The link reaction occurs in the matrix of the mitochondria.
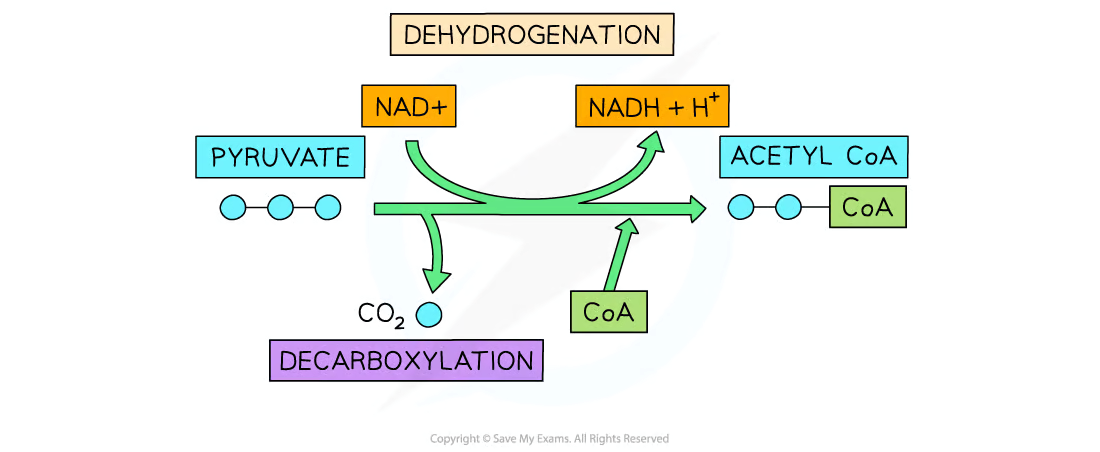
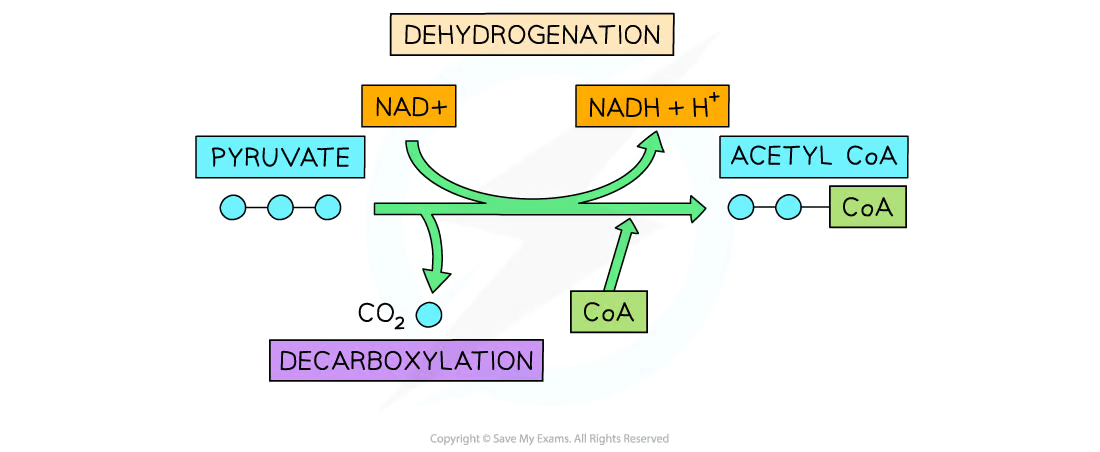
Outline the link reaction with references to decarboxylation, oxidation, and binding of CoA.
C1.2.11 (AHL): Oxidation and decarboxylation of pyruvate as a link reaction in aerobic cell respiration.
There are 3 main steps of the link reaction.
First, in decarboxylation, pyruvate loses a carbon dioxide molecule to produce a 2-carbon acetyl group.
Next, in oxidation, pyruvate is oxidized. It loses electrons and hydrogen while NAD is reduced to NADH (gains pyruvate’s electrons and hydrogen).
Lastly, the acetyl coenzyme A (Acetyl-CoA) is formed when the acetyl group binds to coenzyme A to form acetyl-CoA.
The acetyl group is transferred to the Krebs cycle by coenzyme A (by acetyl-CoA).
Describe how lipids and carbohydrates are metabolized to form acetyl-CoA.
C1.2.11 (AHL): Oxidation and decarboxylation of pyruvate as a link reaction in aerobic cell respiration.
Both lipids and carbohydrates can be metabolized to form acetyl-CoA.
Carbohydrates first undergo glycolysis before the link reaction.
Triglycerides (lipids) can bypass glycolysis, as they can be broken down into glycerol and fatty acids, which enter the mitochondrion to be converted into acetyl-CoA.
State the location of the Krebs cycle.
C1.2.12 (AHL): Oxidation and decarboxylation of acetyl groups in the Krebs cycle with a yield of ATP and reduced NAD.
The Krebs cycle occurs in the matrix of the mitochondrion.
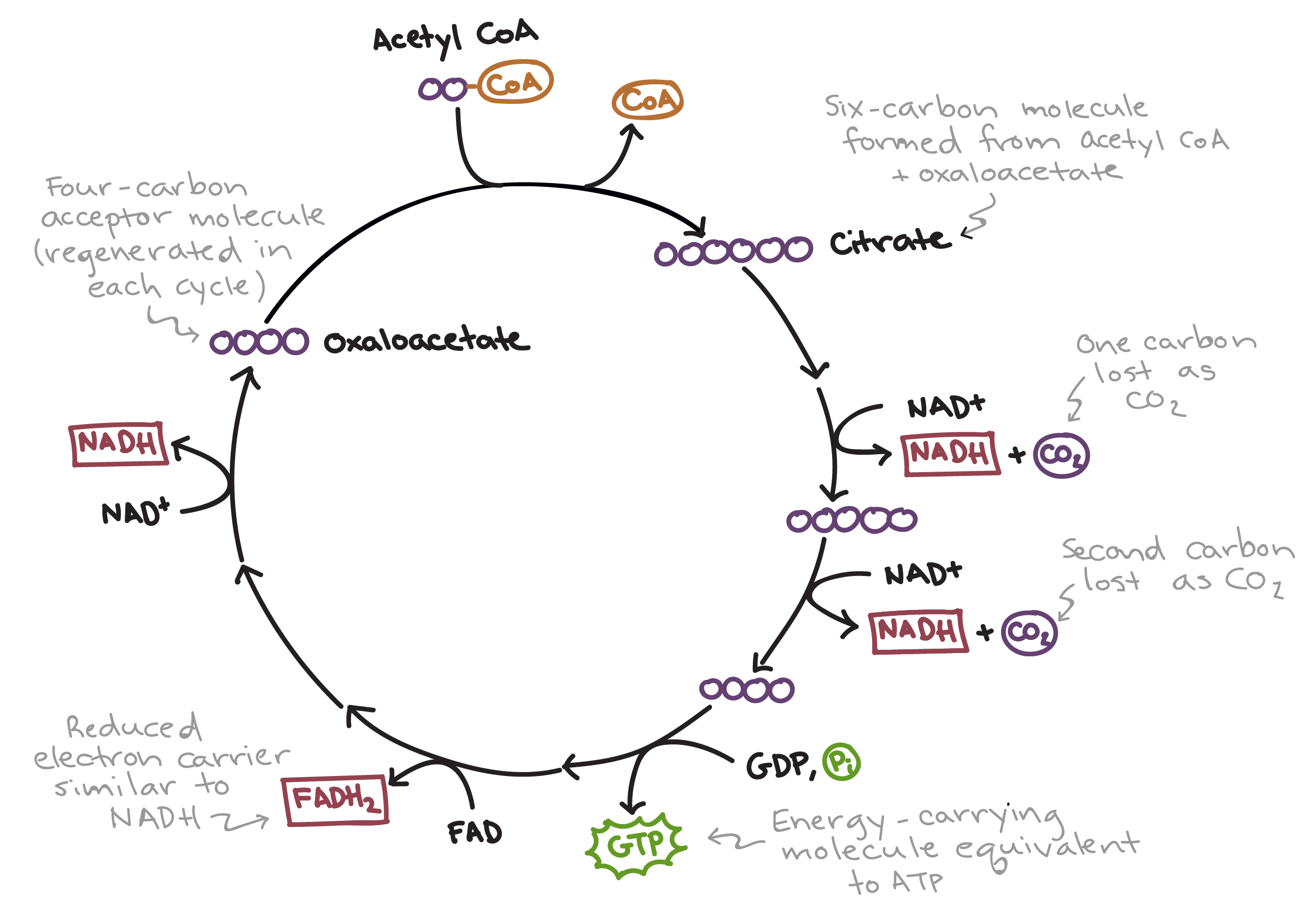
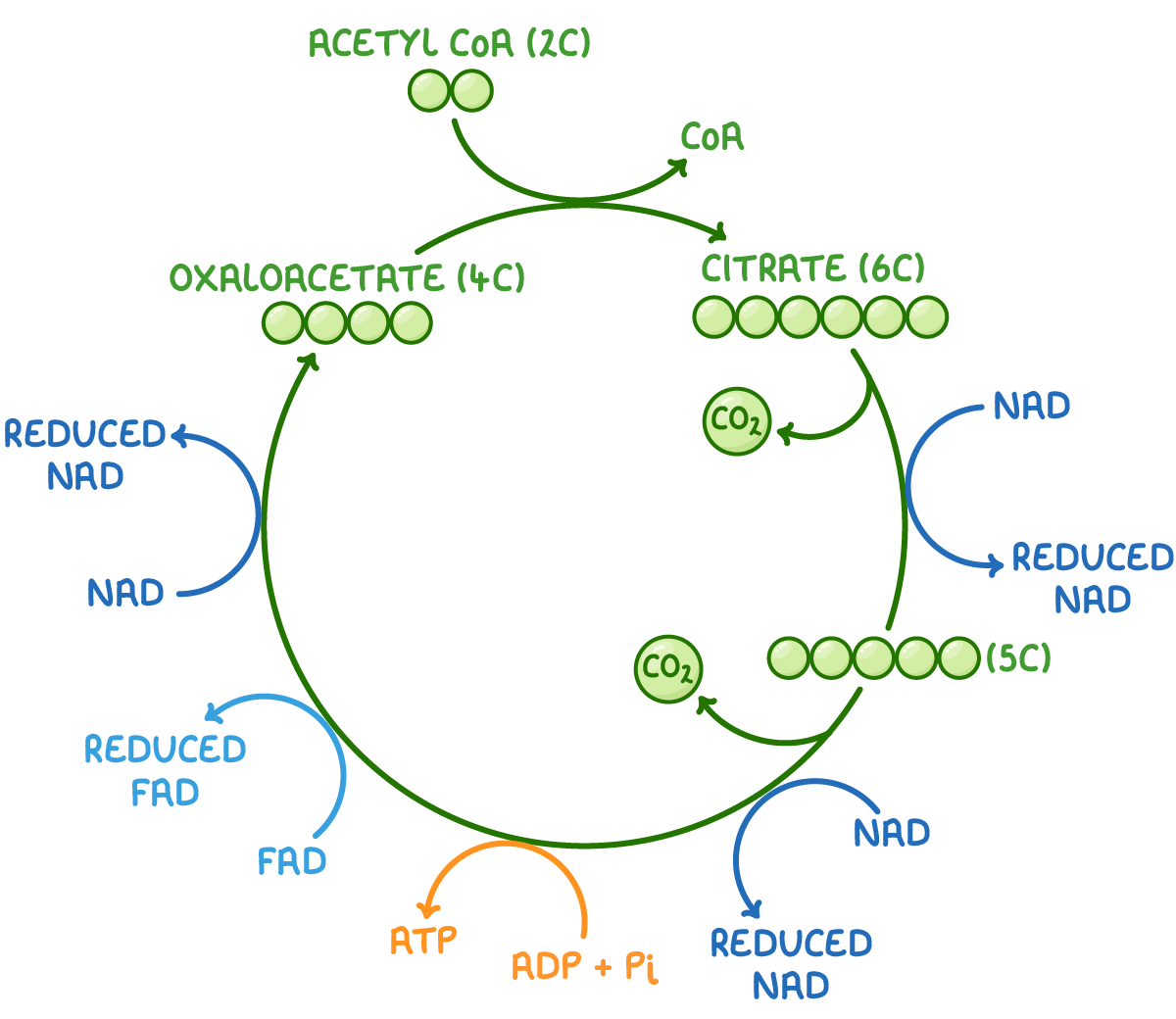
Outline the events of the Krebs cycle, referencing the formation of citrate from oxaloacetate, decarboxylation of citrate to reform oxaloacetate, formation of CO2, formation of ATP and the oxidation reactions that form reduced NAD (NADH) and reduced FAD (FADH2)
C1.2.12 (AHL): Oxidation and decarboxylation of acetyl groups in the Krebs cycle with a yield of ATP and reduced NAD.
First, 2-carbon acetyl group from acetyl-CoA (the coenzyme A is stripped away) combines with the 4-carbon oxaloacetate to form citrate (6-carbon).
Next, citrate is converted back to oxaloacetate through a series of decarboxylations and oxidations. Citrate is decarboxylated and oxidized to release CO2 and form a 5-carbon compound, and then the 5-carbon compound is decarboxylated and oxidized again. During these 2 intermediate stages, NAD is reduced to NADH as it takes electron, which are dehydrogenation reactions for citrate. Thus, a 4-carbon molecule, 2 CO2 and 2 NADH are formed.
Then, the 4-carbon compound is oxidized 2 more times. Once, FAD is reduced to FADH2 and then NAD is reduced to NADH. These 2 decarboxylations and 4 oxidation reactions produce oxaloacetate.
Oxaloacetate is regenerated by the reactions of the Krebs cycle.
State the purpose of reduced NAD and reduced FAD produced in the Krebs cycle.
C1.2.12 (AHL): Oxidation and decarboxylation of acetyl groups in the Krebs cycle with a yield of ATP and reduced NAD.
Reduced NAD and reduced FAD take electrons and hydrogen from citrate (dehydrogenation reactions). They then carry these electrons to the mitochondrial electron transport chain.
List the net products of one turn of the Krebs cycle.
C1.2.12 (AHL): Oxidation and decarboxylation of acetyl groups in the Krebs cycle with a yield of ATP and reduced NAD.
In one Krebs cycle (one 3C pyruvate), 2 CO2, 3 NADH, 1 FADH2, and 1 ATP are produced.
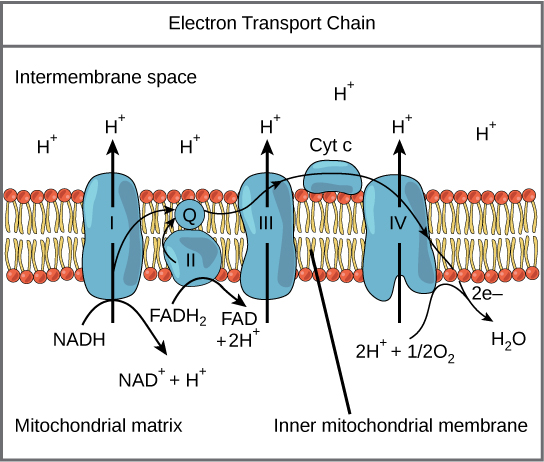
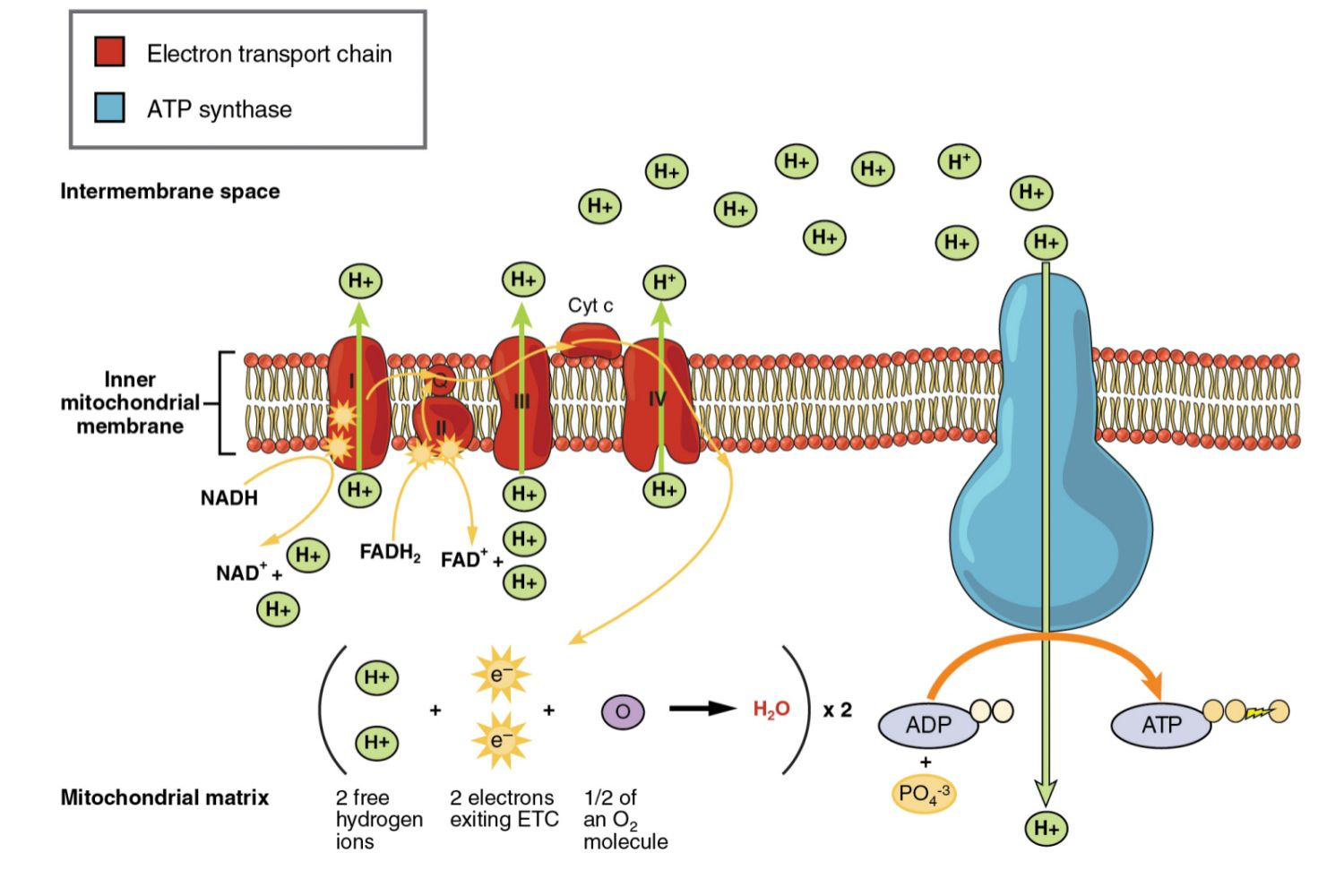
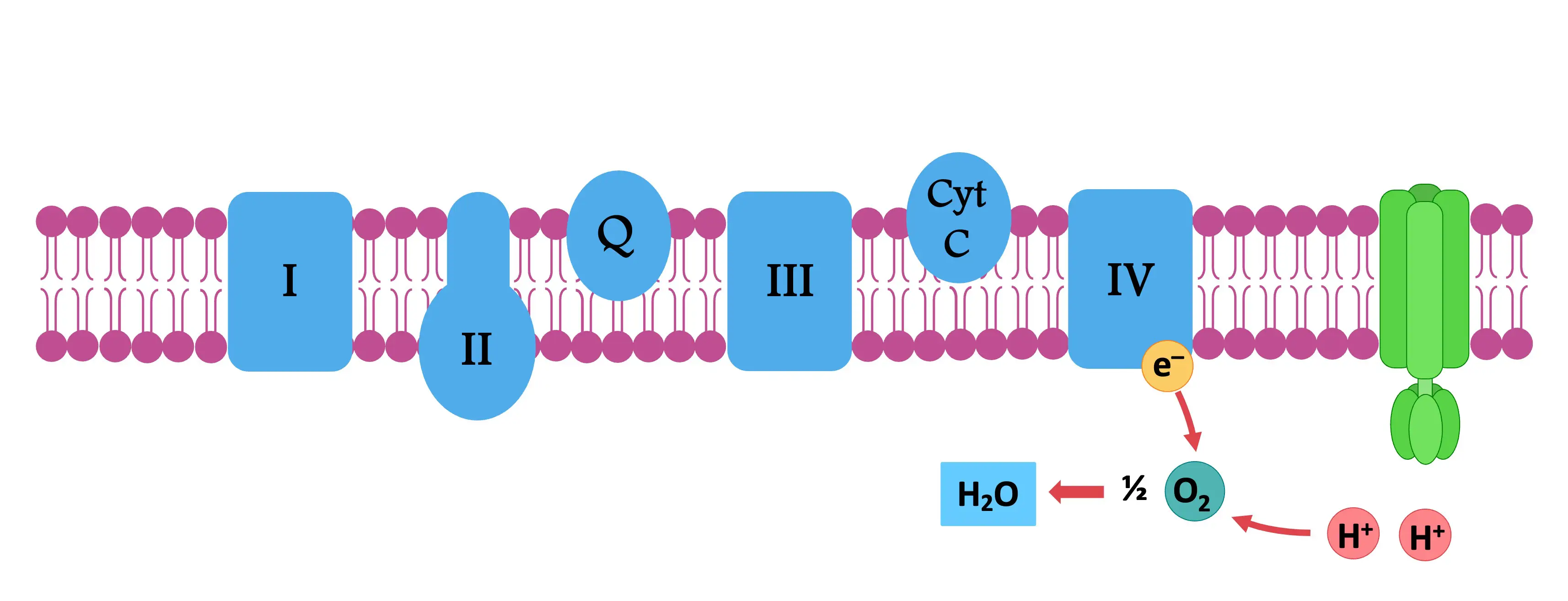
Outline the structure and function of the electron transport chain within a mitochondrion.
C1.2.13 (AHL): Transfer of energy by reduced NAD to the electron transport chain in the mitochondrion.
The electron transport chain involves a series of proteins embedded within the inner membrane of the mitochondrion. These proteins transfer protons between the intermembrane space (the space between the inner and outer membranes) and the mitochondrial matrix.
The function of the electron transport chain is to synthesize ATP by generating an electrochemical gradient.
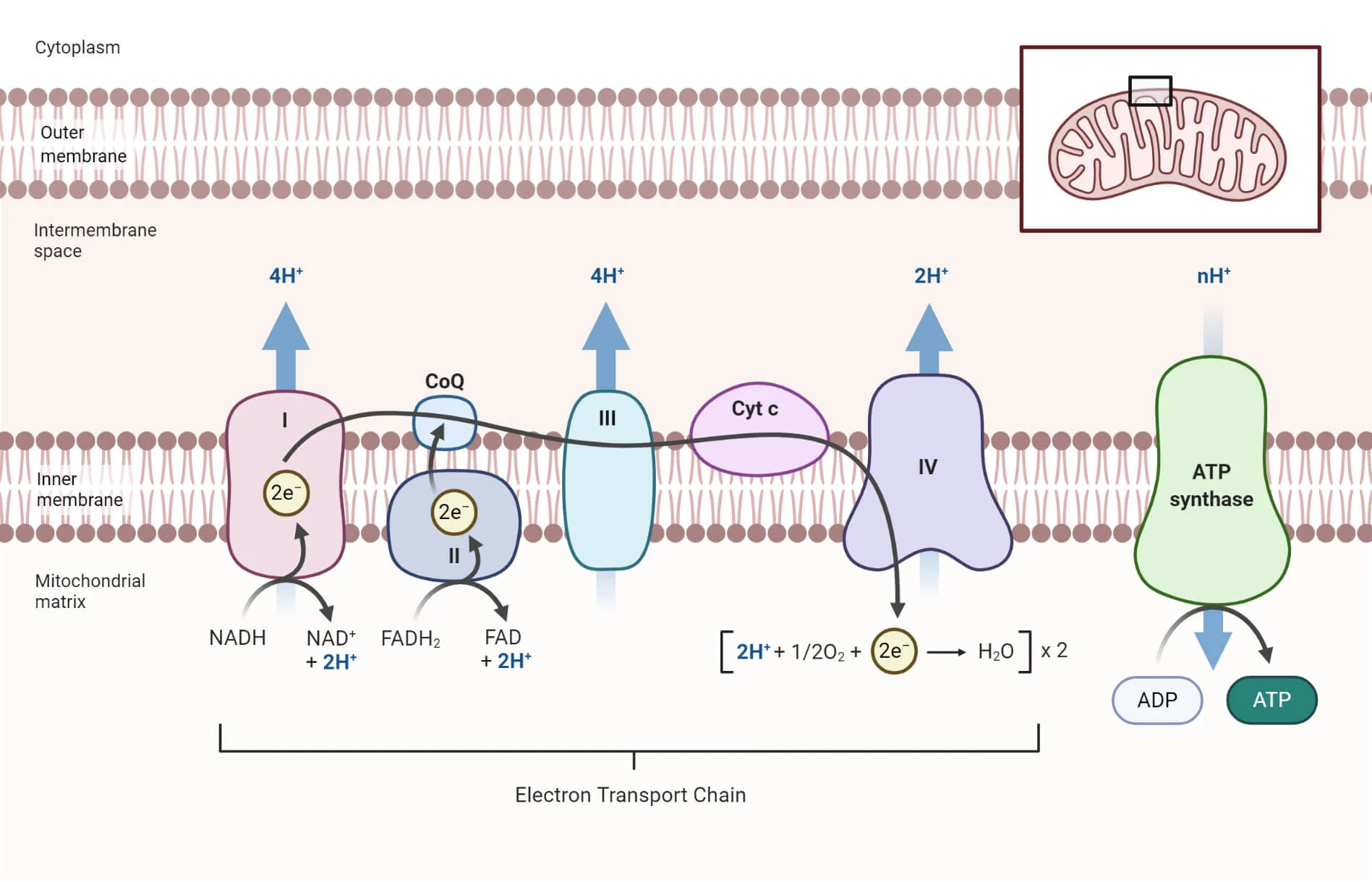
State what happens to NADH and FADH2 at the electron transport chain.
C1.2.13 (AHL): Transfer of energy by reduced NAD to the electron transport chain in the mitochondrion.
Reduced NAD (NADH) and reduced FAD (FADH2) are oxidized with the transfer of electrons to electron carrier protons into NAD + H+ and FAD + 2 H+ (respectively).
List the reactions that generated the reduced NAD and reduced FAD used in the electron transport chain.
C1.2.13 (AHL): Transfer of energy by reduced NAD to the electron transport chain in the mitochondrion.
Glycolysis (2NADH)
Link Reaction (2 NADH)
Krebs Cycle (6 NADH, 2 FADH2)
*Number indicated in parentheses are per glucose molecule
Describe how the movement of electrons through the electron transport chain is used to generate a proton gradient in the intermembrane space.
C1.2.14 (AHL): Generation of a proton gradient by flow of electrons along the electron transport chain.
As NADH and FADH2 are oxidized to produce NAD and FAD and H+, the movement of their electrons provides energy for the active transport of their protons (H+) from the mitochondrial matrix to the intermembrane space.
This develops a high concentration of protons in the intermembrane space (a concentration gradient).
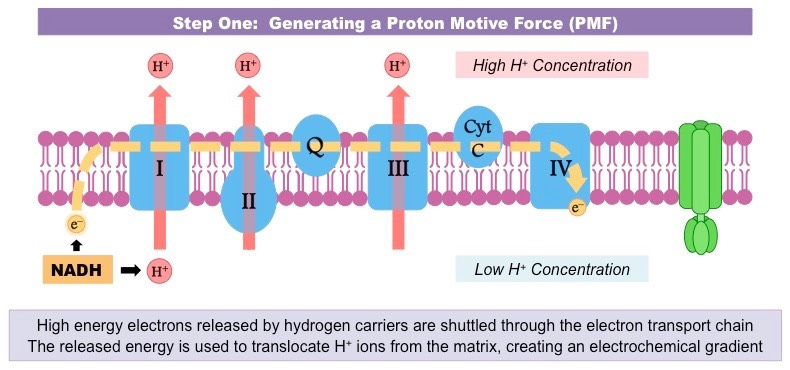
Define chemiosmosis.
C1.2.15 (AHL): Chemiosmosis and the synthesis of ATP in the mitochondrion.
The movement of ions across a semipermeable membrane bound structure down the electrochemical gradient.
In cellular respiration, it is the generation of ATP using kinetic energy from protons moving through ATP synthase.
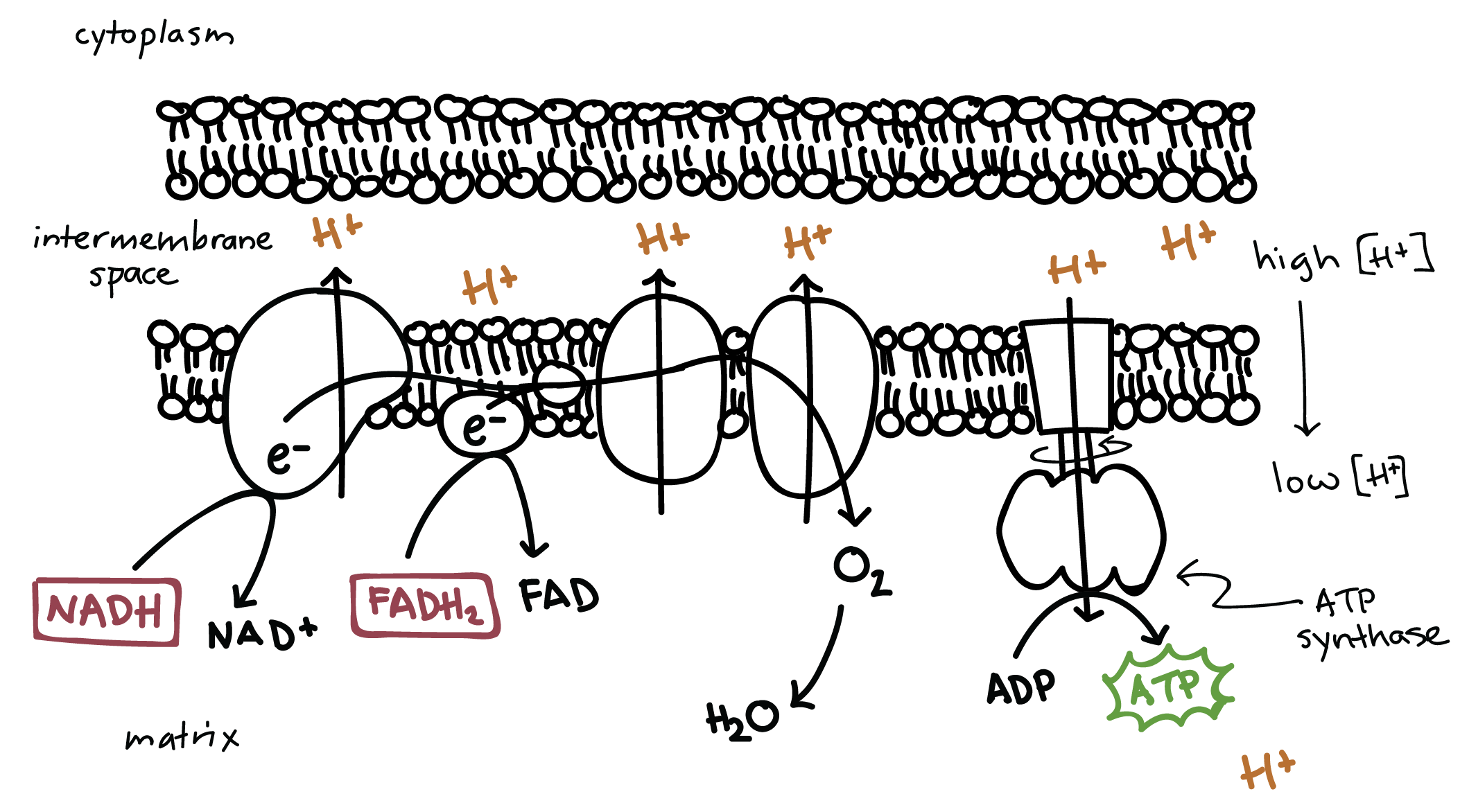
Describe the structure ATP synthase.
C1.2.15 (AHL): Chemiosmosis and the synthesis of ATP in the mitochondrion.
ATP synthase is an inner mitochondrial membrane that functions as a proton channel.
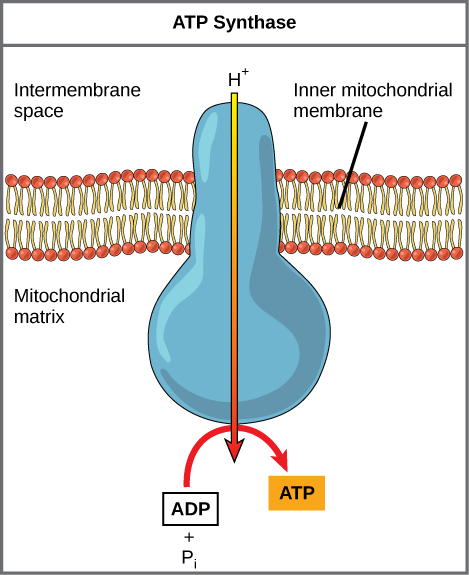
Outline the formation of ATP by ATP synthesis, with reference to movement of protons and phosphorylation of ADP.
C1.2.15 (AHL): Chemiosmosis and the synthesis of ATP in the mitochondrion.
Charged protons cannot pass through other mitochondrial membrane proteins, but they can pass through ATP synthase. As the protons pass through ATP synthase down the concentration gradient (facilitated diffusion), this movement generates energy to phorphorylate ADP and Pi into ATP.
This yields about 30-34 ATP.
Compare the total amount of ATP made from anaerobic and aerobic respiration.
C1.2.15 (AHL): Chemiosmosis and the synthesis of ATP in the mitochondrion.
Net 2 ATP are produced in anaerobic respiration.
Net 38 ATP (2 from glycolysis, 2 from Krebs cycle, and theoretically 34 from the ETC) are produced in aerobic respiration.
State that oxygen is the final electron acceptor in the electron transport chain.
C1.2.16 (AHL): Role of oxygen as terminal electron acceptor in aerobic cell respiration.
Oxygen is the final (terminal) electron acceptor in the electron transport chain.
Oxygen takes electrons from protein IV and 4 H+ from the matrix to form 2 H2O.
Explain why aerobic respiration will stop if oxygen is not present.
C1.2.16 (AHL): Role of oxygen as terminal electron acceptor in aerobic cell respiration.
Aerobic respiration will stop if oxygen is not present because oxygen is the terminal electron acceptor. It accepts electrons and protons (H+) from the mitochondrial matrix to produce water (H2O) and allowing the continual flow of electrons along the chain.
If oxygen is not present, the ETC will not function because the electrons will not be taken. If there are too many electrons, the electrons will get backed up, causing the ETC to halt.
State how the formation of water in the matrix at the end of the electron transport chain impacts the proton gradient between the intermembrane space and the matrix.
C1.2.16 (AHL): Role of oxygen as terminal electron acceptor in aerobic cell respiration.
The formation of water (H2O) helps maintain the proton gradient between the intermembrane space and the matrix by taking away hydrogens in the matrix (keeping the concentration of H+ low in the matrix).
Compare the use of carbohydrates and lipids as respiratory substrates in aerobic and anaerobic respiration.
C1.2.17 (AHL): Differences between lipids and carbohydrates as respiratory substrates.
Only carbohydrates can be used for anaerobic respiration because lipids bypass glycolysis. Lipids are not used in anaerobic respiration.
Carbohydrates are broken down into monosaccharides (like glucose) to use in glycolysis. Lipids are used for long-term source of energy by being broken down into fatty acid chains and then carbon compounds.
However, afterward, both undergo the rest of aerobic respiration. They are broken down and oxidized to form ATP.
Explain the greater energy yield of lipids compared to carbohydrates when used as respiratory substrates.
C1.2.17 (AHL): Differences between lipids and carbohydrates as respiratory substrates.
Lipids produce more energy per gram because they have more carbon chains. These carbon chains have less oxygen and more oxidizable hydrogen and carbon, so they can produce more energy.
Outline the process by which lipids can be a substrate for respiration.
C1.2.17 (AHL): Differences between lipids and carbohydrates as respiratory substrates.
The fatty acid chains are broken into 2-carbon compounds. They bypass glycolysis. This then forms acetyl CoA and continues on with the process of aerobic respiration. This means they can only be digested aerobically.