B Lymphocyte Development & Maturation
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
B&T cell development & maturation
B lymphocyte generation
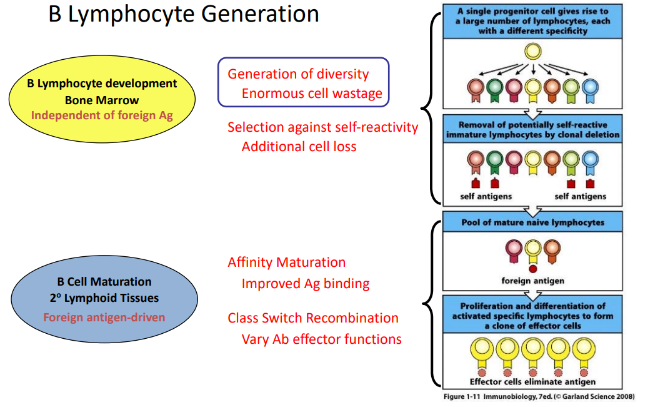
challenges of assembling a working BCR
-VDJ recombination: some V gene segments are pseudogenes (internal stops), rearranging can lead to frame shifts → stop codon, autoreactive BCRs cannot be permitted → considerable cell wastage!
-additional complexities: 2 heavy chain (HC) and 4 light chain (LC) alleles
-generation of BCR repertoire must be regulated
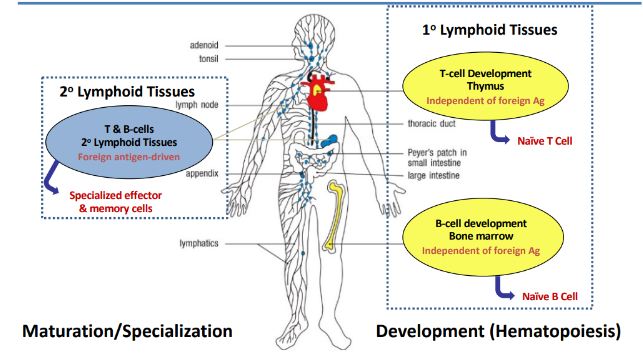
antigen-independent B-cell development → generation of B cells in the bone marrow
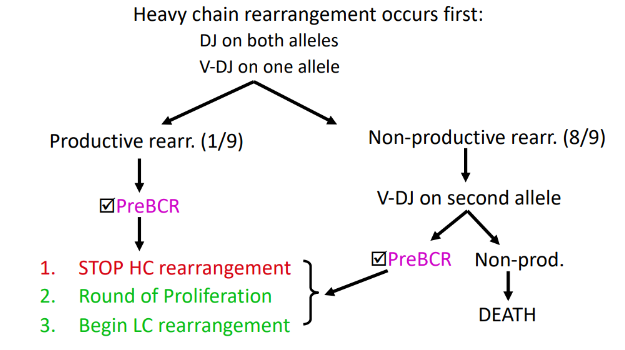
1) DNA rearrangements are orderly: heavy chain first, followed by light chain
2) allelic exclusion ensures that each naive B cell expresses a single antibody on the surface
3) deletion of self-reactive clones protects us from generation of auto-reactive B cells
ordered rearrangement of Ig genes during B-cell development in the bone marrow
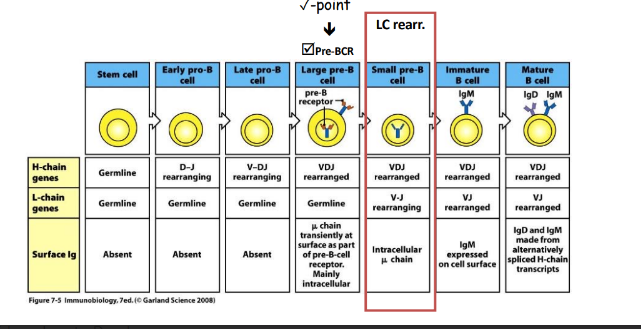
rearrangement at the H-chain loci
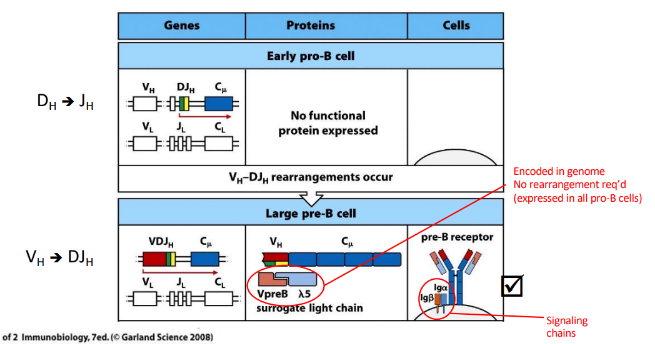
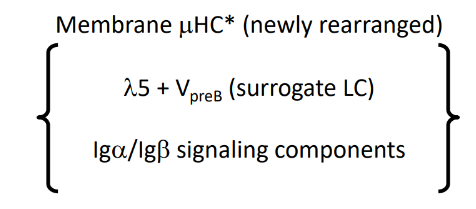
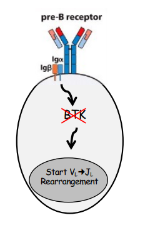
pre-B cell receptor
-”tonic” (always on) signaling once on the cell surface
somatic recombination is highly ordered
failure to pass pre-BCR checkpoint
-X-linnked agammaglobulinemia
-developmental arrest, pre-B cell stage
-no circulating B cells (or very low numbers)
-no IgM, IgD, IgG, IgA, IgE (or very low levels)
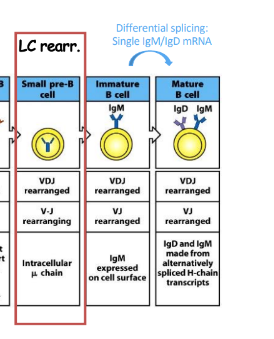
differential splicing
-single IgM/IgD mRNA
B-cell checkpoint #2
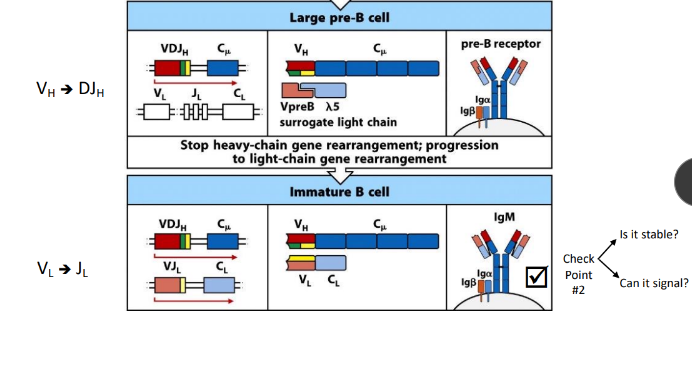
Vl-Jl rearrangement
-lower stakes, multiple “re-dos”
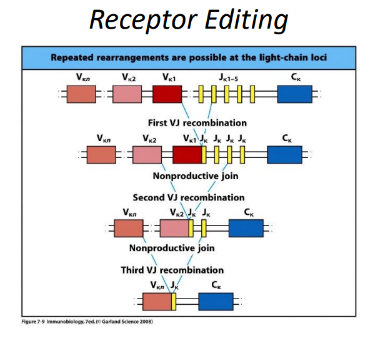
rearrangement of Ig alleles is ordered
-biggest challenge first (H-chain)
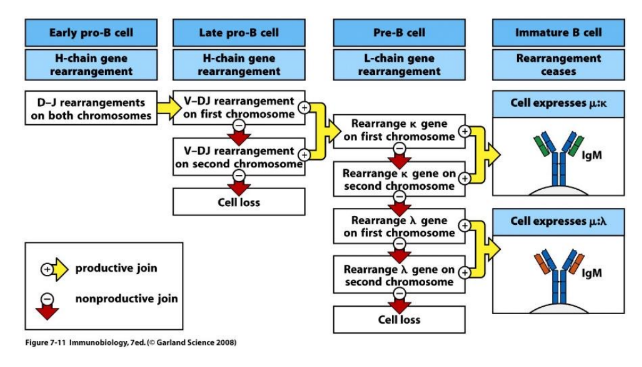
2 checkpoints that confer allelic exclusion
-pre-BCR and BCR
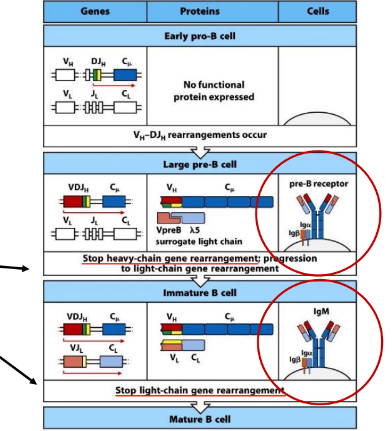
establishment of allelic exclusion
-signaling by pre-BCR or BCR (via Igalpha/Igbeta):
-reduces expression of RAG-1 and RAG-2
-targets RAG-2 for proteasomal degradation
-reduces access of the HC locus to the recombinase machinery (mechanism unclear)
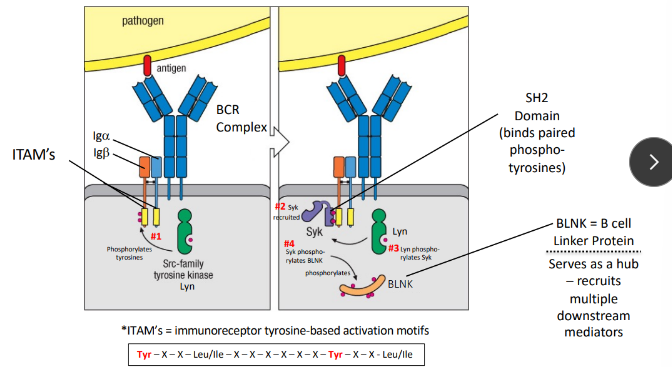
BCR signal induction
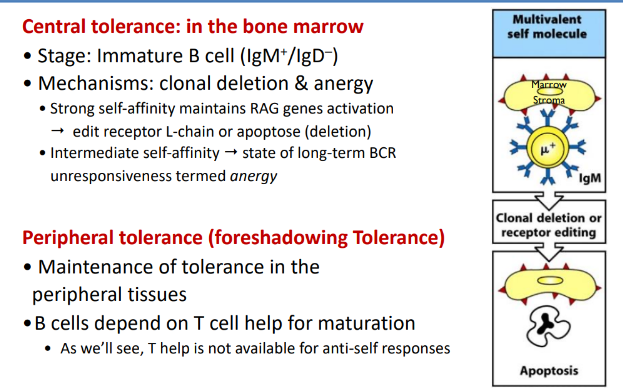
B cell tolerance
-central tolerance: in the bone marrow
-peripheral tolerance (foreshadowing tolerance)
B cell development summary

antigen-dependent B-cell maturation occurs in
-secondary lymphoid tissues
-B cells organize into follicles in secondary lymphoid tissues:
-LN, spleen, MALT; B cell maturation occurs within germinal centers that form within the follicle
antigen-depndent B-cell maturation
-results in high-affinity antibodies with tailored effector fxns
-products include long-lived plasma cells and memory B cells
-step-wise process:
1) antigen binding by BCR → B cell activation; seeks T cell help
2) T cell-B cell recognition and pairing; B cell receives T cell help
3) germinal center formation: B cell proliferation/cloncal expansion (A- somatic hypermutation aka affinity maturation, B- immunoglobulin class switch)
4) differentiation into: plasma cell (antibody-secreting factory), memory B cell (rapid response in case of future encounters)
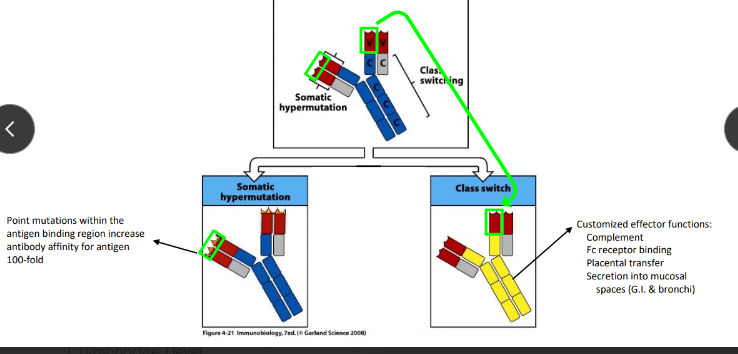
somatic hypermutation v class switch recombination
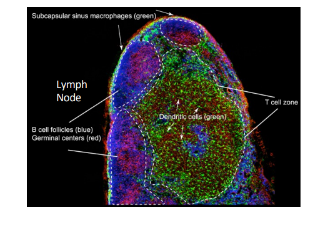
lymph nodes from B cell’s perspective
-B cells gather into follicles in the cortex, adjacent to the T cells in their paracortex
-germinal centers arise in the follicles as a result of a lucky Ag-specific B cell finding Ag-specific T cell help to undergo clonal expansion
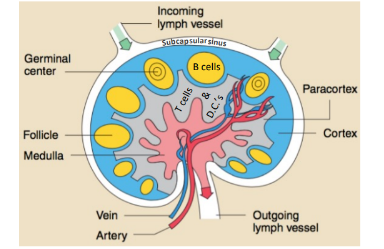
follicular dendritic cells
-non-hematopoietic cells in follicle
-two critical functions: scaffolding the follicle and antigen repository
-use Fc and complement receptors to hold and preserve whole Ag in immune complexes
-Ag clusters on the FDC surface form “bodies” call iccosomes
-iccosomes can break free and be bound by a nearby B cell with a BCR specific for that antigen
match-making of the T-dependent B cell response
-naive B cells hang out in the follicle sampling Ag flowing in lymph
-when a cell’s BCRs bind a polyvalent antigen, the BCRs cluster leading to 2 ingenious outcomes:
1) rapid-phase IgM production- short round of proliferation (clonal expansion) and a few daughter cells differentiate into plasma cells, migrate to LN medullary cords, and begin secreting low-affinity anti-invader IgM
2) those B cells that don’t differentiate into plasma cells become antigen presenters- a) surface BCRs are endocytosed with bound antigen, b) in the lysosome, antigen dissociates and is degraded into peptides loaded onto MHC II, c) B cell then migrates to the BT border looking for an effector CD4+ T cell’s help- effectors CD4+ T cells patrol the BT border surveilling for antigen presented by B cells
B:T match-making
-B-T pairing: an effector CD4+ T cell recognizes peptide presented by our B cell
-T cell activation → LFA-1 (on T cell) tight binding of B cell ICAM
-physical pairing can last 6-12 hours
contact-dependent T cell help for B cells (also macrophages and DCs)
-while paired, T cell expresses CD40-ligand (CD154), the primary mediator of contact-dependent T cell help (to B cells, macs, DCs)
-B cell CD40 binds T cell CD40L completing the B cell’s activation
-required for further maturation (which will take place in a germinal center)
-after the pair parts, B cell migrates into the follicle and begins proliferating, forming a germinal center
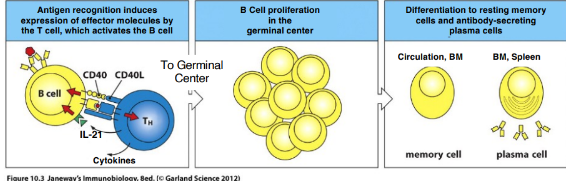
“germinal center rxn”
-intense antigen-driven B cell proliferation
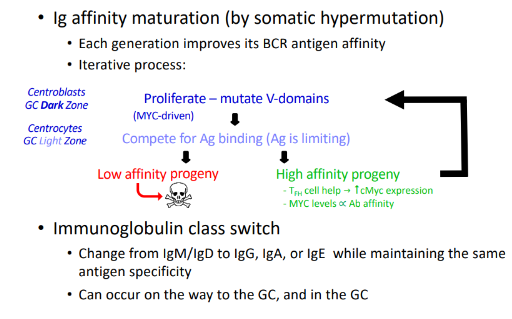
affinity maturation through somatic hypermutation
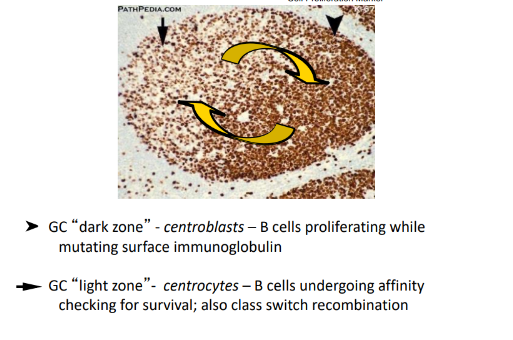
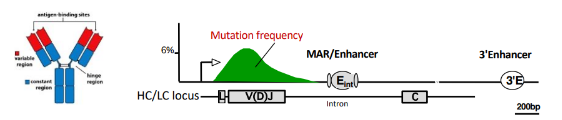
somatic hypermutation (SHM)
-random mutagenesis in V regions during cell cycle, mostly single base changes
-mutation rate is 10^6-fold higher than for normal DNA replication
-critical enzyme involved: activation-induced deaminase (AID)- converts cytosine to uracil; uracil is excised, repair of excision sites is by an error-prone polymerase
-combined with selectin → increased affinity for antigen
expression of alternate isotypes
-two mechanisms for expression of alternate isotypes:
-IgM to IgD via differential RNA splicing
-IgM to IgG, IgA, or IgE by DNA rearrangement
-membrane to secreted Ig via differential RNA splicing
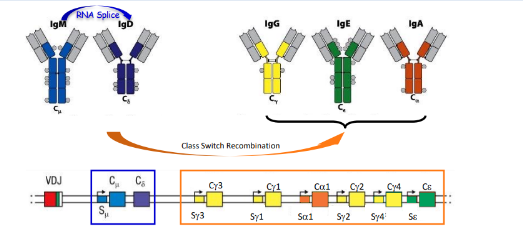
class switch recombination (CSR)
-DNA rearrangement that allows the same VDJ to be expressed with different heavy chain constant regions
-critical enzyme involved: activation-induced deaminase (AID)
-cytokines direct which switch regions are targeted
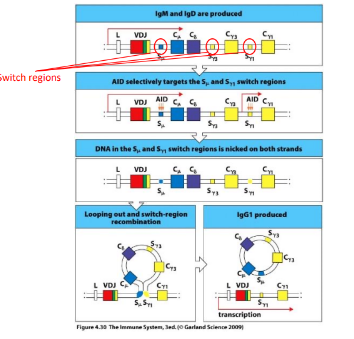
failure to undergo GC maturation
-fail maturation process
-hyper-IgM syndrome
-deficiency of any of the following: T cell CD40L (X-linked, most common), B cell CD40 (AR), B cell DNA modifying enzyme AID (AR) → defect in class switch and affinity maturation, abundant serum IgM- all low affinity, no (or very low) IgG, IgA, IgE
germinal center microenvironment
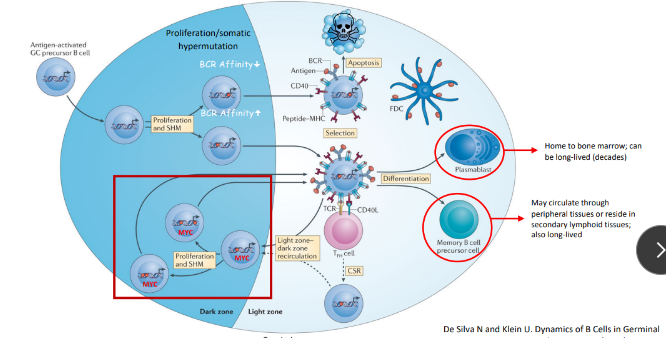
improving with each exposure
-increase in the antibody level and affinity for antigen over time
-switch to an Ig class with the most appropriate functional properties
-even more prominent with subsequent exposures
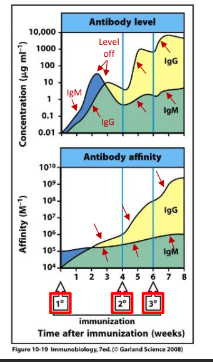
summary
