Core Practical 1: Measure the volume of a gas
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
Overview:
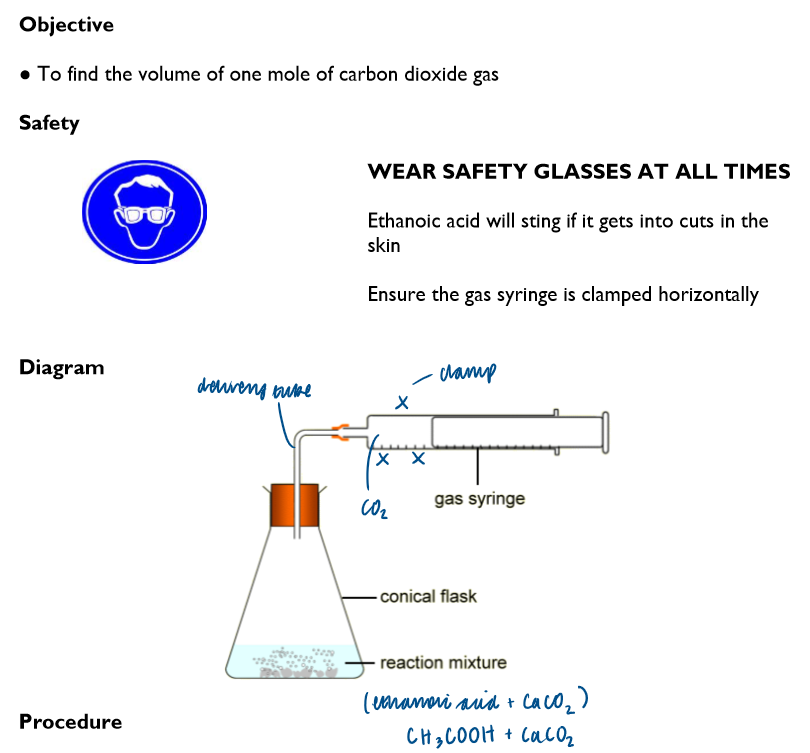
Procedure:

Write a chemical equation for the reaction between ethanoic acid and calcium carbonate:
2CH3COOH (aq) + CaCO3 → Ca(CH3COO)2 (aq) + CO2 (g) + H2O (l)
Why is it more accurate to find the mass of the calcium carbonate used by weighing the sample tube with calcium carbonate in, then tipping it out and reweighing the sample tube, rather than weighing the empty tube at the start?
some calcium carbonate powder may have remained in the weighing boat when it was tipped into the beaker
so not all calcium carbonate powder that was initially weighed would have reacted with the ethanoic acid
What is the major source of error caused by the procedure used?
loss of gas before the bung is replaced in the flask
What change to the apparatus could be made to eradicate the error?
use a different method, such as using a tap funnel
therefore avoiding replacing the bung after tipping in the CaCO3
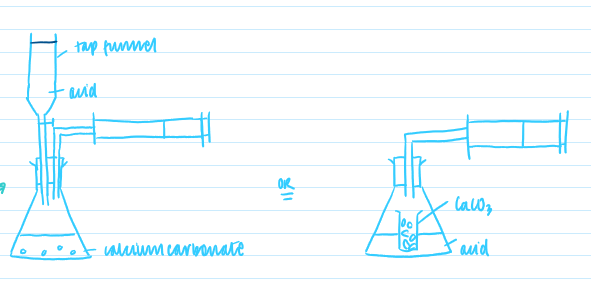
Calculation to show that the ethanoic acid was in excess in all experimental runs:
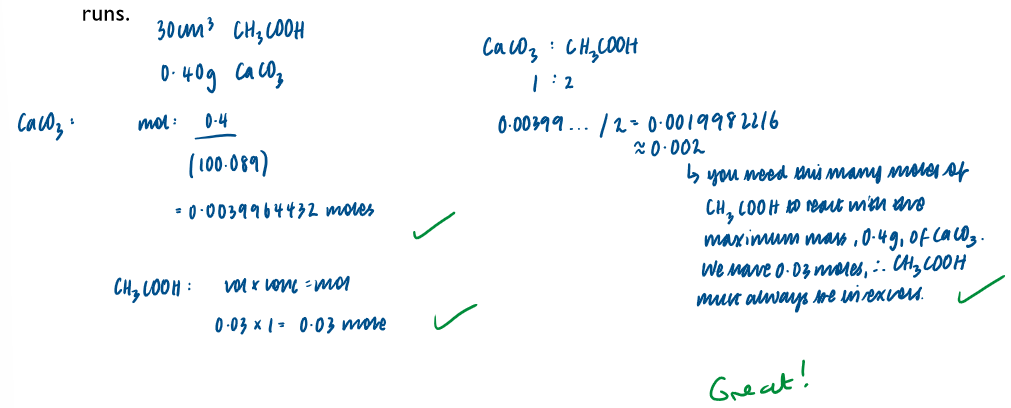
Why did you not exceed 0.40g of calcium carbonate?
the gas syringe did not have the volume capacity for a greater mass than 0.40g
as too great a volume of CO2 gas would be produced
Ethanoic acid is a weak acid. Hydrochloric acid is an example of a strong acid. Suggest why a weak acid should produce better results in this experiment than a strong acid.
ethanoic acid would react more slowly
so the carbon dioxide gas should be produced more slowly
(ethanoic acid only partially dissociates into H+ ions)
so less CO2 gas would escape before the bung was replaced
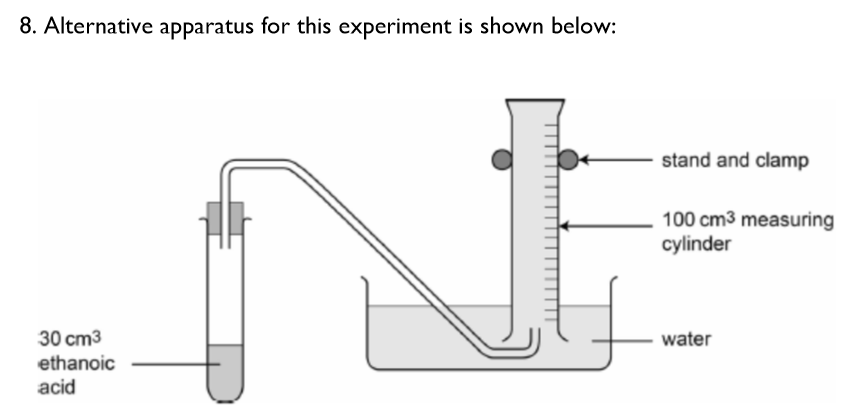
Can you suggest any limitations or problems a student might encounter using this apparatus, as opposed to the conical flask and gas syringe combination that you used?
carbon dioxide gas that is formed might dissolve into the water
forming carbonic acid solution
so the reading obtained would not be as accurate as not enough gas would be collected
(systematic error)