ihs 340 exam 3 content
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
antigen
substance that induces an immune response in the body
substance specifically bound by antibodies or T lymphocyte antigen receptors
passive immunity
the short-term immunity which results from the introduction of antibodies from another person or animal.
featured cells in allergic reactions/atopy
mast cells, Th2 cells, B cells, eosinophils, basophils, dendritic cells, ILC2s
featured signals and receptors in allergic reactions/atopy
IgE, IgG1, FcR, IL-4, IL-5, IL-13, IL-9, IL-10, PGE2, PGD2, leukotrienes, alarmins (IL-33, IL-25, TSLP)
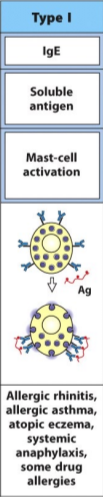
type I hypersensitivity reaction (outdated classification)
immune reactant: IgE
antigen: soluble antigen
effector mechanism: mast-cell activation
examples of reaction: allergic rhinitis, asthma, eczema, anaphylaxis, some drug allergies
aka immediate hypersensitivity response
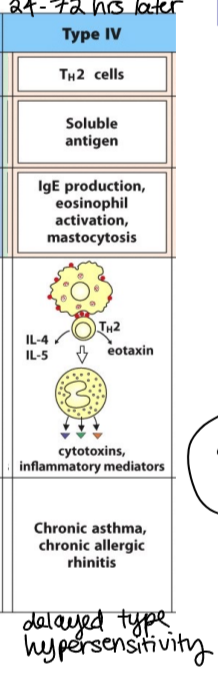
type IV Th2 hypersensitivity reaction (outdated classification)
immune reactant: Th2 cells
antigen: soluble antigen
effector mechanism: IgE production, eosinophil activation, mastocytosis
examples of reaction: chronic asthma, chronic allergic rhinitis
why is describing hypersensitivity reactions as different types “outdated”?
the lines between these types have been blurred as we learn more about immune and allergic responses
what is the main driver of IgE production by B cells?
IL-4
what does it mean for T cells to be polarized?
different T cell types are unique, the cytokines they release are unique to them
i.e. IL-9 only released by Th2 cells, not any other type
IgM principal effector functions
complement activation and neutralization
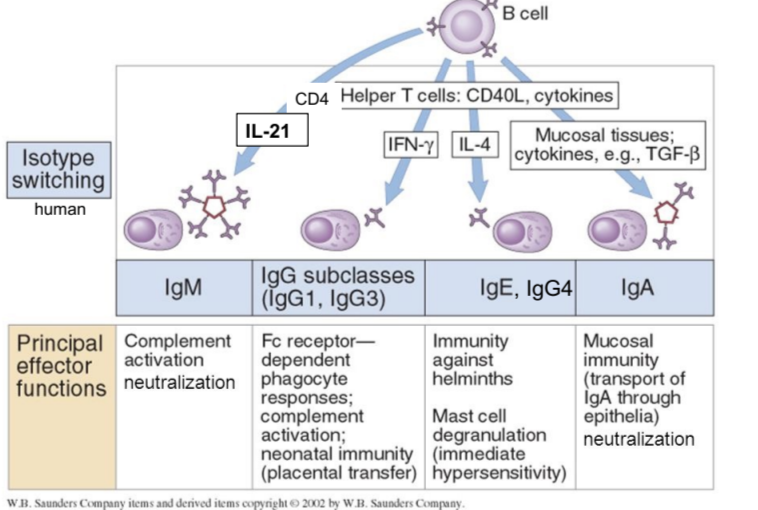
IgG principal effector functions
FcR-dependent phagocyte responses, complement activation, neonatal immunity
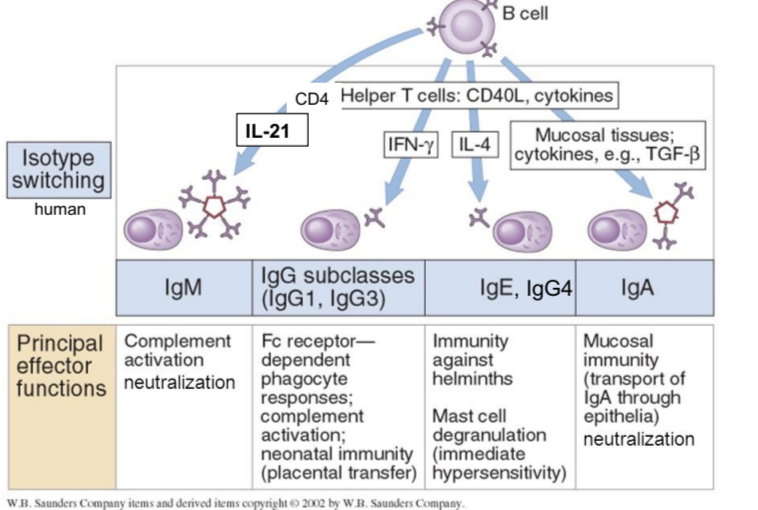
IgE and IgG4 principal effector functions
immunity against helminths, mast cell degranulation (immediate hypersensitivity response)
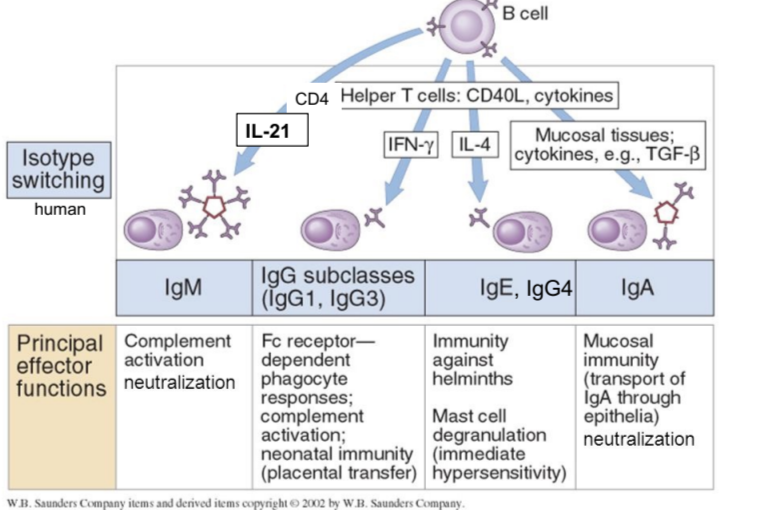
IgA principal effector functions
mucosal immunity, neutralization
how do mast cells and eosinophils contribute to immunity against intestinal parasites?
a worm is marked by antibodies, so that cells know it needs to be removed
eosinophils attach to the Fc portion of these antibodies and stuns the worm with powerful toxins
mast cells release histamine to cause fluid movement to the vessels and activate smooth muscles to expel the worm
describe histamine effects in type I hypersensitivity reactions
they bind to H1 receptors to contract smooth muscles in bronchi, also allows BV dilation and increased permeability, resulting in edema and urticaria (hives)
how is an allergic response similar to the response in expelling a worm?
the body is using similar mechanisms like mast cell degranulation to expel the allergen
what is necessary for an effective immune response?
need engagement of TcR with MHC class II receptors
need engagement of costimulatory molecules between APCs and T cells
need cytokines to allow for T cell differentiation
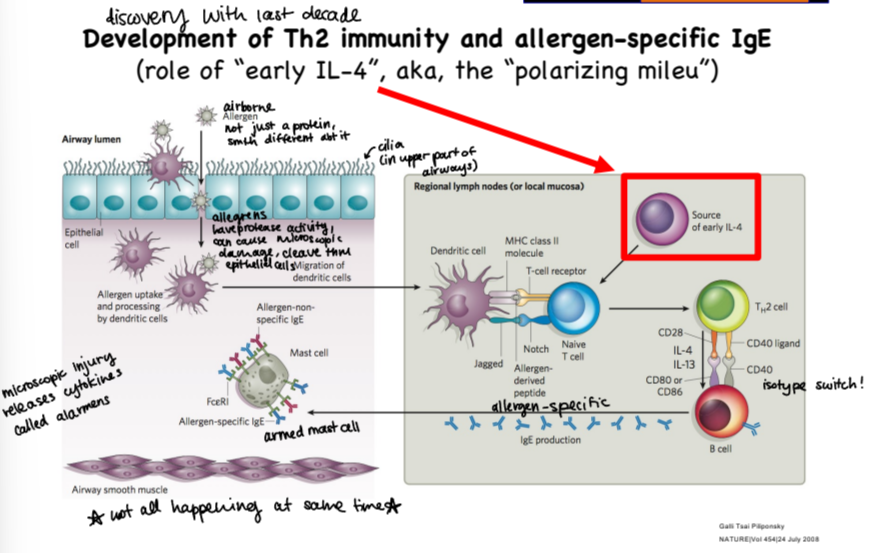
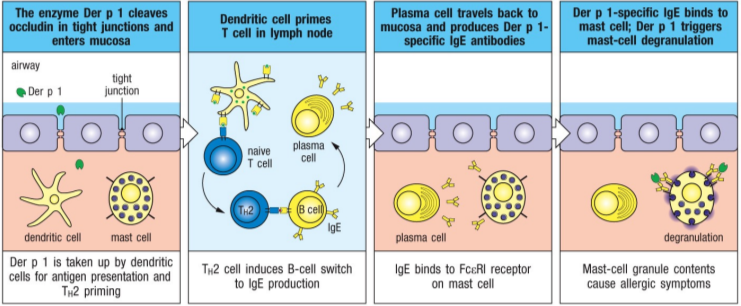
describe the pathway of an airborne allergen through the upper airways and its interaction with the immune system
these allergens have protease activity, it gets through epithelial cells lining the airway lumen cleaving through epithelial cells and causing microscopic damage
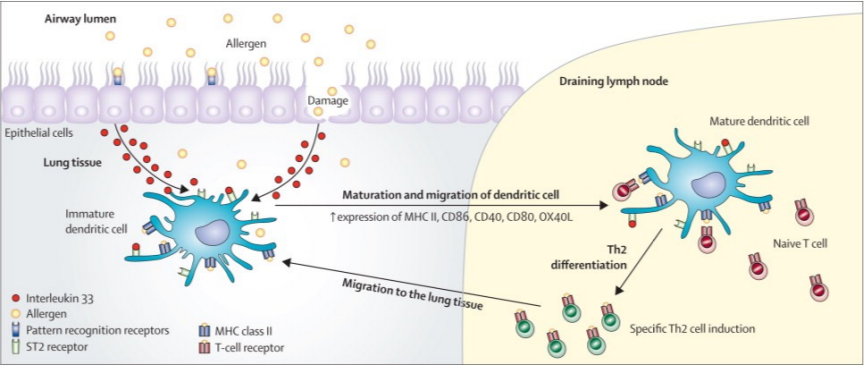
allergen uptake and processing done by dendritic cells, once they have it they migrate to the nearest lymph node
in the lymph node, DC binds with TcR on naive T cell, which then differentiates to Th2 cell
primed Th2 cell binds to B cell which switches isotype to make allergen-specific IgEs
IgEs bind to mast cell, arming it and allowing it to respond to allergen in airway tissue
innate lymphoid cells (ILCs)
an important early source of polarizing cytokines
lack specific antigen receptors (no TcRs, no Ig) and also co-receptor complexes
ILCs develop in bone marrow
expression of transcription factor Id2 in lymphocyte progenitor is required for development of all ILCs, represses other lymphocyte fates
migrate from bone marrow and populate lymphoid tissues (spleen and lymph nodes) and peripheral mucosal organs
what are three major subgroups of ILCs?
ILC1 and NK cells
ILC2
ILC3
how are the major subgroups of ILCs defined?
largely on the basis of the types of cytokines that each produce
group 1 ILCs
similar to Th1 cells, but without TcRs
release IFN gamma
group 2 ILCs
similar to Th2 cells, but without TcRs
secrete IL-13, IL-4, and IL-5 like Th2 cells do
group 3 ILCs
similar to Th3 cells, but without TcRs
release IL-17 and IL-22
what can induce distinct cytokine responses from ILCs?
MAMPs of different microorganisms
DAMPs from injured host cells
cytokine signals from other cells
other environmental signals like pollutants (ILC3 studied for most of this)
why are ILC2s an important early source of polarizing cytokines?
they can play a major role in shaping or responding to their tissue environment
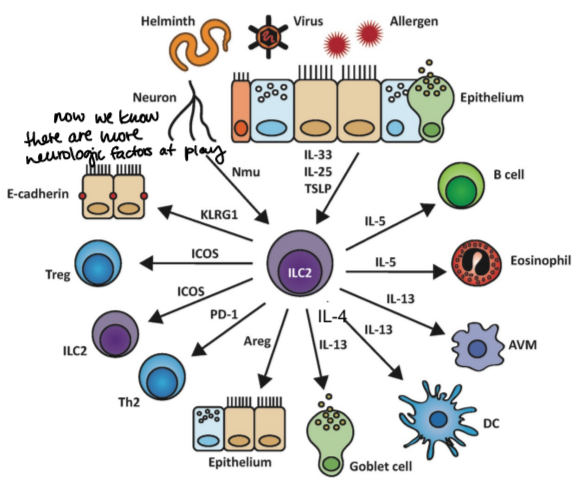
what are type 2 responses characterized by?
actions of ILC2s, IgEs, and innate effector cells (eosinophils, basophils, and mast cells)
what are type 2 responses (Th2, ILC2) induced by and what are they drivers of?
they are induced by multicellular parasites like helminths and are drivers of allergic responses
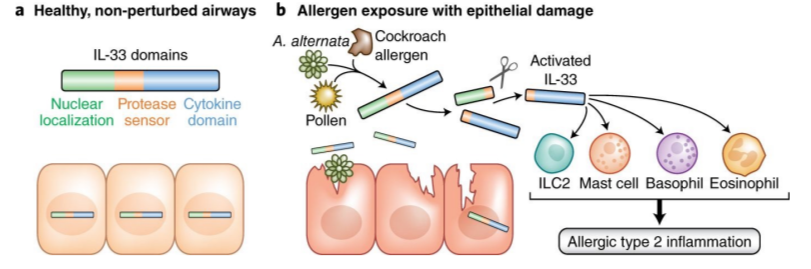
alarmins
proteins that respond to any damage to epithelium
TSLP, IL-33, IL-25
primarily produced by epithelial cells that sense MAMPs common to helminths or DAMPs
what do alarmins activate?
ILC2s
what do ILC2s do after activated by alarmins?
they rapidly produce IL-4, IL-5, and IL-13, it doesn’t make a lot of cytokines, just enough to get the process started
IL-13 effects
stimulates mucus production by goblet cells in the epithelium and mucosal smooth muscle contractions that facilitate worm expulsions, also activate B cells
IL-5 effects
stimulates production and activates eosinophils that can kill worms
IL-4 effects
stimulates class switching to IgE antibodies that arm mast cells and eosinophils
amphiregulin
stimulates tissue repair after a worm is cleared out
what is an example of an airborne allergen possessing enzymatic activity that damages the epithelium?
dust mite allergen Der p1 (cysteine protease)
it cleaves protein (occludin) that helps maintain intracellular tight junctions, damaging barrier function of epithelial cells
the allergen and other dust mite antigens penetrate epithelial barrier and are taken up by DCs
induces specific Th2 cells and IgEs
function of alarmin IL-33
mediator of allergic inflammation
localized in mucosal tissues to respond rapidly to environmental insults like allergens or epithelium damage
how does IL-33 affect dendritic cells?
it activates them like other inflammatory signaling molecules, causing their “dendrites” to retract, and it flows with lymph towards lymph node to mature and do its job
DCs are great at releasing costimulatory molecules and interacting with T cells after maturing in the lymph node
what antibody receptors do mast cells constitutively express?
FcεR1, a high-affinity IgE receptor
also constitutively expressed on basophils and inducible in eosinophils
how are mast cells activated for degranulation?
when a multivalent allergen (multiple epitopes) binds and cross-links two or more IgEs on the surface
this causes fast degranulation because these granules filled with proinflammatory mediators are premade
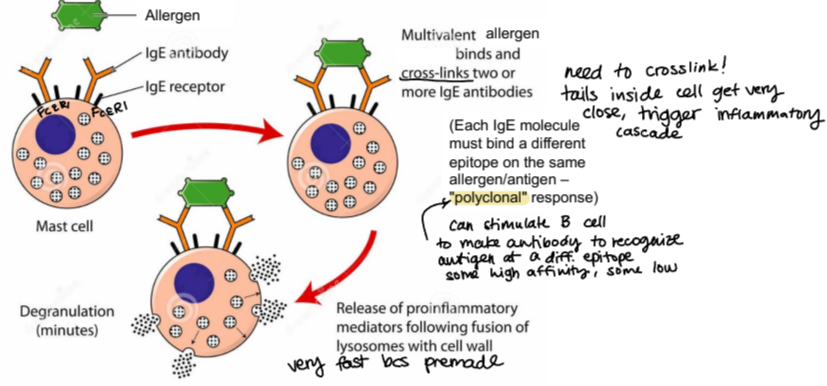
which molecules are released from mast cells rapidly?
enzymes like trypase, toxic mediators histamine and heparin
which molecules are produced and secreted by mast cells after activation?
cytokines IL-4, IL-13, IL-33, TNF-alpha, etc.
chemokines like CCL3
lipid mediators like prostaglandins and leukotrienes
preformed and released molecules from eosinophils
enzymes like eosinophil peroxidase and colleganse, MMP9
toxic proteins like eosinophil cationic protein
molecules synthesized and released by eosinophils after activation
cytokines IL-3, IL-5, TGF-alpha and beta
chemokines like CXCL8 (IL-8)
lipid mediators like leukotrienes and platelet-activating factor (also in mast cells)
describe the central effector cell in allergic responses
mast cells, which express FcεR1 constitutively
prominent in mucosal and epithelial tissues like in respiratory and GI tract
also located in subuendothelial connective tissue (just on outside of blood vessels)
can have local response (limited) or systemic response (life-threatening, shock)
how do mast cells and neurons interact?
mast cell mediator release can affect nociceptors, increasing pain perception
neuronal activation can release NTs which can affect mast cells
direct release of IL-4?
protease allergens can directly induce IL-4 release from mast cells and basophils, a potential mechanism for food sensitivity
IL-18-induced “innate” allergic response
IL-18 can be produced by epithelial cells, macrophages, and other cells
IL-18 can stimulate basophils and mast cells to produce IL-4 and IL-13, even in the absence of FcεR1 cross-linking
leukotrienes
small molecules made of fatty acids that facilitate communication between local groups of cells
potent activator of neutrophilic inflammation
can also activate sensory nerves, smooth muscles
bioactive lipids derived from enzymatic modification of arachidonic acid
made via lipoxygenase activity
early phase of type I hypersensitivity response
occurs within minutes
includes histamine effects, proteases, and eosinophils
late phase of type I hypersensitivity response
occurs after 8-12 hours
includes Th2 cells, basophils, eosinophils, leukotrienes
mast cell-mediated immediate reaction
vascular and smooth muscle responses predominate (occurs within minutes)
molecules released from pre-formed granules/vesicles
mast cell-mediated late-phase reaction
characterized by leukocyte recruitment and inflammation (takes hours to days)
molecules mostly synthesized and secreted after activation
early-phase allergic reaction in the skin
wheal-and-flare response, due to release of histamine
occurs within 5-10, welt with soft swelling with red rim around it because of BV dilation
late-phase allergic reaction in the skin
more widespread swelling response, involves leukocyte recruitment and additional released molecules from mast cell
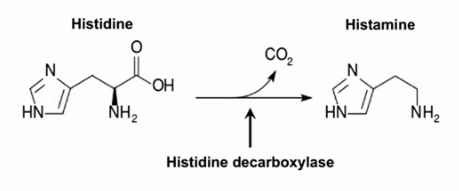
histamine
bioactive amine formed by decarboxylation of AA histidine via histidine decarboxylase
found in almost all tissues of the body, high amounts along mucosa, in tissues of lungs, skin, and gastrointestinal tract (because highly concentrated in mast cells and basophils)
important for sleep cycle, inflammation (H1 receptors), and gastric acid secretion via parietal cells (H2 receptors)
antihistamines
H1 receptor antagonists, blocking histamine binding to H1 receptor
examples of antihistamines
benadryl (diphenhydramine) 1st gen
zyrtec (cetirizine) 2nd gen
allegra (fexofenadine) 2nd gen
claritin (loratidine) 2nd gen
difference in generations of antihistamines
1st gen - discovered first, can cross blood-brain barrier and causes drowsiness
2nd gen - discovered later, don’t cross blood-brain barrier, nondrowsy (only if you take 1 every 24 hours)
specific qualities of zyrtec (cetirizine)
high doses causes it to cross the BBB and can bind to H1 receptors in the CNS with higher affinity, causing brain fog and drowsiness
specific qualities of allegra (fexofenadine)
can be prescribed at higher doses, doesn’t cross BBB even at higher doses
what stimuli can trigger histamine release from mast cells?
allergic reactions, tissue damage, drugs and foreign chemicals
prostaglandins
bioactive lipids derived from enzymatic modifications of arachidonic acid, released from cell membranes by phospholipase A2
made by cyclooxygenase activity (COX-1, COX-2, COX-3)
play a role in inflammation, pain, body temp regulation, gastric acid secretion, immune response development and immune regulation, etc.
target of NSAIDs
how are Th2 cells and prostaglandins related?
Th2 cells (allergy-driving cells) express receptor for prostaglandin D2 and express enzymes to make prostaglandins (COX-1, COX-2)
NSAIDs
non-steroidal anti-inflammatory drugs
provide relief from fever, pain, and inflammation through actions on COX enzymes
ex. are aspirin, ibuprofen, naproxen, acetominophen
COX-1
constitutively expressed in many somatic cell types
is considered a “housekeeping” enzyme, with roles in such processes as vascular hemostasis and gastric (stomach) protection
COX-2
expression primarily induced by factors such as endotoxins, other MAMPs, cytokines, and growth factors
expressed at sites of inflammation and produces prostaglandins that mediate inflammatory and pain sensation responses
COX-3
found in greatest abundance in canine and human brain
selectively inhibited by acetoaminophen, as well as a few other analgesic and antipyretic (anti-fever) NSAIDs
why is acetaminophen more efficacious against headache and fever than other NSAIDs?
it can cross the BBB at a high enough concentration to inhibit COX-3 in the brain
warnings associated with acetaminophen
chronic consumption causes liver damage
consuming alcohol with acetaminophen can cause significant liver disease
why is low-dose aspirin prescribed to some patients?
aspirin blocks formation of some prostaglandins, including thromboxane A2
this prevents activation of platelets for clotting initiation
clotting prevention can protect against diseases caused by clots, like heart attack and stroke
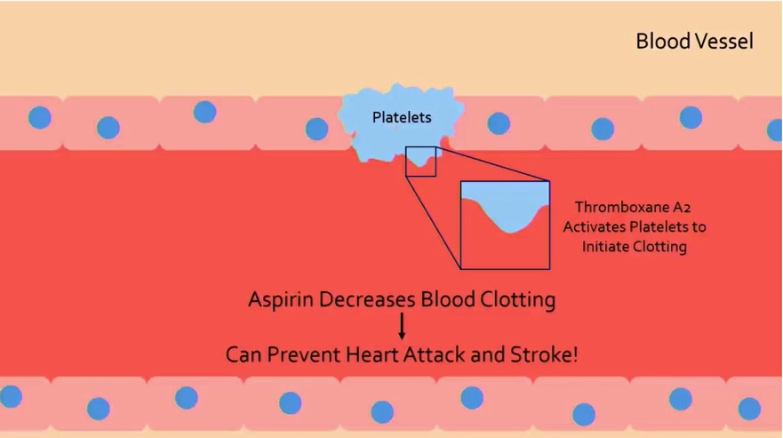
how can aspirin contribute to gastric ulcers and bleeding?
aspirin prevents synthesis of some prostaglandins, which contribute to production of protective mucous in the stomach
less protection and clotting leads to gastric ulcers and bleeding

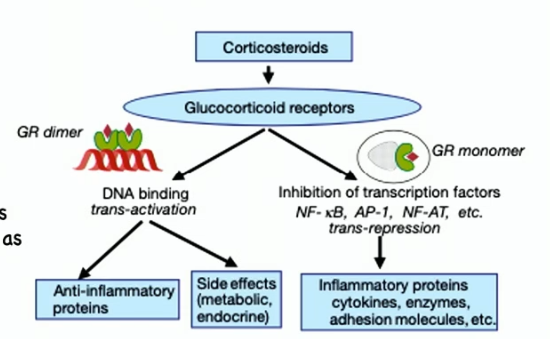
corticosteroids as anti-inflammatory drugs
bind to glucocorticoid receptor in cytosol (bcs steroid)
principal action is to suppress multiple inflammatory genes (like cytokines), inflammatory enzymes, adhesion molecules, and inflammatory mediator receptors
many anti-inflammatory actions of corticosteroids are accounted for by inhibiting transcription factors that regulate inflammatory gene expression
taking a lot of steroids to reduce inflammation leads to risk of adrenal gland stopping production of endogenous corticosteroids, causes dependence and an abrupt withdrawal can cause corticosteroid insufficiency
why do allergic symptoms get worse at night?
corticosteroids can modulate our immune system and does so in a circadian rhythm
higher levels during day and drops off during the night
targets include macrophages, mast cells, neutrophils
what cytokines trigger naive T cells to differentiate into Treg cells?
TGF-beta and IL-2
what cytokines do Treg cells release and what do they do?
TGF-beta and/or IL-10, they dampen the inflammatory response
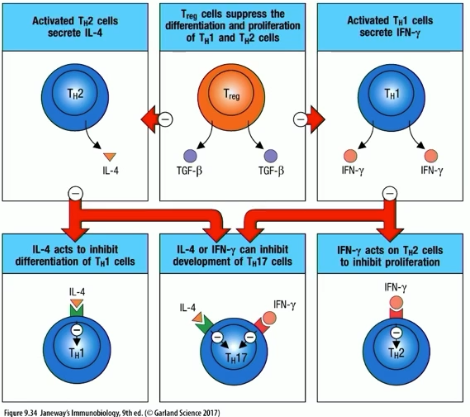
counterregulation in the immune system
Th1 cell production makes IFN-gamma, which acts on Th2 cells and inhibits their proliteration
Treg cells suppress differentiation of Th1 and Th2 cells
Th2 cell production makes IL-4, which acts on Th1 cells and inhibits their proliferation
this all works in a balance, and when you have an allergic response, you lose the balance and Th2 response overwhelms
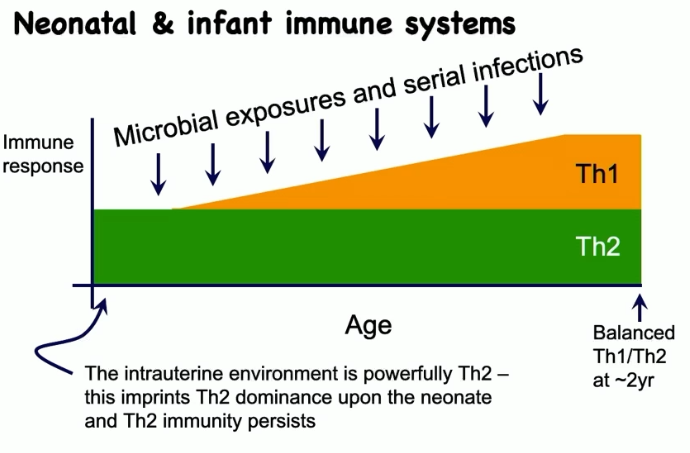
describe the neonate and infant immune system
their initial immune response is predominantly Th2
throughout life with microbial exposures and serial infections, you develop a Th1 response
balanced Th1/Th2 at about 2 years of age
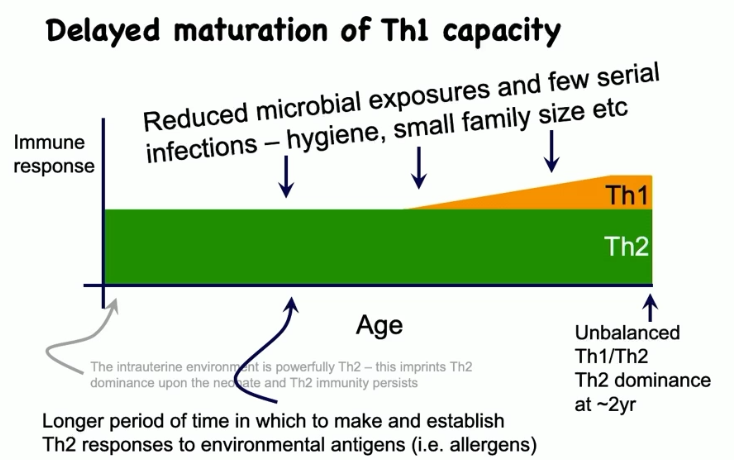
describe the allergic neonate and infant immune system
delayed maturation of Th1 capacity because of reduced microbial exposures and few serial infections (hygiene, small family size, etc.)
unbalanced Th1/Th2, with Th2 predominating
theory of allergies developing through skin
if you develop an immune response through the skin first, subsequent oral exposure has a hard time dampening the immune response
if the first time you see an allergen is orally, then response is much more strongly suppressive of subsequent skin exposure
things that impact hygiene of the skin can impact development of allergic disease, like vitamin D
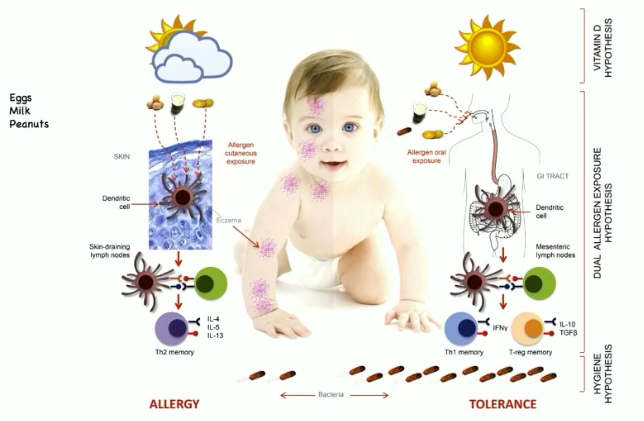
how does vitamin D interact with immune system?
vitamin D (spending time outside) dampens Th2 immune response (allergic response)
which transcription factor triggers induction of Treg cell development from naive T cell?
FOXP3+
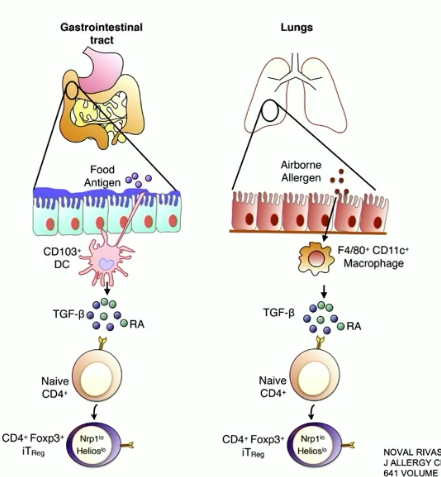
how are Treg cells induced in the GI tract and the lungs?
GI: suppressor dendritic cells along the intestinal mucosa produce TGF-beta, causing FOXP3 to induce Treg cell formation
lungs: macrophages near the mucosa release TGF-beta, same cascade as in GI tract
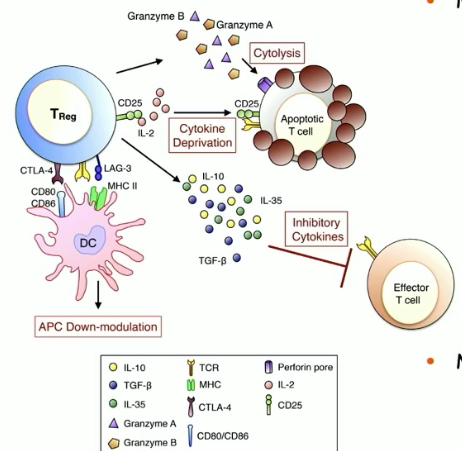
major mechanisms of Treg cell-mediated suppression
production of inhibitory cytokines (IL-10, TGF-beta)
APC inhibition and inhibition of innate signals (ILC2, mast cells, basophils)
promote T cell polarization away from Th2 (downregulate Th2 differentiation and proliferation)
inhibit isotype switching to IgE or promote switching to IgA (noninflammatory antibody)
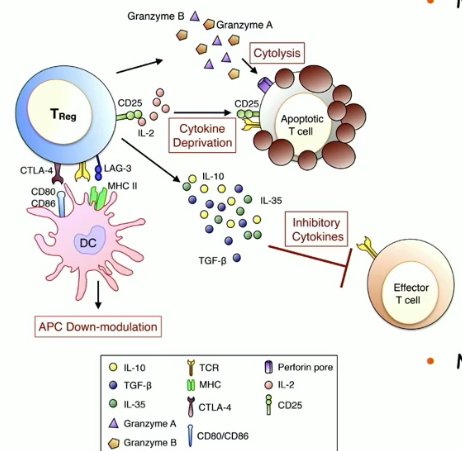
minor mechanisms of Treg cell-mediated suppression
cytokine deprivation
cytolysis
mechanism of preventing allergies, newer discoveries
kill IgE-producing B cells and introduce an adjuvant that produces IgG
form of chemotherapy
what is the signal that drives B-cells to make IgA?
TGF-beta
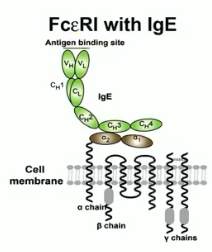
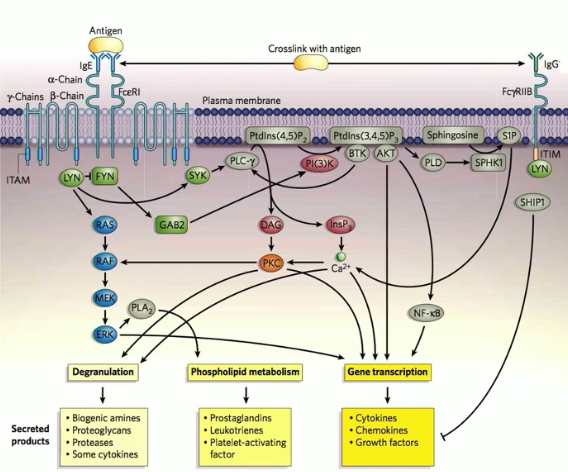
FcεR1
tetrameric receptor complex that binds Fc portion of epsilon heavy chain of IgE
FCɣRIIB
a low affinity receptor fo IgG
expressed on mast cells
crosslinking of antigen between IgE and IgG (via FcεR1 and FCɣRIIB) has inhibitory effect on FcεR1 signaling events, mast cell activation, and product secretion
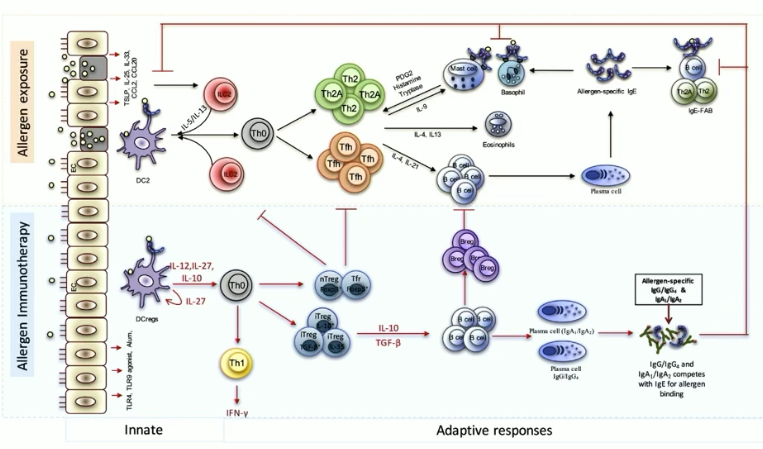
what are allergy shots?
major form of allergy immunotherapy, aka desensitization treatment
theorized mechanism is that it makes more IgG against allergen, promotes inhibitory crosslinking with IgE and IgG
another mechanism is that IgG and IgAs can compete with IgE for allergen binding, inhibiting the allergic response
how do allergy shots work? describe the treatment process
body treats shot like vaccine
body stops producing as many allergic antibodies against allergen
starts with small dosage and builds over time, given weekly for a couple of months and then monthly for 3-5 years
treatment lasts for 5-10 years after allergy shots are stopped
if you stop the shots before 3 years, allergy symptoms typically return more quickly
monoclonal antibodies
Abs with high antigen specificity and target a specific antigen
useful for diagnosis and treatment of diseases
monoclonal antibody and hybridoma treatment
immunize mouse with antigen
fuse one isotype B plasma cell (mono) to an easy-to-grow immortal tumor cell to make a hybridoma, a hyrbid immortal B cell tumor that produces a lot of identical antibodies (clonal) against antigen of interest (monoclonal antibodies)
immortalized plasma cell!
how can you use monoclonal antibodies to treat allergic diseases?
immunize a mouse with IL-4, and so the monoclonal antibody treatment will target and interrupt IL-4 signaling
stopping IL-4 stops Th2 cell response, stopping allergic response
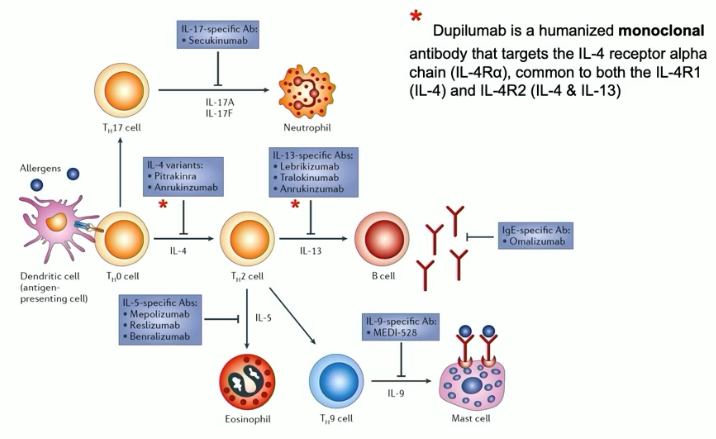
dupilumab (aka dupixent)
FDA-approved monoclonal antibody for eczema, asthma, nasal polyps, and eosinophilic esophagitis
targets IL-4 receptor alpha chain, blocking signaling by IL-4 and IL-13 via steric hindrance (antagonistic)
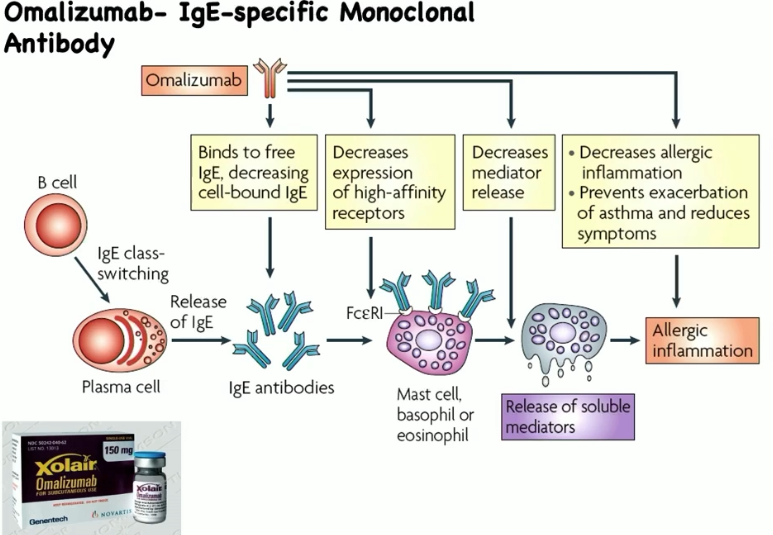
omalizumab (aka xolair)
FDA-approved monoclonal antibody/injection for allergic response
selectively binds to Cε3 domain of IgE molecules, blocks IgE interaction with FcεR1 on mast cells, basophils, eosinophils
inhibits allergen-induced mast cell and basophil degranulation
“big five” for cold and flu meds
acetopminophen - pain reliever (COX-3 inhibitor)
dextromethorphan - antitussive, isomer of semiseynthetic morphine derivative levomethorphan (does NOT act on mu-receptor)
guaifenesin - expectorant, it thins excess mucus in the lungs
phenylephrine - decreases swelling in the nose and ears (alpha-1 adrenergic receptor agonist), decongestant but doesn’t really work
triprolidine/doxylamine succinate - first gen antihistamine (not as effective antihistamine as diphenylhydramine) that makes you drowsy
issues with pseudoephedrine
can make some people hyper
can be used to make speed
conducting zone
areas that filter and warm/humidifies incoming air, not directly involved in gas exchange
what are conducting zones in the human upper airway?
nose/nasal cavity, pharynx, larynx, trachea