EXP 8
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
gas generator
name of the set up

water displacement
what is the set up used for (process)

acetylene gas C2H2 [and calcium hydroxide Ca(OH)2]
what is obtained from this set up?

calcium carbide CaC2 + water H2O
reagents found in the distilling flask

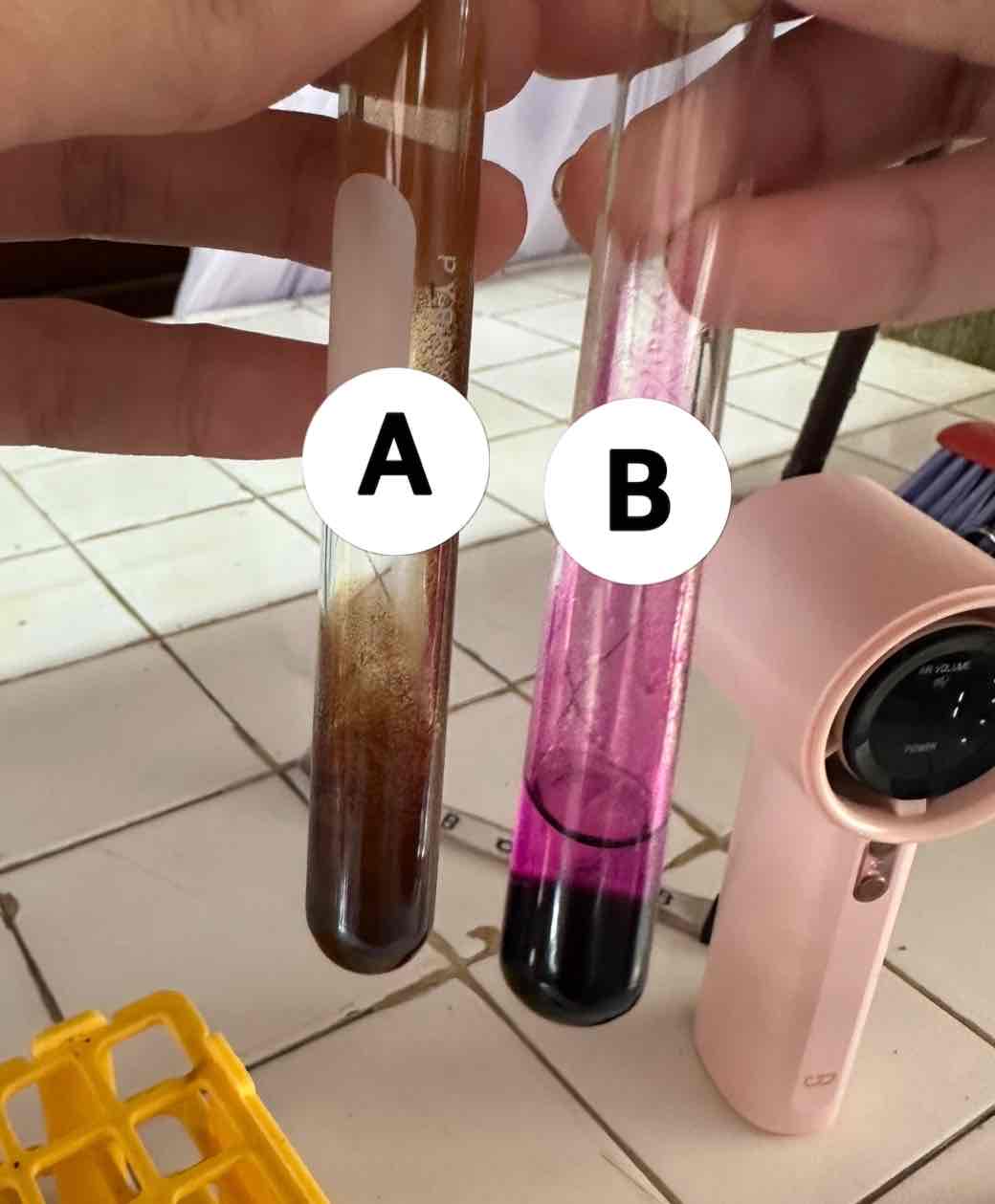
potassium permanganate KMnO4
oxidizing reagent used for this test
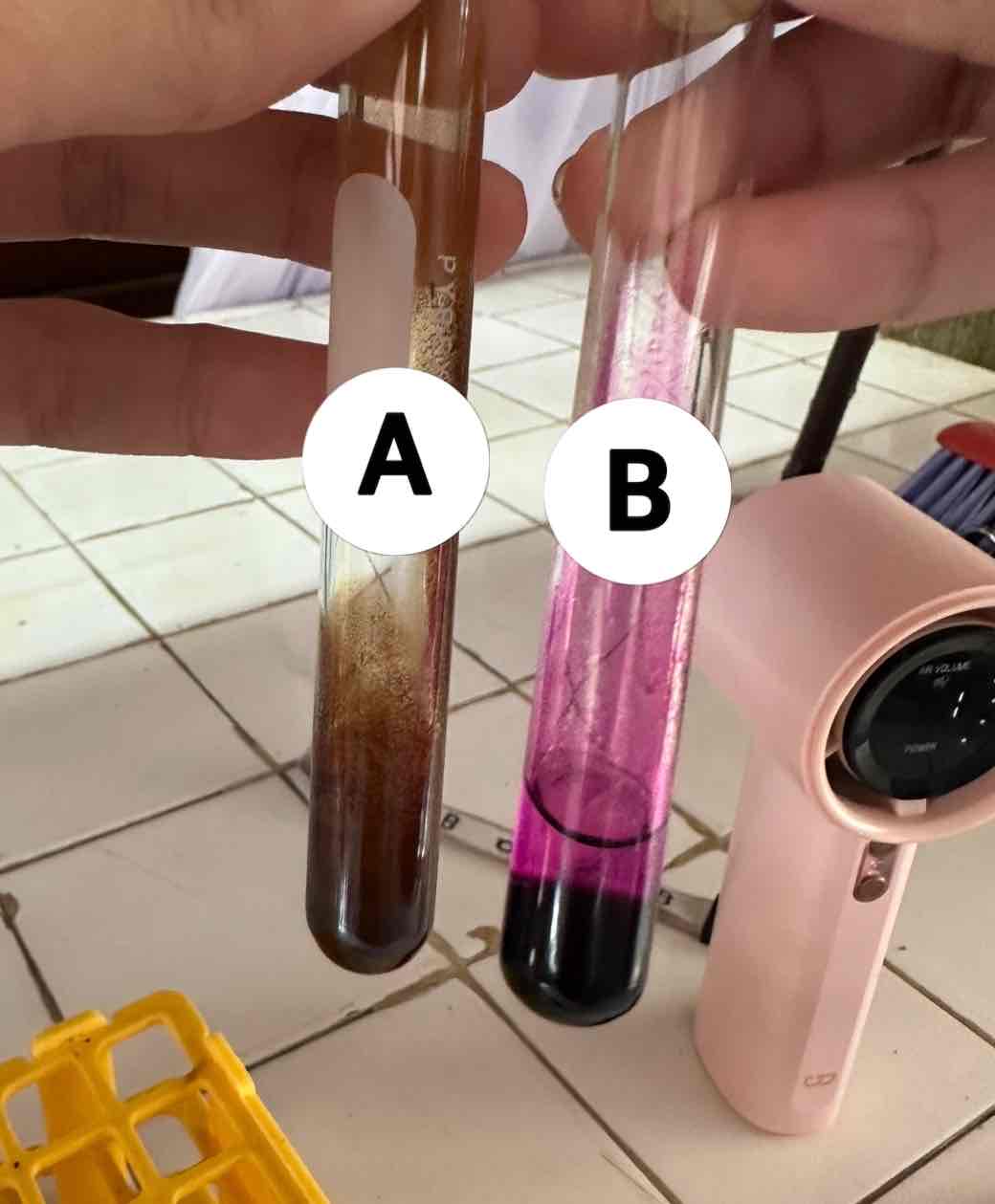
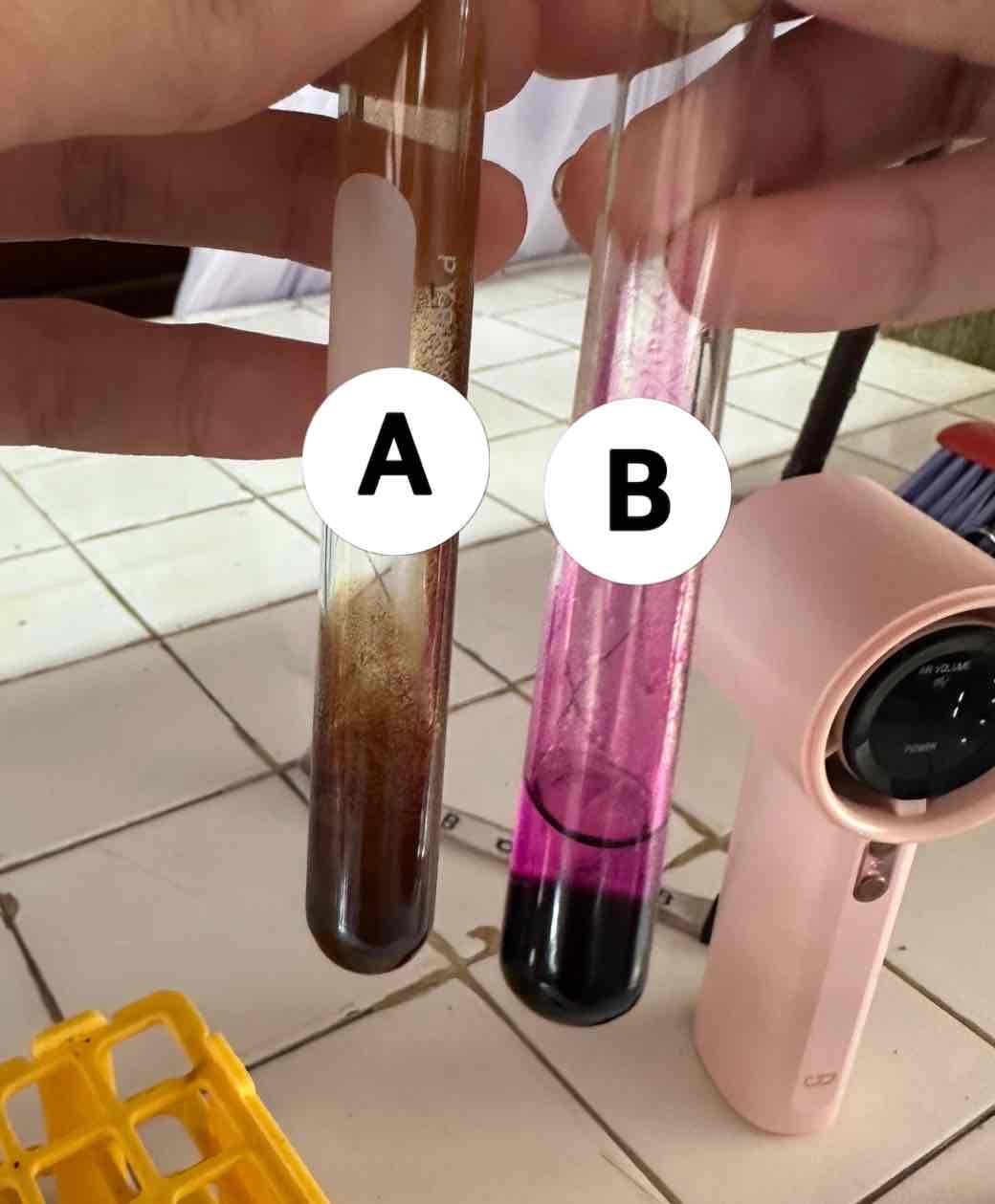
hexane C6H14 + potassium permanganate KMnO4
reagents use for the result in B
NO REACTION (remains purple)
product of the reaction of B
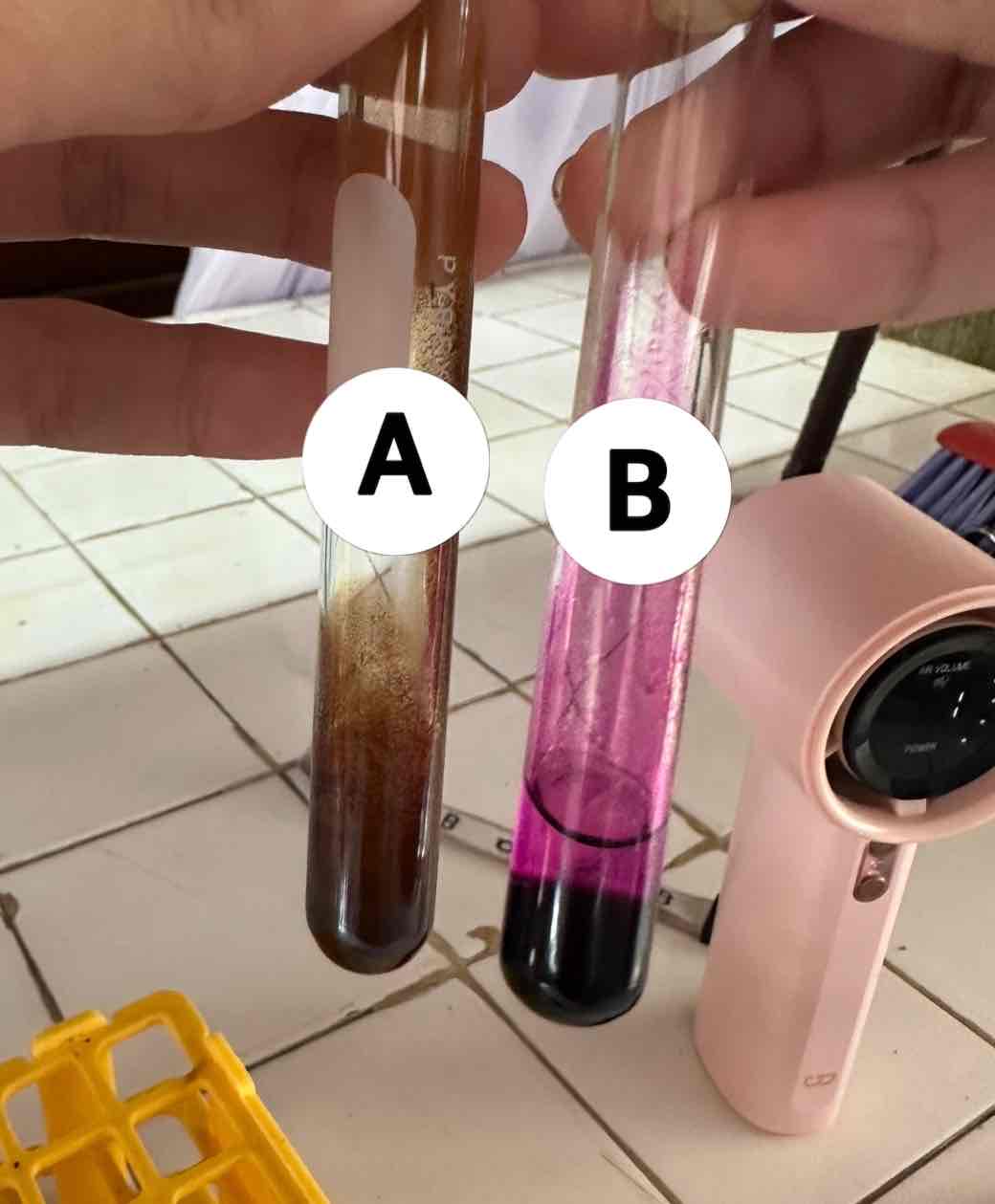
acetylene C2H2 + potassium permanganate KMnO4
reagents use for the result in A
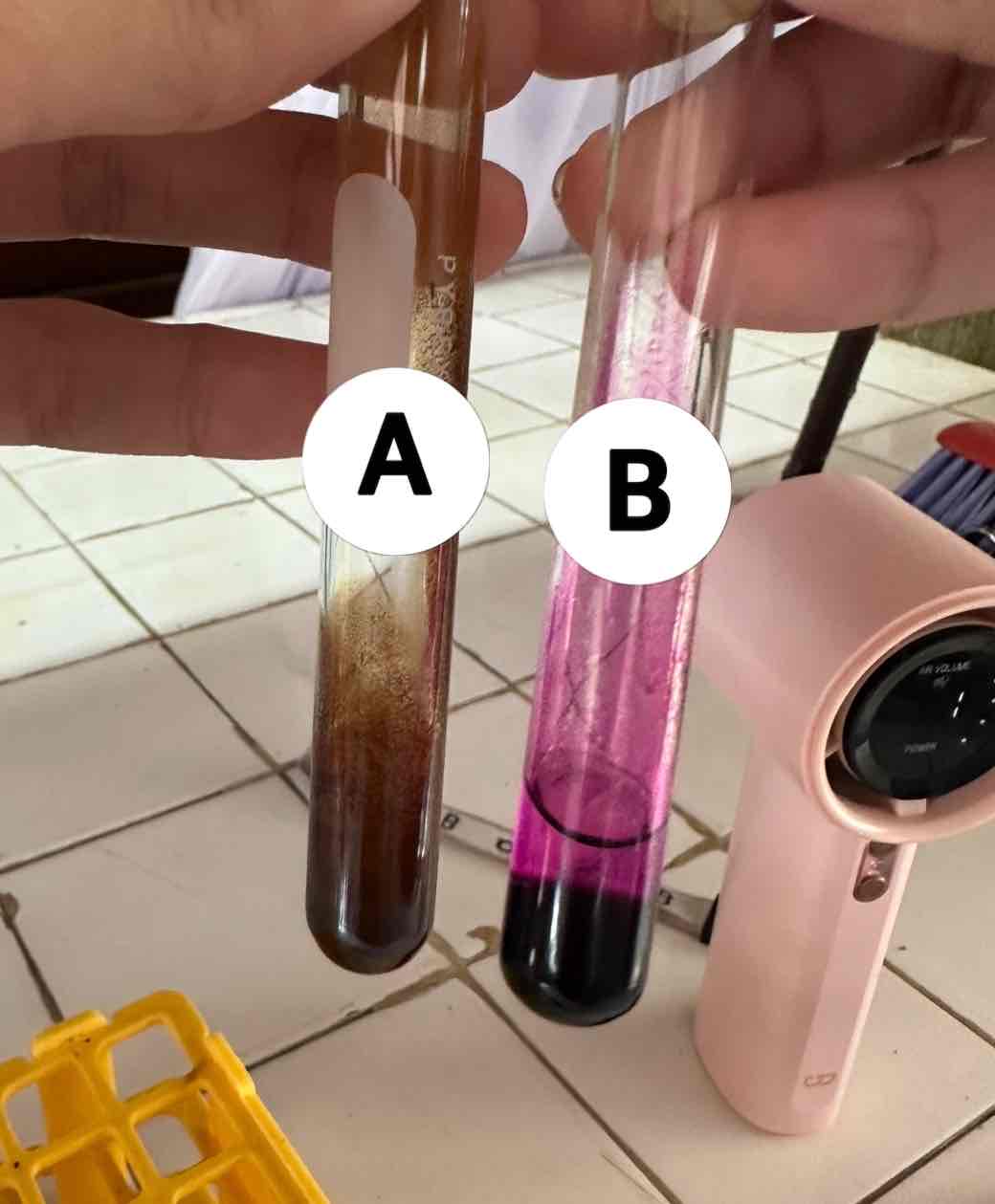
brown ppt: manganese dioxide MnO2
product of the result of A
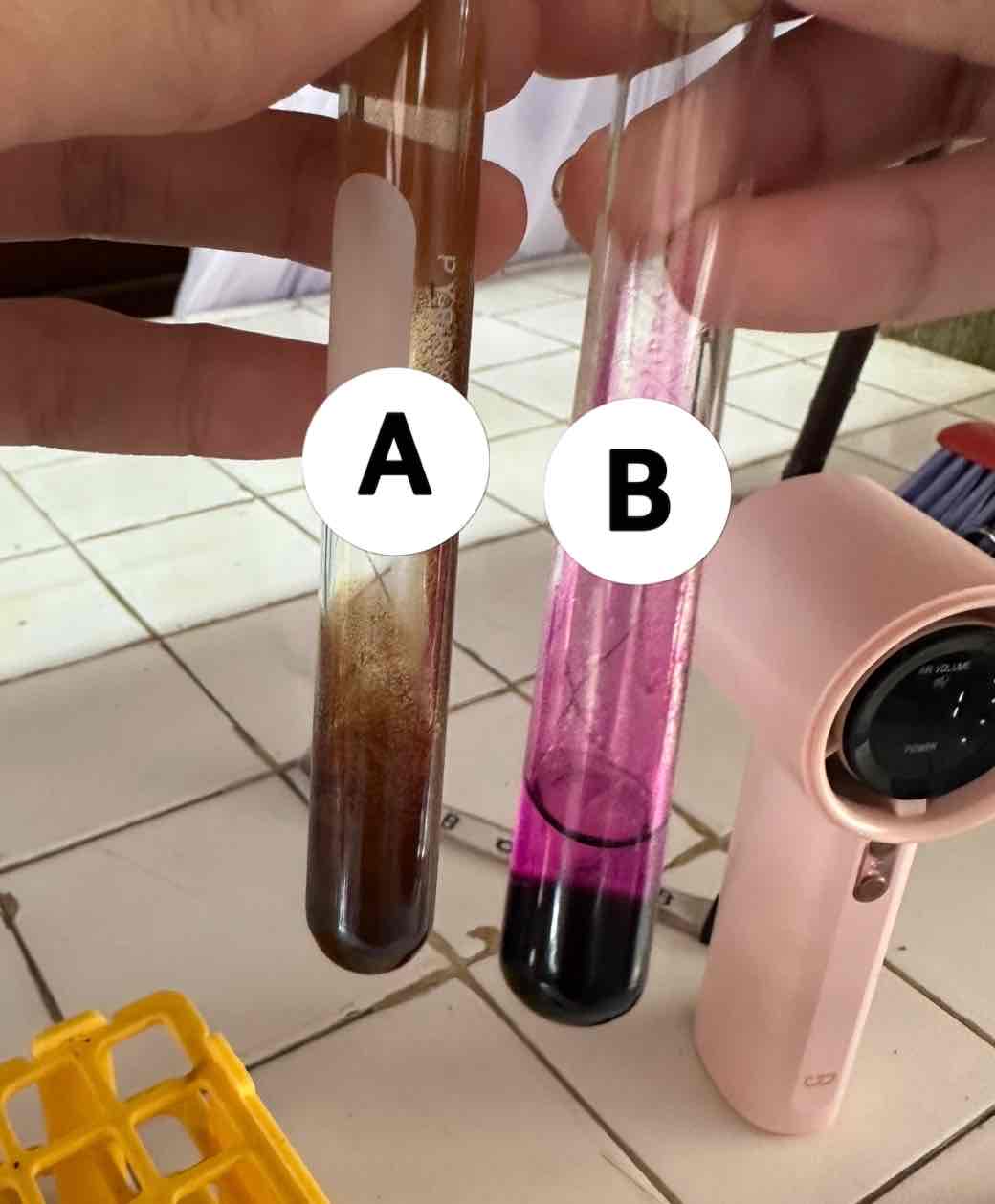
baeyer’s test for unsaturation
name of the test
acetylene C2H2 + bromine Br2
name of reagents used in A

1,1,2,2-tetrabromoethane C2H2Br4
name of the product in A

hexane C6H14 + Bromine Br2 (from bromine water)
name of the reagents used in B

sunlight
name of catalyst used in B

bromohexane C6H13Br
name of the organic product of B

name of A

name of B

acetylene C2H2 + alcoholic iodine
reagents used in A

hexane C6H14 + alcoholic iodine
reagents used in B

hexane C6H14 + oxygen O2
reagents used for this test

carbon dioxide CO2 + water H2O
name of the products from this test

ignition test for alkanes
name of the test used

acetylene C2H2 + ammoniacal solution NH4OH of cuprous chloride Cu2Cl2
reagents used in this reaction

cuprous acetylide Cu2C2
product of this reaction

chocolate brown ppt
color of the product of this reaction
