chapter 20 chem acids and bases`
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is a bronsted lowry acid?
a proton donor
bronsted lowry base
proton acceptor
what is a conjugate acid base pair?
two species that can be interconverted by transfer of a proton.
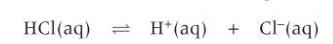
what is the conjugate acid base pair in this equation?
HCl and Cl-
HCl releases a proton to form its conjugate base Cl-.
Cl- accepts a proton to forms its conjugate acid HCl.

conjugate acid base pairs in this?

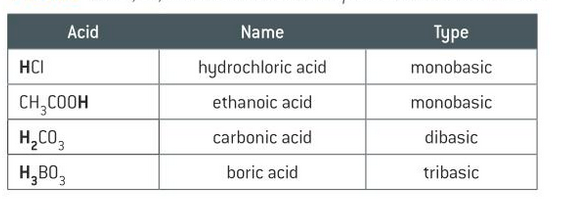
define monobasic, dibasic and tribasic
refer to the total number of protons an acid can donate
acid and carbonate makes?
salt water and CO2
acids with metal oxides?
salt and water
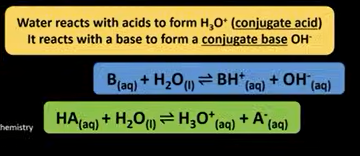
write the equations for how water acts as a base and an acid
pH formula?
-log[H+]
H+ formula?
10^-pH
for pH of monoprotic strong acids?
use the concentration in moldm^-3 directly as the H+ concentration is equal to [HA].
then -log[H+]
for pH of diprotic strong acids?
2[H+] = [Acid]
double the concentration given because they dissociate to give two H+ ions
ionic product of water Kw?
units?
value at 25C?
Kw = [H+][OH-]
mol2dm-6
1× 10-14
dilution factor
new conc = old conc * original vol/total vol
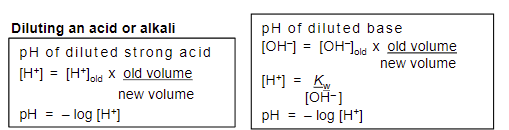
Every time the pH goes up by one…
the concentration of H+ ions goes down by a factor of ten
how to find pH of strong base?
Kw/[OH-] = [H+]
when do you need the Ka formula?
with weak acids because they only dissociate slightly
HA ←> H+ + A-
assumption one for weak acids
only a small amount of the weak acid dissociates so we can assume
[HA]equilibrium = [HA]start
conc at equilibrium = conc at start
assumption 2 for weak acids?
the dissociation of acid is greater than teh dissociation of water. so we can assume all H+ ions come from acid
[H+] = [A-]
what is the Ka formula for weak acids?
[H+]2/[HA]
what is pKa?
another way of measuring strength of acid
similiar to pH
pKa = -log(Ka)
how do you measure pH experiementally?
pH meters measure the pH of a solution however, they must be calibrated correctly.
probe placed in distilled water first
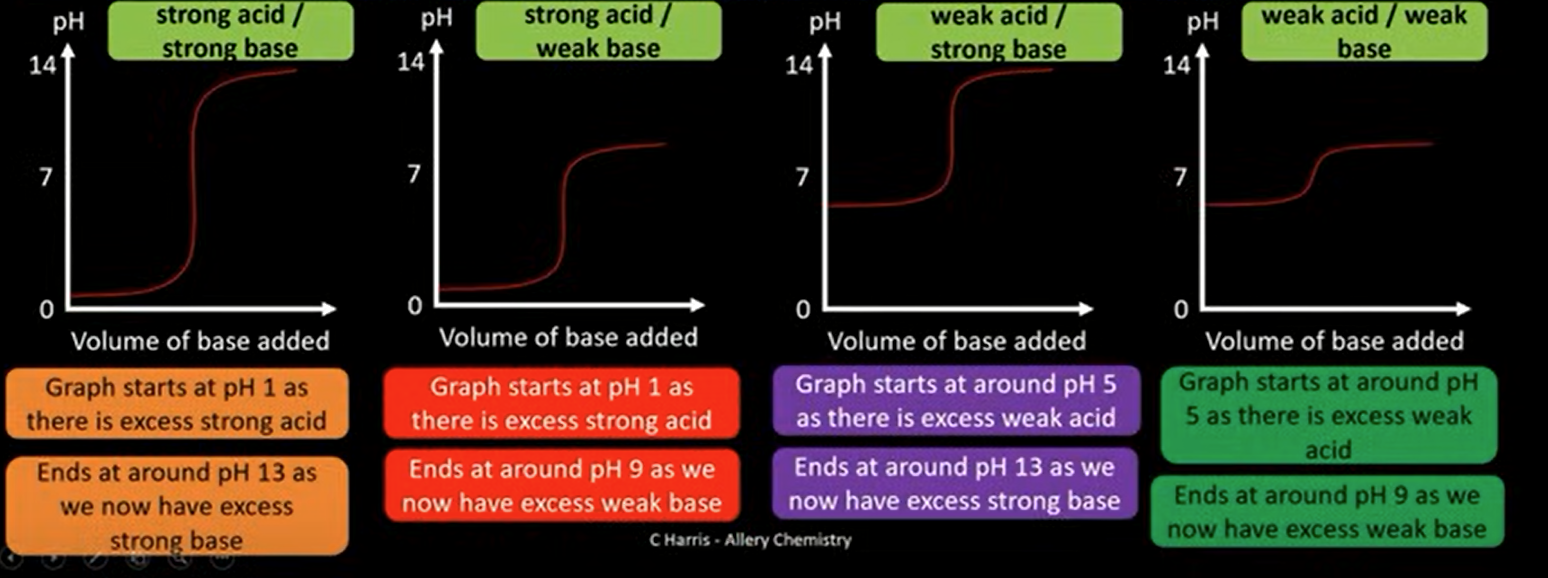
draw out the four titration curves