psychosis and schizophrenia
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
What are the main types of hallucinations experienced in psychosis?
Hallucinations can involve sight, sound, smell, touch, or taste.
In psychosis, which senses can be affected by hallucinations?
Hallucinations may occur in the senses of sight, sound, smell, touch, and taste.
What are common examples of delusions in psychosis?
Common types of delusions include persecutory and grandiose delusions.
In psychosis, what types of delusions might a person experience?
A person may experience persecutory or grandiose delusions
What are signs of confused and disturbed thoughts in psychosis?
Signs include rapid and constant speech, disturbed speech, and loss of train of thought.
How might confused and disturbed thoughts present in someone with psychosis?
They may show rapid and constant speech, disturbed speech, or a loss of train of thought.
What does lack of insight and self-awareness mean in psychosis?
It means the person is unaware that their delusions or hallucinations aren’t real.
In psychosis, what does it indicate when someone shows lack of insight and self-awareness?
It indicates they are unaware that their delusions or hallucinations are not real.
what are causes of psychosis?
•Alzheimer’s, Parkinson’s, Huntington’s diseases
•Depression, bipolar disorder
•Some types of epilepsy
•Stress, trauma
•Lack of sleep
•Drugs
•Schizophrenia
What are the Greek origins of the term schizophrenia and their meanings?
It comes from “schizein” meaning “to split” and “phren” meaning “mind.”
What does schizophrenia actually mean and what does it not mean?
A2:
It means a divided mind or fragmented thinking, not multiple or split personalities.
In schizophrenia, what does “divided mind” refer to, and what common misconception should be avoided?
It refers to fragmented thinking and a division between internal thought and external reality, not multiple or split personalities.
What kind of division does schizophrenia describe?
It describes a division between internal thought and external reality.
n schizophrenia, what is divided between the person’s internal experience and the outside world?
There is a division between internal thought and external reality.
When is schizophrenia considered as a possible diagnosis?
Schizophrenia is a chronic mental illness only considered after a second episode of psychosis.
t what stage can schizophrenia be diagnosed?
It is diagnosed after a second episode of psychosis, reflecting a chronic mental illness.
What are positive symptoms of schizophrenia and what do they represent
Positive symptoms are an increase in abnormal active behaviours, including hallucinations, delusions, disordered thoughts, and disturbed speech.
In schizophrenia, what are examples of positive symptoms and what do they reflect?
They reflect increased abnormal active behaviours such as hallucinations, delusions, disordered thoughts, and disturbed speech.
What are negative symptoms of schizophrenia and what do they indicate?
Negative symptoms indicate the absence of normal active behaviours, such as affective blunting, avolition, anhedonia, poverty of speech, social withdrawal, and neglect of hygiene.
In schizophrenia, which symptoms show the absence of normal active behaviours?
Negative symptoms include affective blunting, avolition, anhedonia, poverty of speech, social withdrawal, and neglect of hygiene.
How do negative symptoms typically relate in timing and persistence to positive symptoms in schizophrenia?
Negative symptoms often occur before positive symptoms and then persist over time.
In schizophrenia, when do negative symptoms usually appear compared to positive symptoms, and how long do they last?
They often occur before positive symptoms and persist even after other symptoms improve.
What are the cognitive symptoms of schizophrenia?
Cognitive symptoms involve disturbance of normal thought processes, including poor executive function, poor decision making, poor cognitive flexibility, recognition deficits, memory problems, and attention deficits.
Which cognitive disturbances are common in schizophrenia?
Poor executive function, poor decision making, poor cognitive flexibility, recognition deficits, memory problems, and attention deficits
What is known about the overall cause of schizophrenia?
The cause is largely unknown, but it is likely to be multifactorial, involving both genetic and environmental factors.
How do genetic factors contribute to schizophrenia?
Schizophrenia is strongly inherited, with about a 50% concordance in monozygotic twins. No specific gene is identified, but over 100 susceptibility genes have been implicated.
What evidence supports a genetic basis for schizophrenia?
It shows strong inheritance, about 50% concordance in monozygotic twins, and involvement of more than 100 susceptibility genes, though no single gene has been identified.
What environmental factors are associated with schizophrenia?
No specific environmental factors are confirmed, but possible influences include pregnancy and delivery complications, prenatal and childhood virus infections, urban birth and residence, and psychosocial factors such as a dysfunctional family environment.
What role does the frontal cortex play in schizophrenia, and how does dopamine affect it?
A1:
The frontal cortex (prefrontal cortex) controls cognition, decision-making, and executive function. Low dopamine (hypodopaminergia) here contributes to negative symptoms and cognitive deficits.
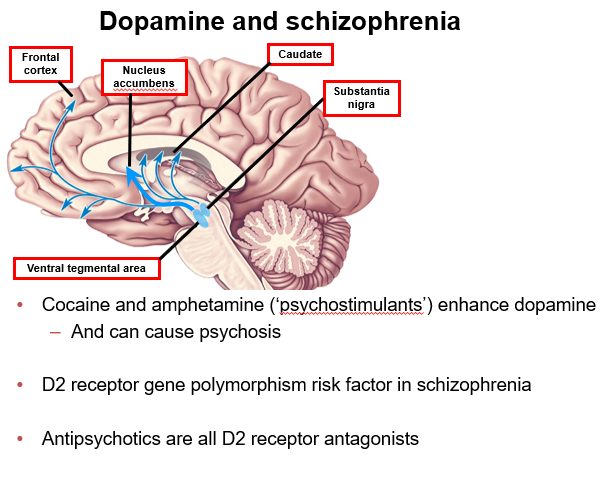
How does dopamine activity in the frontal cortex relate to schizophrenia symptoms?
Reduced dopamine in the frontal cortex leads to negative symptoms like affective blunting, avolition, and anhedonia, and cognitive deficits.
What is the function of the nucleus accumbens and how does dopamine affect schizophrenia symptoms?
The nucleus accumbens, part of the ventral striatum, regulates reward and motivation. Hyperdopaminergia here causes positive symptoms such as hallucinations and delusions.
In schizophrenia, how does dopamine in the nucleus accumbens influence behaviour?
Too much dopamine in the nucleus accumbens contributes to positive symptoms, including hallucinations and delusions.
What is the role of the caudate in dopamine pathways and schizophrenia?
The caudate, part of the dorsal striatum, controls motor function and habit formation. Dopamine blockade here (by antipsychotics) can cause extrapyramidal side effects (EPS).
How does dopamine in the caudate relate to schizophrenia treatment?
Blocking dopamine in the caudate (nigrostriatal pathway) can lead to movement-related side effects (EPS) during antipsychotic therapy.
What roles do the substantia nigra and ventral tegmental area (VTA) play in dopamine pathways?
Substantia nigra → dorsal striatum: controls motor functions
VTA → nucleus accumbens & frontal cortex: regulates reward, motivation, cognition
Dysregulation contributes to psychosis.
Which dopamine-producing regions are important in schizophrenia, and what are their roles?
Substantia nigra (motor control) and VTA (reward, motivation, cognition) produce dopamine. Dysregulation in these pathways can lead to psychosis.
How do cocaine and amphetamine affect dopamine and psychosis?
They enhance dopamine release or block reuptake in the nucleus accumbens and frontal cortex, which can cause psychosis.
Why can psychostimulants like cocaine and amphetamine trigger psychosis?
They increase dopamine activity in the mesolimbic (nucleus accumbens) and mesocortical (frontal cortex) pathways, potentially causing psychosis.
What is the role of D2 receptors in schizophrenia and its treatment?
D2 receptor gene polymorphisms are a risk factor for schizophrenia. All antipsychotics block D2 receptors (D2 antagonists), particularly in the mesolimbic pathway, reducing positive symptoms.
How do D2 receptors relate to schizophrenia risk and antipsychotic action?
D2 receptor variations increase schizophrenia risk. Antipsychotics antagonize D2 receptors, which decreases positive symptoms like hallucinations and delusions.
How do dopamine pathways explain schizophrenia symptoms?
Mesolimbic (VTA → nucleus accumbens): ↑ dopamine → positive symptoms
Mesocortical (VTA → frontal cortex): ↓ dopamine → negative/cognitive symptoms
Nigrostriatal (substantia nigra → caudate): normal → motor control; D2 blockade → EPS
Tuberoinfundibular pathway: normal → regulates prolactin; D2 blockade → hyperprolactinemia
Which dopamine pathways are linked to positive, negative, and motor symptoms in schizophrenia?
Mesolimbic ↑ dopamine → positive symptoms
Mesocortical ↓ dopamine → negative/cognitive symptoms
Nigrostriatal → motor control; D2 blockade → EPS
Tuberoinfundibular → prolactin regulation; D2 blockade → hyperprolactinemia
How does the mesolimbic pathway and D2 receptor activity relate to schizophrenia symptoms?
Mesolimbic pathway (VTA → nucleus accumbens) has increased D2 receptor activity, causing positive symptoms like hallucinations and delusions.
Which dopamine pathway and receptor are associated with positive symptoms in schizophrenia?
Hyperactive D2 receptors in the mesolimbic pathway lead to positive symptoms.
What is the role of the mesocortical pathway and D1 receptor activity in schizophrenia?
Mesocortical pathway (VTA → frontal cortex) has reduced D1 receptor activity, leading to negative symptoms (avolition, anhedonia) and cognitive symptoms (poor executive function, working memory deficits).
Which dopamine pathway and receptor contribute to negative and cognitive symptoms?
Hypoactive D1 receptors in the mesocortical pathway produce negative and cognitive symptoms.
How do D1 and D2 receptor activity in dopamine pathways explain schizophrenia symptoms?
Mesolimbic D2 hyperactivity → positive symptoms
Mesocortical D1 hypoactivity → negative and cognitive symptoms
Antipsychotics block D2 receptors → reduce positive symptoms; negative/cognitive symptoms less responsive.
Why do antipsychotics mainly improve positive symptoms in schizophrenia?
They antagonize D2 receptors in the mesolimbic pathway. Negative/cognitive symptoms remain because mesocortical D1 receptor activity is low.
What is the primary mechanism of action of typical (first-generation) antipsychotics?
They are high-affinity D2-receptor antagonists, blocking dopamine in the mesolimbic pathway.
How do first-generation antipsychotics work at the receptor level?
They antagonize D2 receptors with high affinity, reducing dopamine activity in the mesolimbic pathway.
Give two examples of typical (first-generation) antipsychotics.
Chlorpromazine and haloperidol are examples.
Which drugs are considered first-generation antipsychotics?
Chlorpromazine and haloperidol.
Which schizophrenia symptoms do typical antipsychotics mainly treat?
They are effective only against positive symptoms, such as hallucinations and delusions.
Are typical antipsychotics effective for negative symptoms?
No, they treat only positive symptoms; negative and cognitive symptoms are largely unaffected.
What are extrapyramidal side effects of typical antipsychotics?
EPS are movement disorders caused by D2 receptor blockade in the nigrostriatal pathway, leading to Parkinson’s-like or Huntington’s-like symptoms.
How do typical antipsychotics cause extrapyramidal side effects?
Blocking D2 receptors in the nigrostriatal pathway disrupts normal motor control, resulting in acute dystonias or tardive dyskinesia.
What is an example of an acute extrapyramidal side effect?
Acute dystonias, which are Parkinson’s-like movement disorders (e.g., muscle spasms, rigidity).
Which EPS resembles Parkinson’s disease in patients on typical antipsychotics?
Acute dystonias, presenting as Parkinson’s-like symptoms.
What is an example of a chronic extrapyramidal side effect?
Tardive dyskinesia, which is Huntington’s-like, including involuntary, repetitive movements, often irreversible.
Which EPS resembles Huntington’s disease in patients taking typical antipsychotics?
Tardive dyskinesia, a chronic, involuntary movement disorder.
How do typical antipsychotics cause increased prolactin release?
They block D2 receptors in the tuberoinfundibular pathway, removing dopamine’s inhibitory effect on prolactin, leading to hyperprolactinemia.
Which pathway and receptor are responsible for antipsychotic-induced galactorrhea?
D2 receptor blockade in the tuberoinfundibular pathway increases prolactin, causing galactorrhea.
What is a clinical sign of increased prolactin due to typical antipsychotics?
Galactorrhea (milk secretion), menstrual disturbances in women, and sexual dysfunction in men.
Which serious side effect of typical antipsychotics presents as milk secretion in non-lactating patients?
Galactorrhea, caused by hyperprolactinemia.
Which receptors are responsible for sedation from antipsychotics?
Histamine H1 and muscarinic receptor antagonism cause sedation.
Why do many antipsychotics cause drowsiness or sedation?
Because they block H1 histamine and muscarinic receptors.
Which receptor antagonism leads to hypotension with antipsychotic use?
α-adrenergic receptor antagonism causes peripheral vasodilation and hypotension.
What is the mechanism of antipsychotic-induced hypotension?
Blockade of α-adrenergic receptors leads to low blood pressure.
Which receptors mediate blurred vision, dry mouth, and constipation in patients on antipsychotics?
Muscarinic receptor antagonism causes these peripheral autonomic side effects.
How do antipsychotics cause dry mouth, blurred vision, and constipation?
By blocking muscarinic receptors, affecting the autonomic nervous system
What is the D2 receptor activity of atypical antipsychotics?
They are D2 receptor antagonists with relatively low affinity, which reduces motor side effects compared to typical antipsychotics.
How do atypical antipsychotics differ from typical ones regarding D2 receptors?
They block D2 receptors less strongly, making them effective for positive symptoms with fewer extrapyramidal effects.
Give four examples of atypical antipsychotics.
Clozapine, olanzapine, risperidone, aripiprazole.
Which drugs are considered second-generation antipsychotics?
Clozapine, olanzapine, risperidone, aripiprazole.
Which schizophrenia symptoms are treated by atypical antipsychotics?
They are effective against both positive (hallucinations, delusions) and negative symptoms (avolition, anhedonia).
Are atypical antipsychotics effective for negative symptoms?
Yes, unlike typical antipsychotics, they treat both positive and negative symptoms.
Which serotonin receptor do atypical antipsychotics strongly antagonize?
High-affinity 5HT2 receptor antagonism; a high 5HT2:DA block ratio may improve efficacy and reduce motor side effects.
How does 5HT2 antagonism contribute to the atypical antipsychotic profile?
It enhances efficacy, especially for negative symptoms, and reduces extrapyramidal side effects.
What are common side effects of atypical antipsychotics?
Weight gain (H1 and 5HT2C antagonism)
Diabetes
Agranulocytosis / leukopenia (especially clozapine)
Which atypical antipsychotic side effects are linked to histamine, serotonin, and blood effects?
Weight gain (H1/5HT2C), diabetes, and agranulocytosis (clozapine).
When is clozapine prescribed?
Clozapine is reserved for treatment-resistant schizophrenia, given only if >2 other antipsychotics fail, because of serious side effects.
Why is clozapine considered a last-resort antipsychotic?
It is effective where others fail but has high risk of agranulocytosis and other side effects, so only used if >2 antipsychotics are ineffective.
What are the limitations of selective D2 receptor antagonists?
Cause serious dopaminergic-related side effects (extrapyramidal symptoms)
Only control positive symptoms (hallucinations, delusions)
How can 5HT2 receptor activity affect psychosis?
5HT2 agonists (e.g., LSD) can induce psychotic episodes
5HT2A antagonists may increase dopamine release, improving negative symptoms with fewer motor side effects
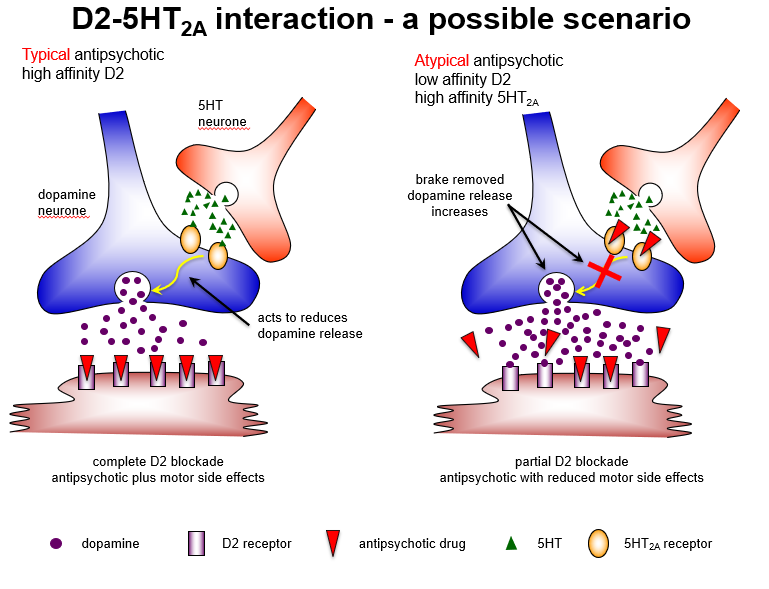
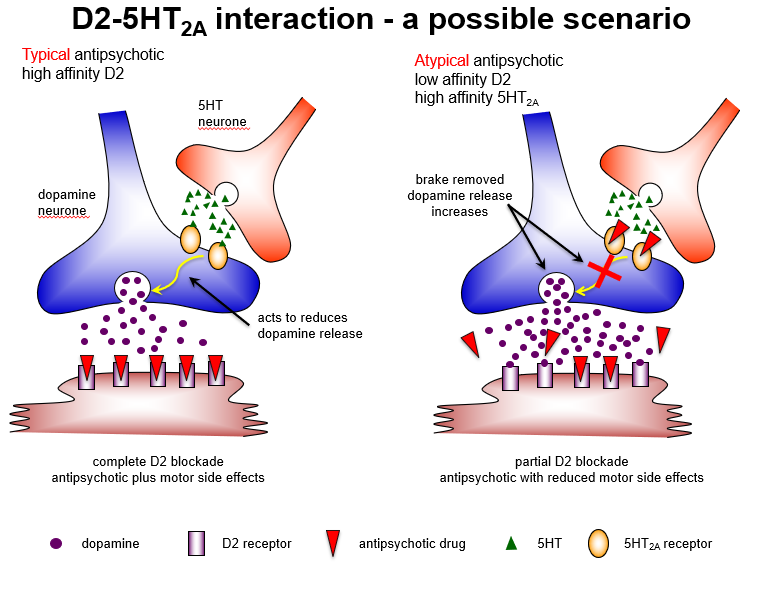
Why might blocking 5HT2A receptors be useful in atypical antipsychotics?
5HT2A antagonism removes inhibition of dopamine release, enhancing D1 receptor activity in cortex, reducing negative symptoms, and minimizing extrapyramidal side effects.
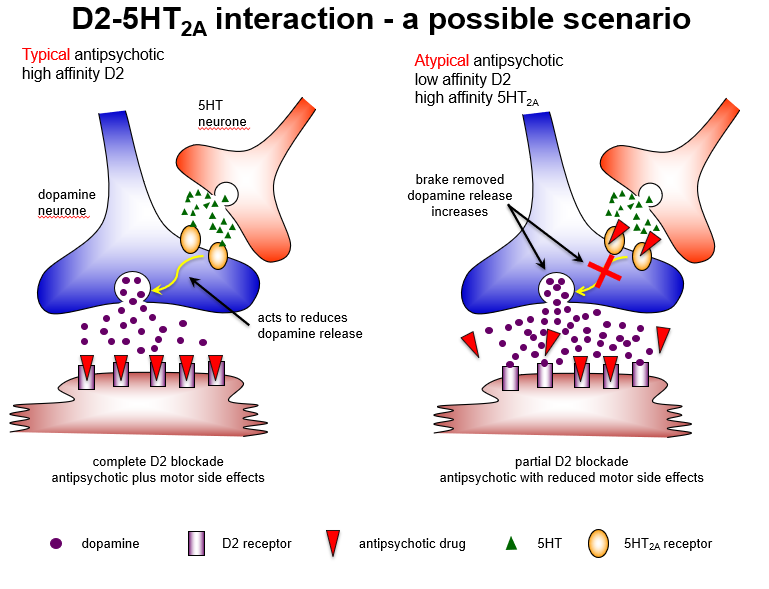
Compare typical and atypical antipsychotic mechanisms regarding D2 and 5HT2A receptors.
Typical: high-affinity D2 blockade → strong antipsychotic effect + motor side effects
Atypical: low-affinity D2 + high-affinity 5HT2A blockade → antipsychotic effect + reduced motor side effects
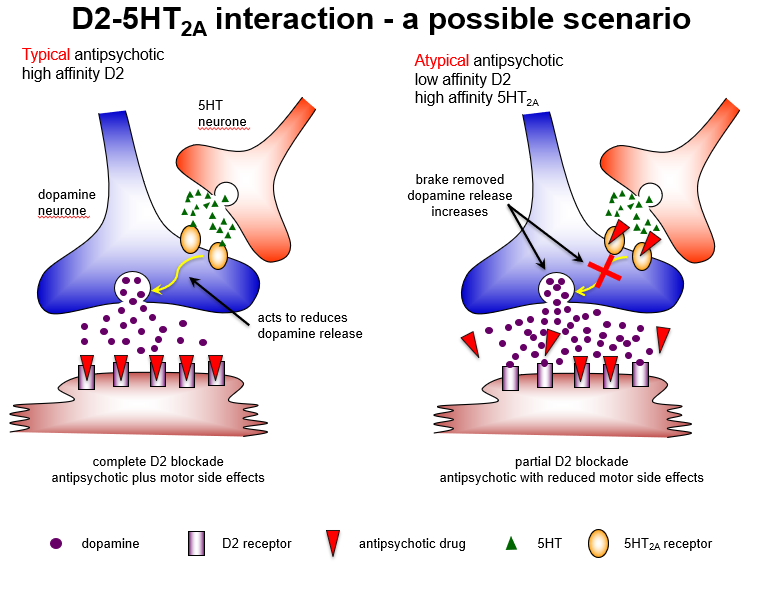
How do typical and atypical antipsychotics differ in receptor interactions?
Typical: complete D2 blockade → good positive symptom control, high extrapyramidal risk
Atypical: partial D2 + strong 5HT2A blockade → maintains antipsychotic effect, reduces motor side effects.
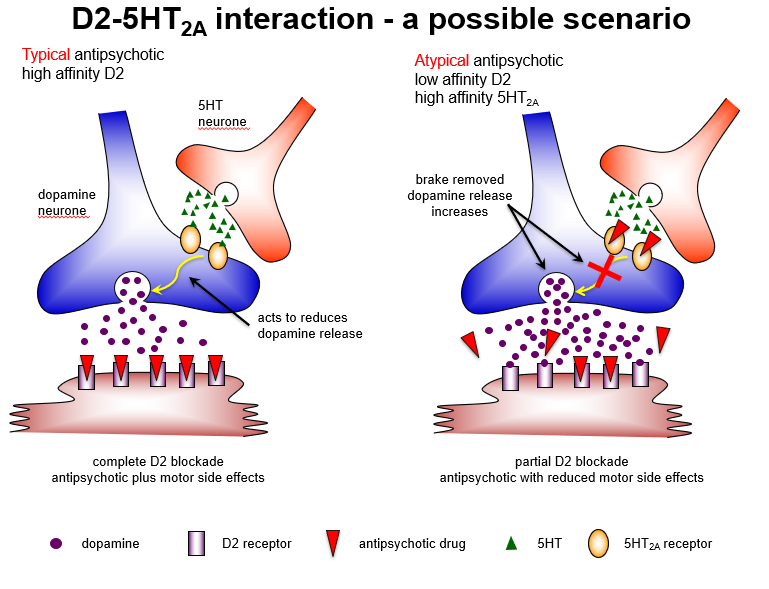
Why do atypical antipsychotics cause fewer extrapyramidal side effects?
Because 5HT2A antagonism predominates in the nigrostriatal pathway, increasing dopamine release, leading to partial D2 blockade and reduced EPS.
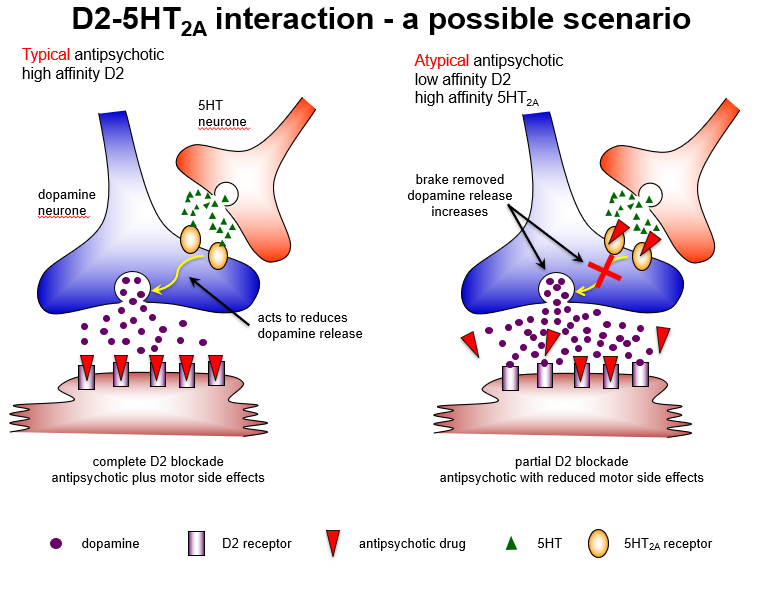
Which brain pathway explains why atypical antipsychotics have fewer motor side effects?
Nigrostriatal pathway: 5HT2A blockade increases dopamine, allowing partial D2 blockade and fewer extrapyramidal symptoms.

How do atypical antipsychotics affect mesolimbic and cortical pathways?
Mesolimbic: few 5HT2A receptors → still get D2 blockade, so positive symptoms controlled
Cortex: mostly D1 receptors → increased dopamine via 5HT2A antagonism → improved negative symptom
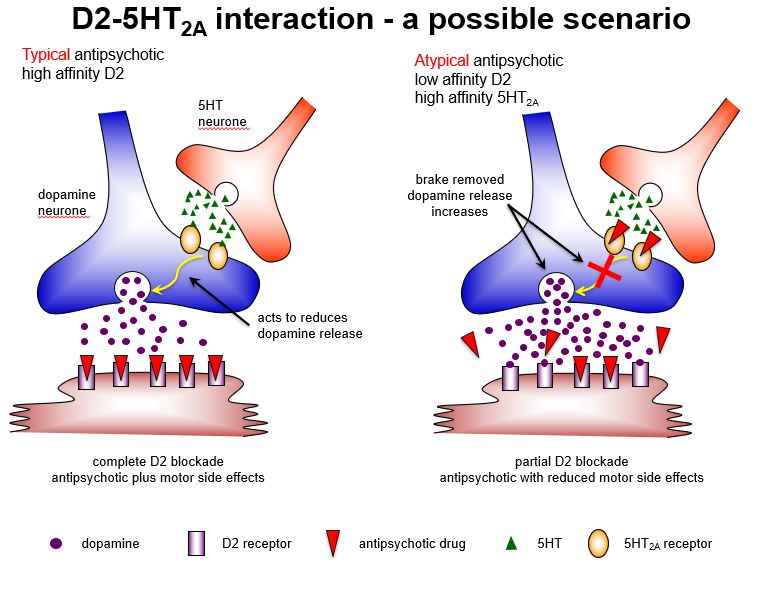
Why do atypical antipsychotics improve negative symptoms in the cortex?
5HT2A antagonism increases dopamine, activating D1 receptors, which reduces negative and cognitive symptoms.