NST 103 MT 2 (Sul)
1/146
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
147 Terms
In fasting/starvation…
Low insulin & High glucagon and epinephrine in the blood
Protein synthesis is suppressed
Cholesterol synthesis is low
FA & TAG synthesis are negligible
while they last, glycogen supplies to maintain plasma glucose levels and used for energy
FFA are released from stored TAG, β-oxidized to acetyl CoA and (1) used directly for energy or (2) converted to KB (liver) which can be used by other tissues for energy
Protein catabolism releases AAs that can be used for energy either directly (via pyruvate and/or TCAC) or indirectly (converted to glucose in liver, utilized for energy in different tissues
cAMP and glucagon + epinephrine
glucagon & epinephrine bind to receptors on the membrane, which activates G protein
GDP converted to GTP (active form)
GTP stimulates adenylyl cylcase (AC, enzyme in the membrane), which converts ATP into cAMP
cAMP stimulates PKA, which phosphorylates proteins, influencing their activity
substrates in GNG
Lactate:
Produced by anaerobic glycolysis in muscles and red blood cells
converted into pyruvate in the liver
Glycerol:
Released from the breakdown of triglycerides in adipose tissue
an be converted into DHAP (intermediate)
Amino acids (especially alanine):
During periods of fasting or starvation, muscle proteins break down, releasing amino acids.
converted into intermediates such as pyruvate or oxaloacetate
Propionate:
breakdown of certain fatty acids and can be converted into succinyl-CoA.
GNG vs glycolysis
GNG | glycolysis | |
purpose | synthesize glucose from non-carbohydrate precursors | break down glycose into pyruvate to produce ATP |
when | fasting | high energy demand |
location | liver, kidneys | all cells, esp muscle and brain |
starting material | lactate, glycerol, glucogenic amino acids | glucose |
end products | glucose | pyruvate (lactate in anaerobic conditions) |
energy requriment | consumes 4 ATP and 2 GTP | produces net gain of 2 ATP p |
key regulatory enzymes | pyruvate carboxylase, PEP carboxykinase, fructose-1,6-biphosphate, glucose 6-phosphatase | hexokinase, PFK1, pyruvate kinase |
hormonal regulation | glucagon, cortisol: promote glucose production during fasting | insulin: romotes glucose breakdown to provide energy or store as fat. |
3 bypass steps in GNG
Gluconeogenesis involves bypassing the irreversible steps of glycolysis.
Pyruvate → Oxaloacetate → Phosphoenolpyruvate (PEP):
Enzymes: Pyruvate carboxylase and PEP carboxykinase.
Fructose-1,6-bisphosphate → Fructose-6-phosphate:
Enzyme: Fructose-1,6-bisphosphatase.
Glucose-6-phosphate → Glucose:
Enzyme: Glucose-6-phosphatase.
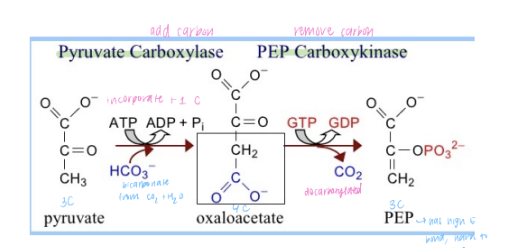
Pyruvate to Phosphoenolpyruvate (PEP)
glycolysis: Pyruvate kinase (converts phosphoenolpyruvate to pyruvate)
bypass:
Pyruvate carboxylase: Converts pyruvate to oxaloacetate in the mitochondria. This step requires ATP.
Phosphoenolpyruvate carboxykinase (PEPCK): Converts oxaloacetate to phosphoenolpyruvate (PEP). This step requires GTP.
This bypass requires energy (1 ATP and 1 GTP) and occurs in two steps, starting in the mitochondria and moving to the cytosol.
Fructose-1,6-bisphosphate to Fructose-6-phosphate
Glycolysis Enzyme: Phosphofructokinase-1 (PFK-1) (converts fructose-6-phosphate to fructose-1,6-bisphosphate)
Bypass Enzyme in Gluconeogenesis:
Fructose-1,6-bisphosphatase: Converts fructose-1,6-bisphosphate to fructose-6-phosphate.
This step is a simple hydrolysis reaction and does not require ATP.
Glucose-6-phosphate to Glucose
Glycolysis Enzyme: Hexokinase/glucokinase (converts glucose to glucose-6-phosphate)
Bypass Enzyme in Gluconeogenesis:
Glucose-6-phosphatase: Converts glucose-6-phosphate to glucose.
This step occurs in the endoplasmic reticulum (ER) and allows free glucose to be released into the bloodstream.
regulation of glycolysis by glucagon and epinephrine
Glucagon and epinephrine trigger a cascade that leads to the phosphorylation of key enzymes
PFK-2 is phosphorylated and becomes inactive, which reduces levels of F-2,6-BP
leads to decreased stimulation of PFK-1, reducing the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate.
Pyruvate kinase is phosphorylated and inactivated. This enzyme normally converts PEP (phosphoenolpyruvate) to pyruvate in glycolysis, and its inhibition further slows glycolysis.
regulation of glycogenolysis by glucagon and epinephrine
Glycogen synthase (the enzyme that builds glycogen) is phosphorylated, which inactivates it.
Glycogen phosphorylase, the enzyme responsible for breaking down glycogen into glucose-1-phosphate, is phosphorylated and activated.
promotes glycogenolysis, leading to the breakdown of glycogen and an increase in glucose availability.
regulation of gluconeogenesis by glucagon and insulin
Glucagon and epinephrine stimulate gluconeogenesis (GNG) when glucose is needed, such as during fasting.
Insulin inhibits GNG during the fed state, promoting glucose storage and usage instead of glucose production.
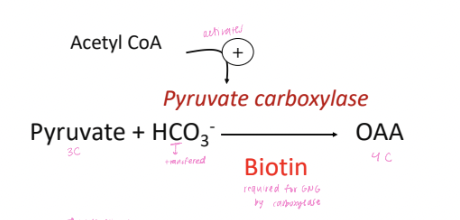
factors that influence pyruvate carboxylase
ATP required for conversion to oxaloacetate
Acetyl CoA is an allosteric activator, directing pyruvate into GNG
levels rise during beta-oxidation (fasting)
PEPCK regulation by gene expression
Glucagon (during fasting) increases the expression of PEPCK, promoting gluconeogenesis.
Insulin reduces PEPCK expression, inhibiting gluconeogenesis in the fed state when glucose availability is high.
factors that regulate Fructose-1,6-bisphosphatase-1 (F16BPase-1)
AMP acts as an allosteric inhibitor, reducing the activity of F16BPase-1, preventing gluconeogenesis when cellular energy levels are low.
F-2,6-BP is also an allosteric inhibitor. During fasting, phosphorylation inhibits PFK-2, decreasing F-2,6-BP levels in the liver, which helps promote gluconeogenesis.
factors that influence Glucose-6-phosphatase
enzyme is located on ER and is influenced by substrate availability.
high Km: glucose-6-phosphate (G6P) must accumulate in higher concentrations for it to be converted to glucose.
Glucagon increases and insulin decreases the gene expression of glucose-6-phosphatase, similar to PEPCK, to regulate glucose output during fasting or feeding.
regulatory factors for gluconeogenesis
Glucagon stimulates gluconeogenesis during fasting by increasing the expression of key enzymes like PEPCK and glucose-6-phosphatase.
Insulin inhibits gluconeogenesis during feeding by reducing the expression of these enzymes.
Allosteric regulators like AMP and F-2,6-BP inhibit gluconeogenesis when cellular energy or glucose is abundant, providing feedback to balance energy metabolism.
how glucagon and epinephrine affects the regulation of glycolysis and gluconeogenesis through the levels of fructose-2,6-bisphosphate (F-2,6-BP)
low F-2,6-BP levels due to gluycagon and epinephrine:
phosphorylation of PFK-2 redcues F-2,6-BP], removing its stimulatory effect on PFK-1, and inhibitory effect on F1,6-BPase-1
When F-2,6-BP is low (due to PFK-2 phosphorylation), F1,6-BPase-1 activity increases, promoting the conversion of fructose-1,6-bisphosphate (F-1,6-BP) back to fructose-6-phosphate (F-6-P) in gluconeogenesis. This favors the production of glucose.
the reduction in F-2,6-BP reduces the activity of PFK-1, slowing down glycolysis (
gluconeogenesis pathway
Pyruvate to Oxaloacetate (OAA):
In the mitochondria, pyruvate is converted to oxaloacetate (OAA) by the enzyme pyruvate carboxylase. This reaction requires ATP and produces CO₂.
Aspartate can also be converted to OAA through transamination reactions.
OAA to Phosphoenolpyruvate (PEP):
OAA cannot cross the mitochondrial membrane, so it must be reduced to malate (or converted to aspartate) and transported to the cytosol.
Once in the cytosol, malate is oxidized back to OAA.
OAA is then converted to PEP by the enzyme phosphoenolpyruvate carboxykinase (PEPCK), which requires GTP.
Fructose-1,6-bisphosphate to Fructose-6-phosphate:
The process continues through reversible steps of glycolysis until fructose-1,6-bisphosphate (F-1,6-BP) is converted to fructose-6-phosphate (F-6-P) by the enzyme fructose-1,6-bisphosphatase.
Glucose-6-phosphate to Glucose:
In the final step, glucose-6-phosphate (G6P) is converted to free glucose by the enzyme glucose-6-phosphatase in the liver. This step occurs in the endoplasmic reticulum and allows glucose to be released into the bloodstream.
why Acetyl-CoA can’t be used for glucose synthesis
Although intermediates in the TCA cycle (such as oxaloacetate and fumarate) can be used as substrates for gluconeogenesis, Acetyl-CoA itself cannot contribute to the net synthesis of glucose because its carbons are lost as CO₂ during the TCA cycle.
Even though fatty acids break down to generate Acetyl-CoA, this breakdown does not contribute carbons for glucose synthesis since the carbons from Acetyl-CoA are lost in the cycle.
Acetyl-CoA and TCA cycle
Acetyl-CoA (2 carbon atoms) enters the TCA cycle (also known as the citric acid or Krebs cycle) by combining with oxaloacetate (OAA) (4 carbon atoms) to form citrate (6 carbon atoms).
As the cycle progresses, two carbon atoms are lost as CO₂ during the conversion of citrate to α-ketoglutarate and succinyl-CoA. This means that for every molecule of Acetyl-CoA that enters the cycle, two carbon atoms are lost, which prevents the net synthesis of carbon-containing molecules like glucose.
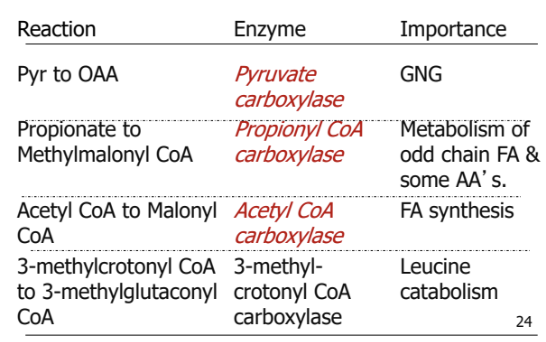
nutrient requring reactions in GNG
magnesiun: associated with ATP and GTP
Niacin: component of NAD/NADH
biotin
in foods:
widely distributed
present in free and bound forms
avidin (protein): biotin inhibitor found in egg and can be inactivated by heating
in lumen
bound biotin hydrolyxed via pancreatic biotinidase, releasing free biotin
absorprtion of free biotin
simple diffusion
na dependent secondary active transport
role of biotin as a coenzyme
in carboxylation reactions by serving as a carrier of a carboxyl group (from bicarbonate) to various substrates.
how is biotin used as a coenzyme
Biotin is attached to a lysine residue of an enzyme to form a biotinyl-lysine-enzyme complex.
In the presence of ATP, HCO₃⁻ is activated and attached to biotin, forming the carboxybiotinyl enzyme complex.
involves the consumption of ATP, which is converted to ADP Pi
The carboxyl group (COO⁻) is transferred from the biotinyl-enzyme complex to a receiving molecule, which completes the carboxylation reaction.
Once the carboxyl group is transferred, biotin is regenerated in the enzyme complex, allowing it to participate in further reactions.
what determines our energy needs
basal metabolic rate
thermic effect of food
thermoregulation (thermogenesis)
physical activity (daily life and exercise)
energy expenditure measurments (BMR)
direct calorimetery: measure heat release from body by measuing changes in the temperature of the environment
indirect calorimetry: measure oxygen uptake and CO2 release as surrogate for heat production
use doubly habelled H2O - based on difference between turnover rate of H and O of body H2O as a function of CO2 production (by product from cell respiration)
what determines BMR/RMR
lean body mass
hormone levels (thyroid, catecholamines)
body surface area
abnormal body temperature (fever)
age (decreased lean body mass)
pregnancy
caffiene & tobacco use
gender: males > females
Kleier’s equation
regression equation: log M = 0.756logW + 1.87
simplified metabolic body size (W3/4): M = 70 x W3/4
energy balance
= intake - expenditure
positive balance: weight gain
negative balance: weight loss
1 lb gain in body fat requires ~3500 kcal
if the gain is over 1 year, energy balance averages +10 kcal/day
energy from diet and body stores to meet daily needs
dietary macronutrients (CHO, fat, protein)
absorbed nutrients
metabolic fuels
stored energy in complex molecules
use of metabolic fuels
fuel needs are tissue specific
maintaining an adequate glucose supply to meet the needs of the brain is central
glucose sparing: utilization of non-glucose substrates for energy by tissues that are not obligatory glucose users
synergistic relationships among organs are essential
what organs use glucose as energetic substrates
brain
RBC & retina
adipose
skeletal muscle
intestine
kidney
liver
heart
what organs use ketone bodies as energetic substrates
brain
skeletal muscle
intestine
kidney
liver
heart
what organs use fatty acids as energetic substrates
adipose
skeletal muscle
kidney
liver
heart
what organs use glycogen as energetic substrates
skeletal muscle
liver
what organs use amino acids as energetic substrates
skeletal muscle (BCAA)
intestine (Gln)
Kidney (Gln)
Liver
what organs use Lac as energetic substrates
kidney
liver
heart
obligatorily glycolytic tissues
brain
RBC and retina
coordination between liver and other organs through metabolite cycles
glucose-lactate (cory cycle)
glucose-alanine (cahill cycle)
glucose-fatty acid (randle cycle): inhibition of glc utilization when FA’s are present (heart, muscle)
importance: spare glucose by peripheral tissues for utilization by brain
insulin, glucagon, epinephrine/norepinephrine receptors and their action
bind to cognate plasma membrane receptors and activate intracellular signaling (kinase/phosphatase, Ca++ signaling)
peptide, water soluble hormones
glucocorticoids & thyroid hormone receptors and their action
bind to cognate receptors (GR and TR) and affect transcription via hormone/receptor binding to specific DNA element in nucleus
lipid soluble hormones
major hormones that influence energy metabolism
anabolic
insulin (muscle, adipose, other)
counter regulatory
glucagon (liver)
catecholamines (liver, adipose, muscle, and other)
glucocorticoids (most tissues)
thyroid hormone (most tissues)
influence of starvation on blood levels of substrates and hormones
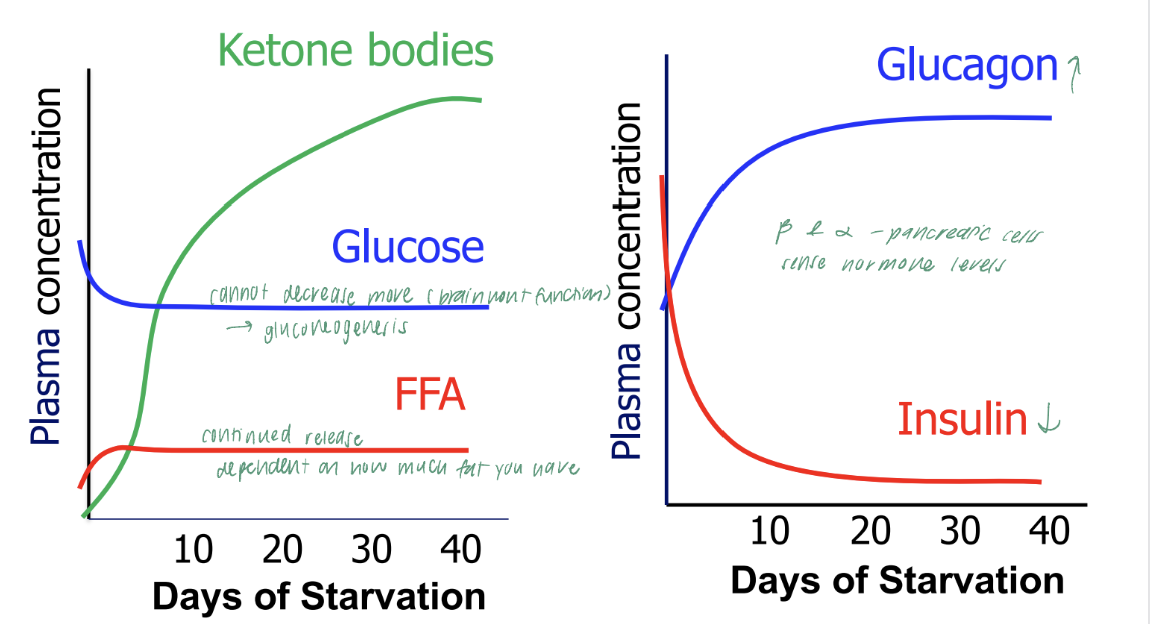
insulins role in glucose homeostasis: high plasma insulin concentration
minimize elevations in plasma glucose concentration
INCREASED
glucose uptake by peripheral cells
glucose metabolism
glycogen and protein synthesis
to move glucose out of blood
DECREASED
gluconeogenesis by liver
glycogen breakdown
lipolysis by adipose sugar
reduce glucose entry into blood
glucagons role in glucose homeostasis: high plasma glucagon concentration
minimize the decrease in plasma glucose concentration
INCREASED
glycogen breakdown
gluconeogenesis
ketogenesis (reduces glucose utilization)
DECREASED
glycogen synthesis
metabolic effects of catecholamines are similar to those of glucagon
catecholamines (epinephrine/norepinephrine from adrenal medulla and norepinephrine from nerve endings) are released in the fight or flight response to stress and stimulate
release of glucose from glycogen in liver
release of FAs and glycerol from TAG in adipose tissue
glucocorticoids
secreted by adrenal cortext in response to stress, including fasting and exercise
results in..
increased GNG (from muscle protein breakdown)
increased lypolysis (release FA for energy, spare glc)
increased ketogenesis (used for energy, spare glc)
decreased synthesis of protein & TAG
does thyroid hormone regulate plasma glucose levels
not directly
stimulatory effect on BMR: stimulates uncopuling and increases Na+/K+ ATPase activity
thyroid hormone effects
from thyroid glands
increases glucose utilization
increases protein synthesis, promoting growth,
stimulates FA synthesis and esterification to TAG
enthalpy and its relationship to nutrition and metabolism
change in enthalpy (ΔH) = heat absorbed (q) - work done (w)
heat (q) warms the body, sparing energy to perform work (w)
burning food in a bomb calorimeter will provide a measure of ΔH because w = 0 under these conditions
Why is measuring ΔH not accurate for true energy value
factors cause the biological energy value to be less than the value predicted by measuring ΔH
completeness of absorption
completeness of oxidation
efficiency with which W can be captured during metabolism under physiological conditions
Atwater factors
Protein: 4 kcal/g
Fat: 9 Kcal/g
Carbohydrate: 4 kcal/g
values are roughly correct for…
1) difference in completeness of chemical vs biological oxidation
2) absorption efficiency
Gibbs Free Energy
predicts the direction of reaction / spontaneity
ΔG = ΔH - TΔS
if ΔG ~0: rxn is near equilibrium and direction can be easily reversed with a change of substrate or product concentration
if ΔG is ±: it is non equilibirum and proceeds in forward (-ΔG) or reverse (+ΔG) direction
How does the body use ΔH?
Energy in enthalpy must be packed into ATP that the body can utilize for metabolic purposes
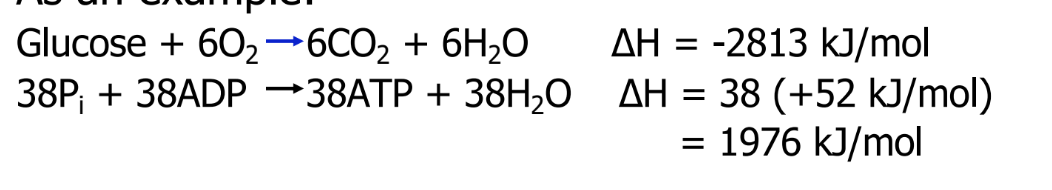
2 reactions are linked: 70% of the chemical energy is converted to biological energy
hydrolysis of phosphate bonds
releases high energy
energy released is not constant for all phosphate bonds
influenced by resonance stability, charge repulsion, and ionization
ΔGo for selected compounds
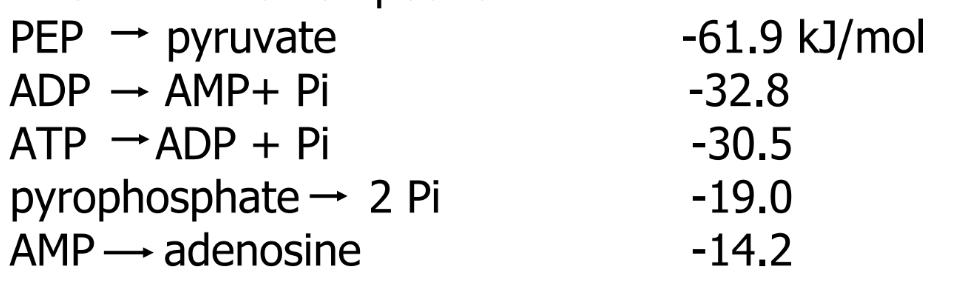
thioester
R-S-CO-R'
have large, negative ΔGo
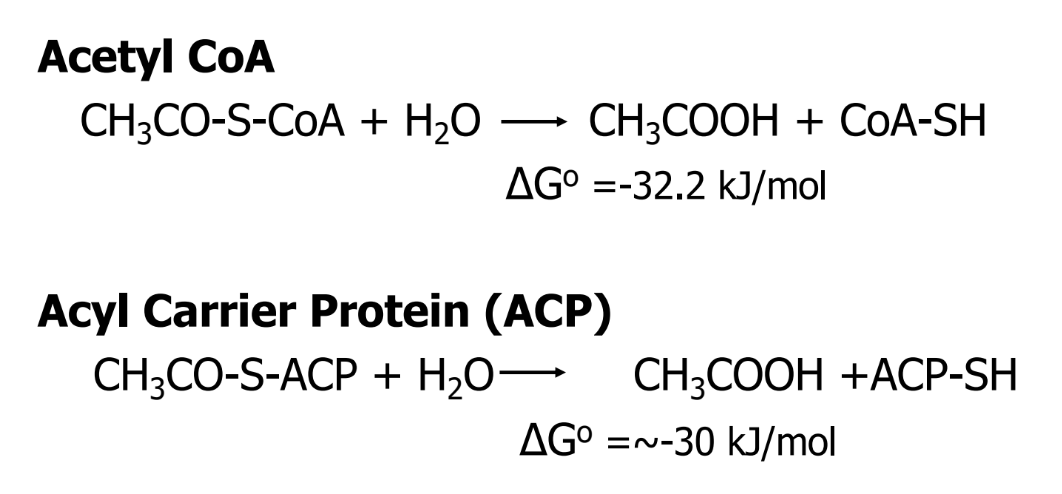
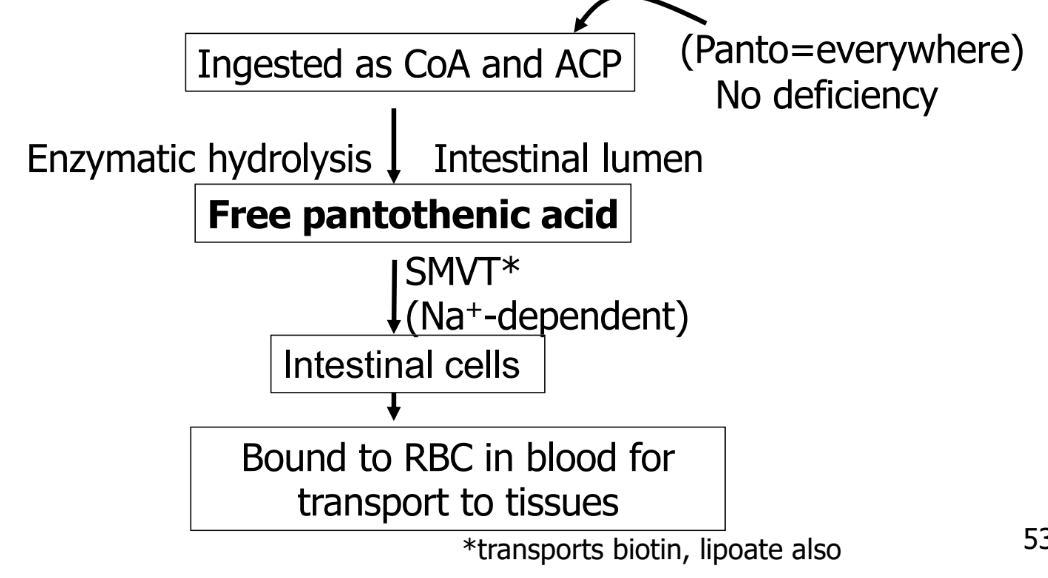
Pantothenic acid
component of ACP and CoA
essential nutreint
obtain from foods and intestinal microflora
UCP1
unique to brown adipose tissue with high mitochondria
permits reentry of H+ into mitochondrial matrix
generates heat rather than ATP for body temperature
coupling respiration from ATP production by UCP1 dissipates heat (thermogenesis)
Nutrients required for universal electron carriers
NAD+, NAPD+: Niacin
FMN, FAD: riboflavin
Nutrients required for electron transport chain
I NADH-dehydrogenase complex-Q: niacin, riboflavin, Fe-S
II Succinate dehydrogenase complex -Q: riboflavin, Fe-S
III cytochrome b-c1 complex cytochrome c: Fe (heme), Fe-(S)
IV cytochrome oxidase complex: Fe (heme), Cu
acquiring niacin
ingested as nicotinic acid and nicotinamide
widely distributed
bound to carbohydrates in corn, released with lime (CaOH2, alkali treatment)
may be synthesized from tryptophan (60 mg tryptophan = 1 NE)
transport of niacin
absorbed from lumen via 2 active transporters
transported to blood and cells
niacin deficiency
pellegra (rough skin)
symptoms: dermatitis, diarrhea, dementia (3Ds)
was endemic with corn from new world
now rare, associated with corn (low in tryptophan) without lime treatment to release niacin, or sorgym (low in niacin and tryptophan)
riboflavin
component of FMN and FAD
ingested as FMN and FAD from meat and green leafy vegeables
enzymatic hydrolysis releases free riboflavin in lumen
transported to blood, where it binds to proteins
riboflavin deficiency
ariboflavinosis
stunted growth
dermatitis
burning of mucous membranes
anemia
neuropathy
assessing riboflavin status
measure FAD-dependent glutathione reductase activity in erythrocytes
GSSG + NADPH + H —→ 2GSH + NADP+
catalyzed by glutathione reductase
with and without FAD for activity coefficient
high number = low endogenous FAD
anaplerotic function of the TCA cycle
TCA cycle intermediates can serve as precursors for synthesis of new compounds
entry of new carbon into the cycle is accompanied by exit via intermediates
any carbon that enters TCAC via an anaplerotic pathway will not result in net oxidation, but used in anotehr route
anaplerosis
reactions that allow the entry of carbon into the pools of TCAC intermediates (other than via acetyl-CoA)
process replenishes intermediates that are used for other biosynthetic purposes, allowing the TCA cycle to continue running efficiently.
example of anaplerosis
glutamine metabolism feeds into the TCA cycle via α-ketoglutarate, which can eventually replenish oxaloacetate, allowing for gluconeogenesis (the synthesis of glucose).
glutamate for TCA cycle in cancer cells
Glutamine in circulation is the highest among amino acids
Glutamine used for biosynthesis of purine & pyrimidine (DNA synthesis for cell proliferation)
Glutamine for FA synthesis (membrane phospholipid synthesis)
pyruvate dehydrogenase (PDH)
catalyzes the irreversible oxidative decarboxylation of pyruvate, converting it into acetyl-CoA
decarboxylation of pyruvate
oxidation of 2C fragment, NADH formed
acetyl coa formation: 2C + CoA
complex of 3 enzymes
requires 5 coenzynes
CoA: panthanoic acid
NAD+: niacin
FAD: riboflavin
lipoic acid
thiamine pyrophosphate: Vit B1
regulated by phosphorylation and dephosphorylation
PDH kinase inactivates PDH by phiosphoylation when energy is high
PDH phosphatase activates PDH by dephosphorylation when ADP, pyruvate, or calcium is high
5 coenzymes required by PDH
CoA: panthanoic acid
NAD+: niacin
FAD: riboflavin
lipoic acid
thiamine pyrophosphate: Vit B1
pyruvate dehydrogenase complex
E1: Pyruvate dehydrogenase (use thiamine) E2: Dihydrolipoamide transacetylase (use lipoic acid)
E3: Dihydrolipoyl dehydrogenase (use riboflavin & niacin)
E1: Pyruvate dehydrogenase
catalyzes the decarboxylation of pyruvate producing a 2-carbon hydroxyethyl group attached to TTP
thiamine pyrophosphate (TPP). TPP is derived from vitamin B1 (thiamine)
stabilizes the reaction intermediate during the decarboxylation.
E2: Dihydrolipoamide transacetylase
transfers the hydroxyethyl group from E1 onto lipoic acid (a cofactor attached to E2), which gets reduced in the process.
then transfers the resulting acetyl group to Coenzyme A (CoA), forming acetyl-CoA.
E3: Dihydrolipoyl dehydrogenase
regenerates the oxidized form of lipoic acid, allowing the cycle to continue.
uses FAD to oxidize the reduced lipoic acid, and the FADH₂ generated is subsequently reoxidized by NAD⁺, producing NADH.
α-Ketoglutarate Dehydrogenase (αKGDH)
parallel enzyme system that operates similarly to the PDH complex but works on α-ketoglutarate in the TCA cycle.
uses similar cofactors and mechanisms to convert α-ketoglutarate into succinyl-CoA, generating NADH and CO₂ in the process.
Co Factors used in what stages of PDH Complex
Thiamine (B1) for E1.
Lipoic acid for E2.
Riboflavin (B2) and Niacin (B3) for E3.
thiamine
Thiamine pyrophosphate (TPP) is essential in:
Decarboxylation of a-keto acids (PDH, aKGDHand metabolism of BCAA’s)
Transketolation reactions (pentose phosphate pathway)In foods (and cells) thiamine is phosphorylated and present as TPP, which must be dephosphorylated to cross membranes
Thiamine deficiency
Thiamine is readily available in whole grain cereals, mostly in the outer layers of the grain, but is removed with milling & refining.
If the “white” flour (or rice) is not fortified, thiamine deficiency can occur.
can also occur with alcoholism
deficiency disease: berberi
nervous and cardiovascular disorders
confusion
anorexia
weakness followed by loss of muscular functions
following thiamine treatment, rapid improvemnet occurs
How is thiamine absorbed
free thiamine can be absorbed in free form
jejunum lumen: TPP must be dephosphorylated to be absorbed in the body
pyrophosphatase and phosphatase dephosphorylatte TPP, resulting in free thiamine
Once free thiamine is formed, it can be absorbed into the enterocytes (intestinal cells) via both passive and active transport mechanisms
in enterocytes, thiamine is phosphorylated to form thiamine diphosphate (TDP, active form)
Ii tissues, thiamine can be dephosphorylated back into its free form to enter the bloodstream or be rephosphorylated depending on cellular needs
Thiamine typically circulates as free thiamine in the plasma, while its active diphosphate form (TDP) is predominant inside cells.
Lipoic acid
function
energy production
reduce oxidative damage to membrane lipids (antioxidant)
can be synthesized
regulation of flux through PDH and TCA cycle
Energy status (most important)
NAD+/NADH
Substrate activation
Product inhibition
Phosphorylation
Allosteric regulation
Regulation of E1 subunit of PDH
PDH Kinase phosphorylates and inactivates PDH in response to high energy levels (high NADH, ATP, acetyl-CoA).
PDH Phosphatase dephosphorylates and activates PDH in response to signals like calcium (indicating muscle contraction) and insulin (indicating the fed state).
Inhibitory energy indicators
PDH
NADH
ATP
GTP
CS
NADH ATP
ICDH
NADH
ICDH:
NADH
ATP
alphaKDGH
NADH
ATP
energy indicators that activate..
PDH
NAD
ADP
AMP
CS: N/A
ICDH:
NAD+
AMP
ADP
alphaKDGH
AMP
high mitochondrial [citrate] effect on TCA cycle flux
When citrate levels in the mitochondria are high, it acts as a feedback inhibitor to slow down the TCA cycle, preventing unnecessary energy production.
The citrate is exported to the cytosol, where it is used for fatty acid synthesis
avoidance of citrate inhibition of CS
Pyruvate is generated when glucose flux through glycolysis is high.
High [pyruvate] in mitochondria activates PDH (over-riding energy status signals), and acetyl-CoA is converted to citrate.
If flux through ICDH is saturated, citrate is transported out to cytosol.
High cytoplasmic [citrate] activates acetyl-CoA carboxylase (ACC) stimulating lipogenesis
energetic stresses that activate AMPK
glucose deprivation
oxygen deprivation
ischemia
metabolic posions
exercise (muscle contraction)
activation of AMPK
AMP binds AMPK with very low Kd (ultrasensitive)
High [ATP] antagonizes AMP/AMPK binding
Ca activates CaMKKb which phosphorylates AMPK
PhosCr (high energy status of muscle) inactivates AMPK
metabolic effects of AMPK activation
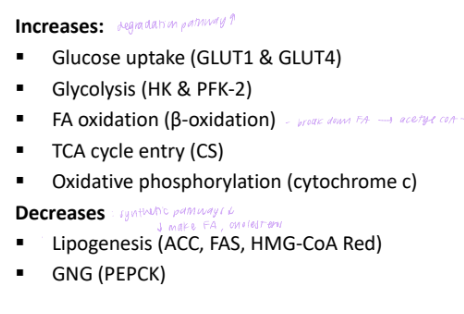
AMPK and insulin sensitivituy
AMPK activation is independent of insulin action
Thus, AMPK activation stimulates uptake of glucose into cells in a manner that is insulin independent
This may be one mechanism by which metformin increases insulin sensitivity in type 2 diabetes.
ADPK and adipokines
White adipose tissue (WAT) secretes adipocyte hormones (adipokines) to regulate energy metabolism.
Leptin: increases as WAT expands.
decreases food intake, Increases energy expenditure & thermogenesis.
One of the leptin action is by inhibiting AMPK in hypothalamus to decrease food intake.
Adiponectin: Increases food intake by activating AMPK in hypothalamus.
How does NADH enter the mitochondria for ETC?
Malate-Aspartate Shuttle: transfer of reducing equivalents from cytosolic NADH to mitochondrial NADH without loss of energy,
Glycerophosphate Shuttle: Transfers reducing equivalents from NADH to FADH₂, which results in lower ATP yield
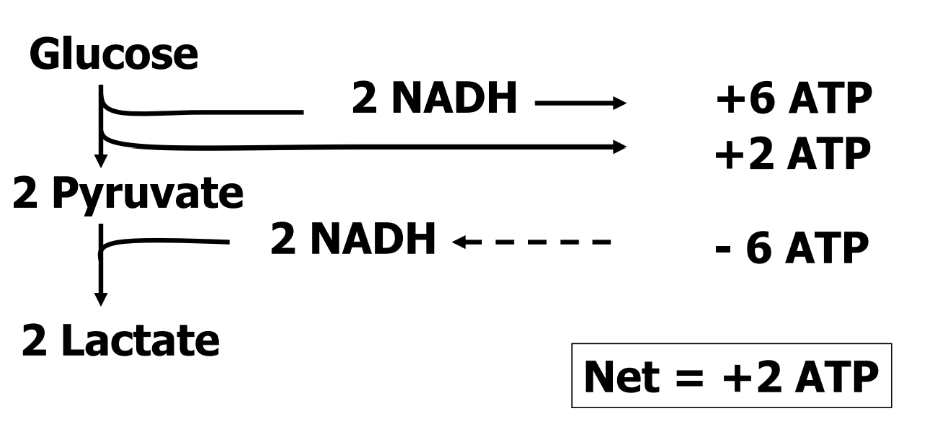
Net Energy Yield From Anaerobic Metabolism of Glucose (Glycolysis)
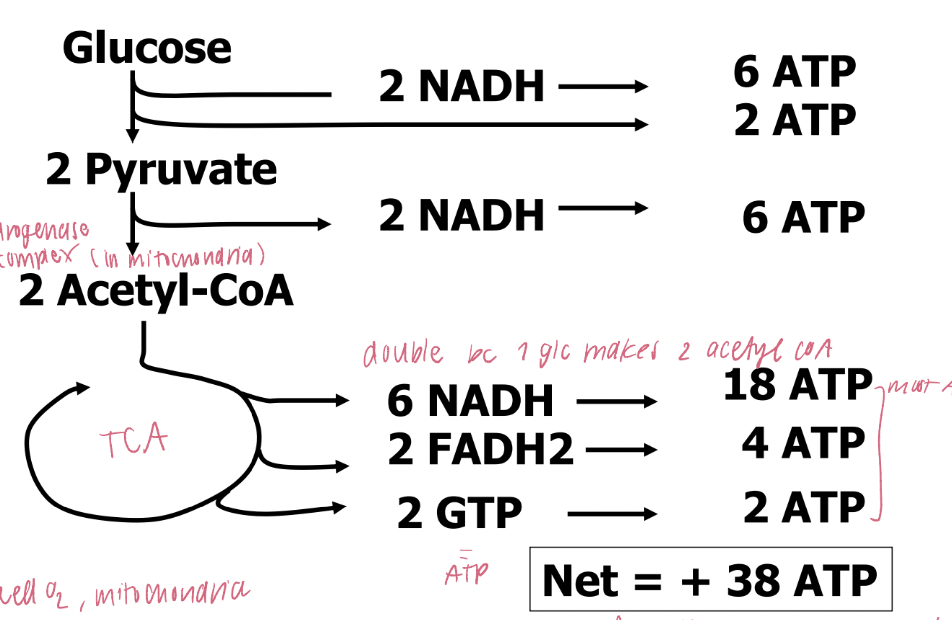
Net Energy Yield From Aerobic Metabolism of Glucose (TCAC)
key non-equilibrium reactions for TCA cycle
Pyruvate Dehydrogenase (PDH):
Converts pyruvate to acetyl-CoA and generates NADH.
links glycolysis to the TCA cycle.
releases CO₂ and generates NADH,
Citrate Synthase:
Catalyzes the condensation of acetyl-CoA and oxaloacetate to form citrate.
Isocitrate Dehydrogenase (ICDH):
Converts isocitrate to α-ketoglutarate, generating NADH and releasing CO₂.
α-Ketoglutarate Dehydrogenase (αKGDH):
Catalyzes the conversion of α-ketoglutarate to succinyl-CoA, generating NADH and releasing CO₂.
production of produce NADH, FADH₂, and ATP in TCA
NADH is generated in the steps catalyzed by pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase.
FADH₂ is generated in the conversion of succinate to fumarate by succinate dehydrogenase.
ATP (or GTP) is generated in the conversion of succinyl-CoA to succinate by succinyl-CoA synthetase.