Properties of Water
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Which type of bond is found within water (H2O)?
Covalent
Why do covalent bonds hold the atoms of water together?
Because they share electrons between each other.
What are the properties of the type of covalent bond that is not found in water?
Nonpolar covalent bonds
What is a hydrogen bond?
The bond between two water molecules, where the polar parts of each molecule attracts opposite charges
What is the difference between cohesion and adhesion?
Cohesion is the ability for water molecules to stick together, and adhesion is the ability of water molecules to stick to other surfaces.
Why does adhesion have to be stronger than cohesion in capillary action?
Because cohesion experiences gravity more than adhesion, therefore water needs to have more adhesion to move up in capillary action rather than submit to gravity.
Why does surface tension happen?
Because water molecules on the surface don’t need to hydrogen bond to more water
What is high specific heat?
High specific heat is the ability of water to withstand higher temperatures without boiling.
Why is water able to resist changes in temperature?
Through high specific heat and evaporative cooling
How does the density of solid water compare to the density of liquid water?
It is less dense
Why is water less dense as a solid?
Because the hydrogen bonds orient in a more organized manner, pushing the molecules farther apart, which lowers the density.
Water has a _______ volume as a solid compared to a liquid
higher
What is a solvent?
A solvent is a substance that can break down other similar substances.
Why is water significant as a universal solvent?
Because it can dissolve and transport molecules necessary for life functions
Like dissolves ____
like
When water dissolves substances, what does it look like? What is it called?
Water molecules, orienting according to Coulomb’s Law, surround the charged particle—separating the charged particle from the other charged particles in the original substance. Hydration shell.
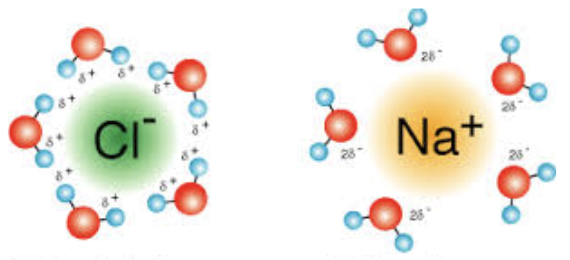
Does the oxygen in water have a partial negative or positive charge?
negative
Does the hydrogen in water have a partial negative or positive charge?
positive
Are fatty acids polar or nonpolar?
nonpolar
Why are fatty acids nonpolar? Are they hydrophilic or hydrophobic?
Because the carbons in the fatty acids are completely bonded with hydrogen (no free valence electrons available for bonding so they are hydrophobic
Why do coastal areas have more stable temperatures than inland areas?
Coastal areas experience more stable temperatures due to the high specific heat capacity of water, which moderates temperature fluctuations by absorbing and releasing heat gradually.