Uworld Gen Chem-Atomic Theory and Chemical Composition
When deciding which species is the smallest ion what Periodic Trend do we use?
Decrease in size across a period and increase down a group on the periodic table
Out of these isoelectric ions (Which of these is the smallest ion)?
1. Na+
2. F-
3. Mg ^2+
4. O^2-
Mg 2+ is the smallest since it had the most left on the PT and was more proton than Na+.
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
When deciding which species is the smallest ion what Periodic Trend do we use?
Decrease in size across a period and increase down a group on the periodic table
Out of these isoelectric ions (Which of these is the smallest ion)?
1. Na+
2. F-
3. Mg ^2+
4. O^2-
Mg 2+ is the smallest since it had the most left on the PT and was more proton than Na+.
What is the Molecular weight or Atomic mass of Ammonium Carbonate?
(NH4)2CO3
(NH4)2CO3
1. N2: 2x14= 28
2. H8: 1x8= 8
3. C: 12.0
4. O3= 16x3= 48
28.0+8.0+12.0+48.0= 96.0
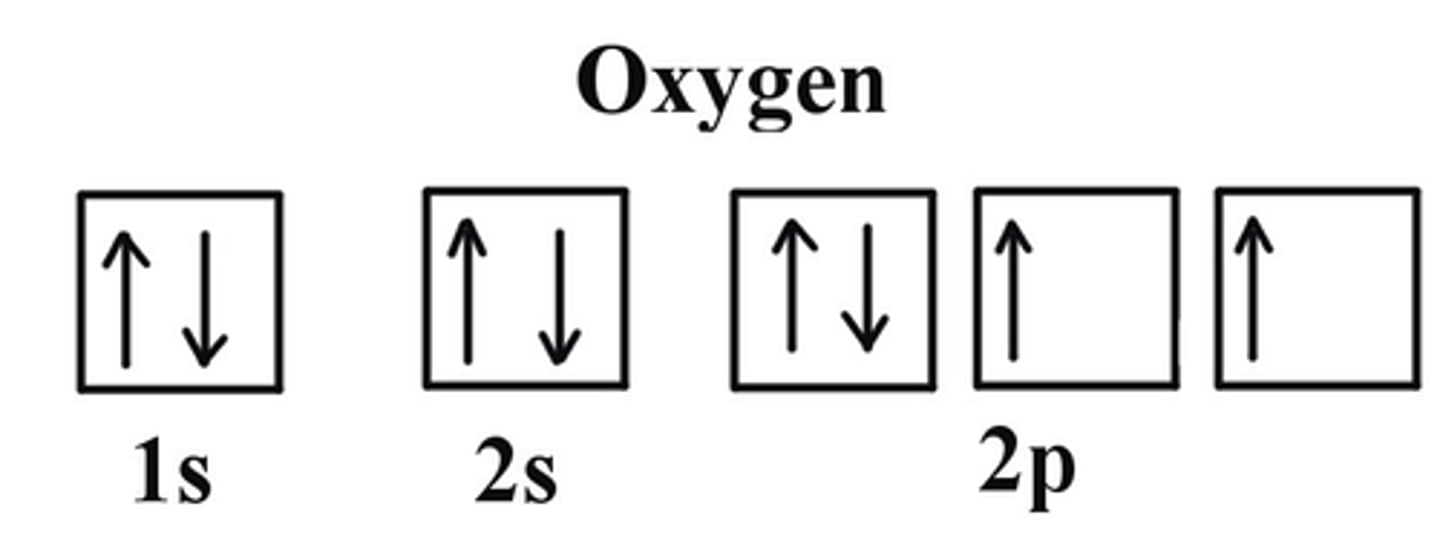
How do you write the electron orbital Diagram for Oxygen?
1. Find the Electron Configuration of Oxygen:
1s2 2s2 2p4
2. There are a total for 8 electrons
3. Make sure to fill in the electrons (arrows) in EACH box first then you can go back and fill the rest in:
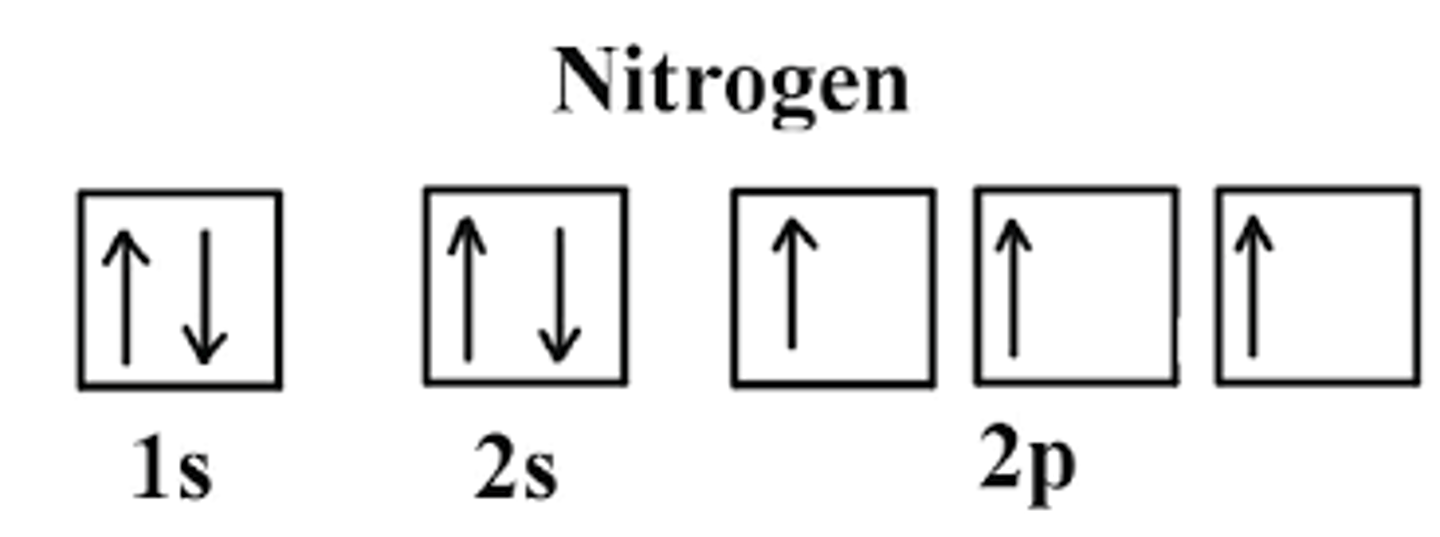
Who do you write the electron orbital Diagram for Nitrogen?
1. Find the Electron Configuration of Oxygen:
1s2 2s2 2p3
2. There are a total for 8 electrons
3. Make sure to fill in the electrons (arrows) in EACH box first then you can go back and fill the rest in:
What does a Line Spectra measure?
Line Spectra of each element is unique and can be used like fingerprints to identify elements
If a sample contains more than one of these elements, which of the following spectra would correspond to a sample containing both S and Se?
How would I solve this?
1. Look at the line Spectra that contains "S" and look at the spectra that contains "Se"
2. Find these wavelengths points in the new Line Spectra that you are trying to look for
3. Se: Has a line on the 585nM
4. S: Has a line on the 630 nM
5. These two lines should be placed on the 585 and the 630 nM
What occurs Radioactive Decay?
In a Radioactive Decay, the half-life is the TIME for the amount of a given isotope that decreases by HALF (1/2):
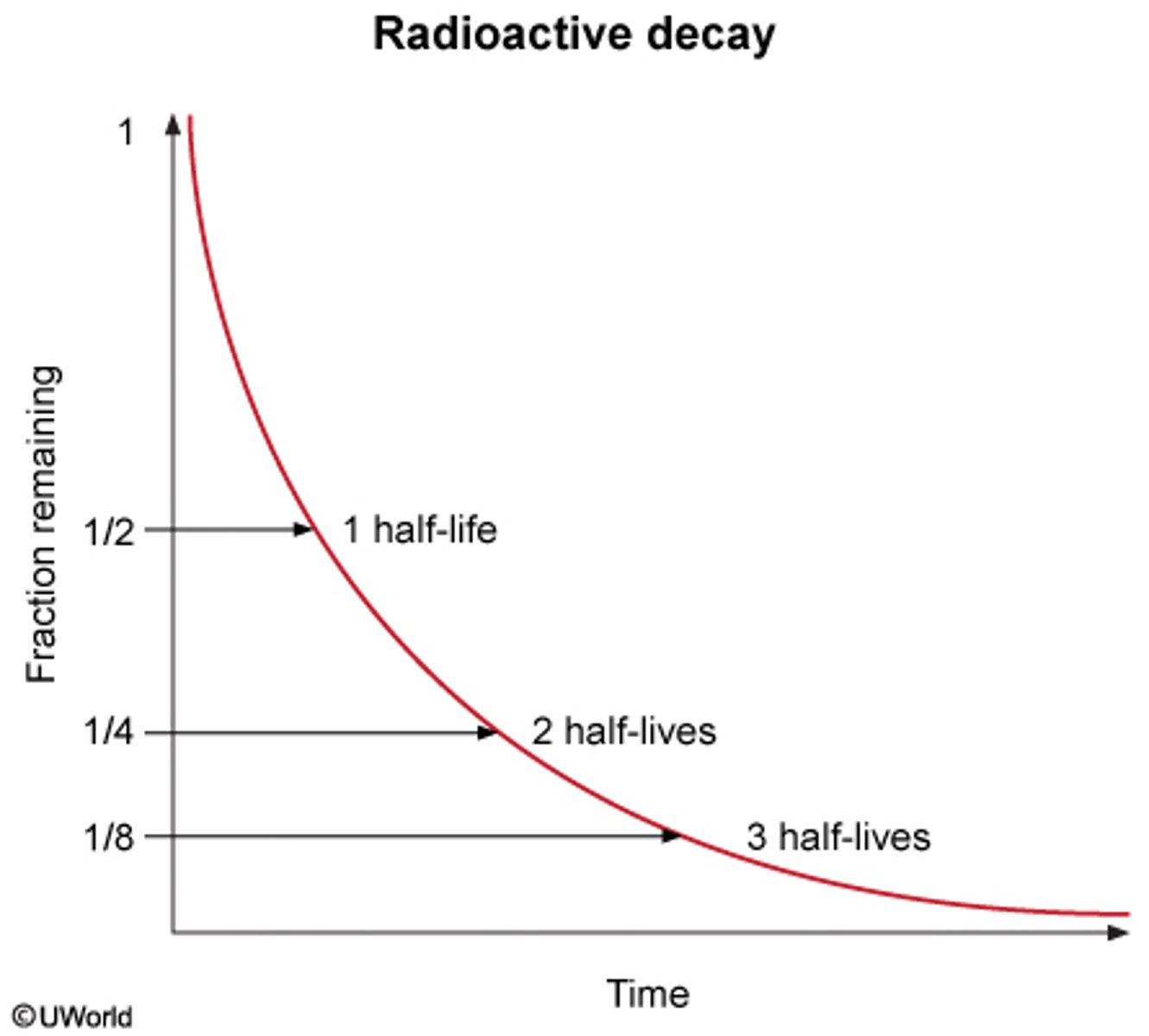
What is the value that is decreased in each of the half-life intervals?
With each half-life interval that passes by is 1/2 from the original amount:
Start amount:
1st half life: 1/2
2 half life: 1/4
3rd half life: 1/8
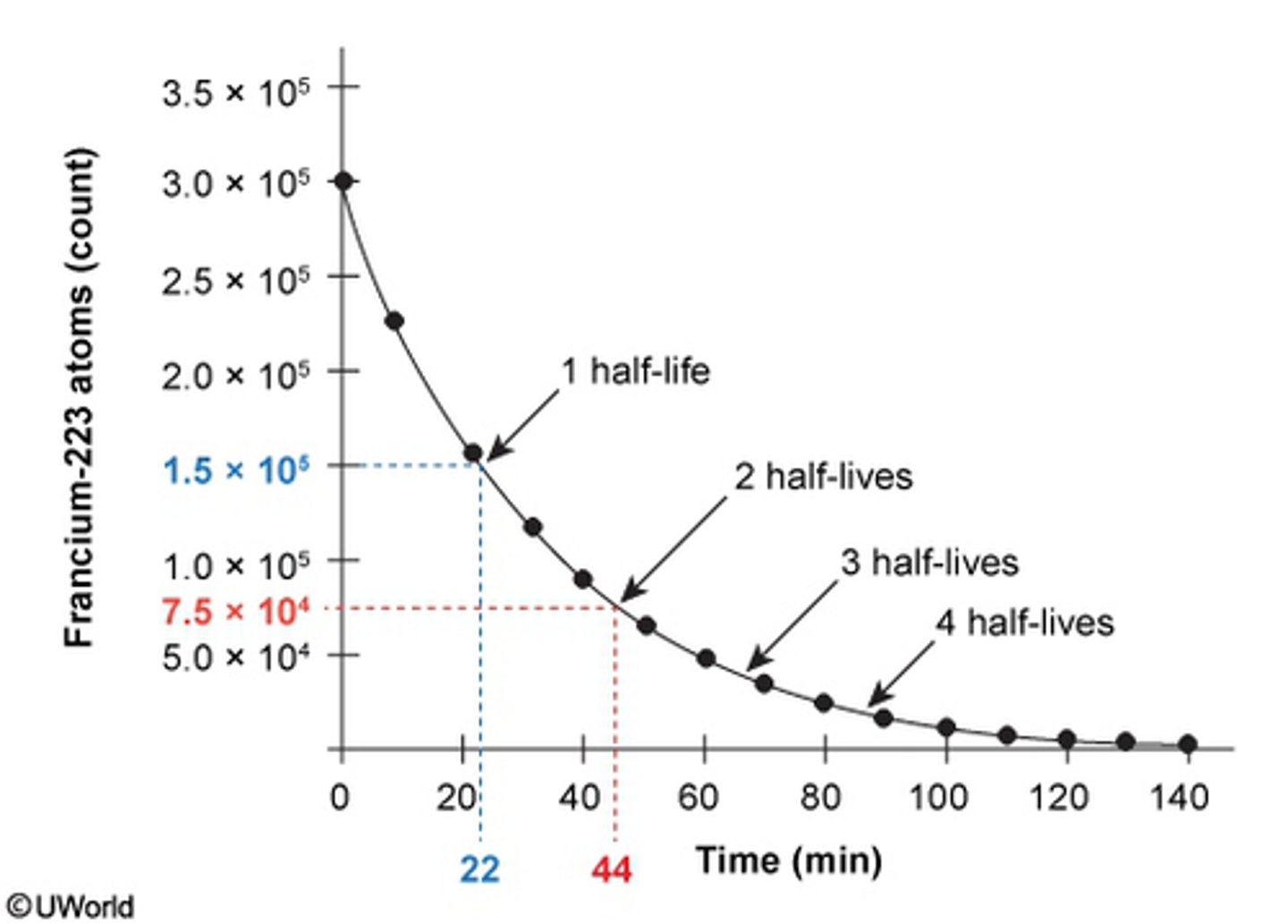
The graph below illustrates the radioactive decay of a population of francium-223 atoms. How long did it take for two half-lives of francium-223 to elapse?
A. 20 Minutes
B. 44 Minutes
C. 65 Minutes
D. 90 Minutes
The question is asking for two half-lives of Francium 223:
1. Determine the first 1/2 half life:
1.5 x 10^ 5= 22 minutes
2. Next you can determine the second half life by multiplying 22 x (1/2)= 44 minutes:
3. B. 44 Minutes
What occurs in a half-life?
A half life is the time required for the amount of a given radioactive isotope to DECREASE by half (1/2):
With each 1/2 that passes by, there is another half that decreases, eventually becoming zero
Alkali metals (Group 1A) have low or high ionization energy?
Which Alkali metal has the highest first ionization energy?
Low Ionization
Lithium
What is the First Ionization Energy?
The amount of energy required to remove an electron
What is the periodic trend of the first Ionization Energy?
Increase across a period and decrease moving own on a group on the periodic table:
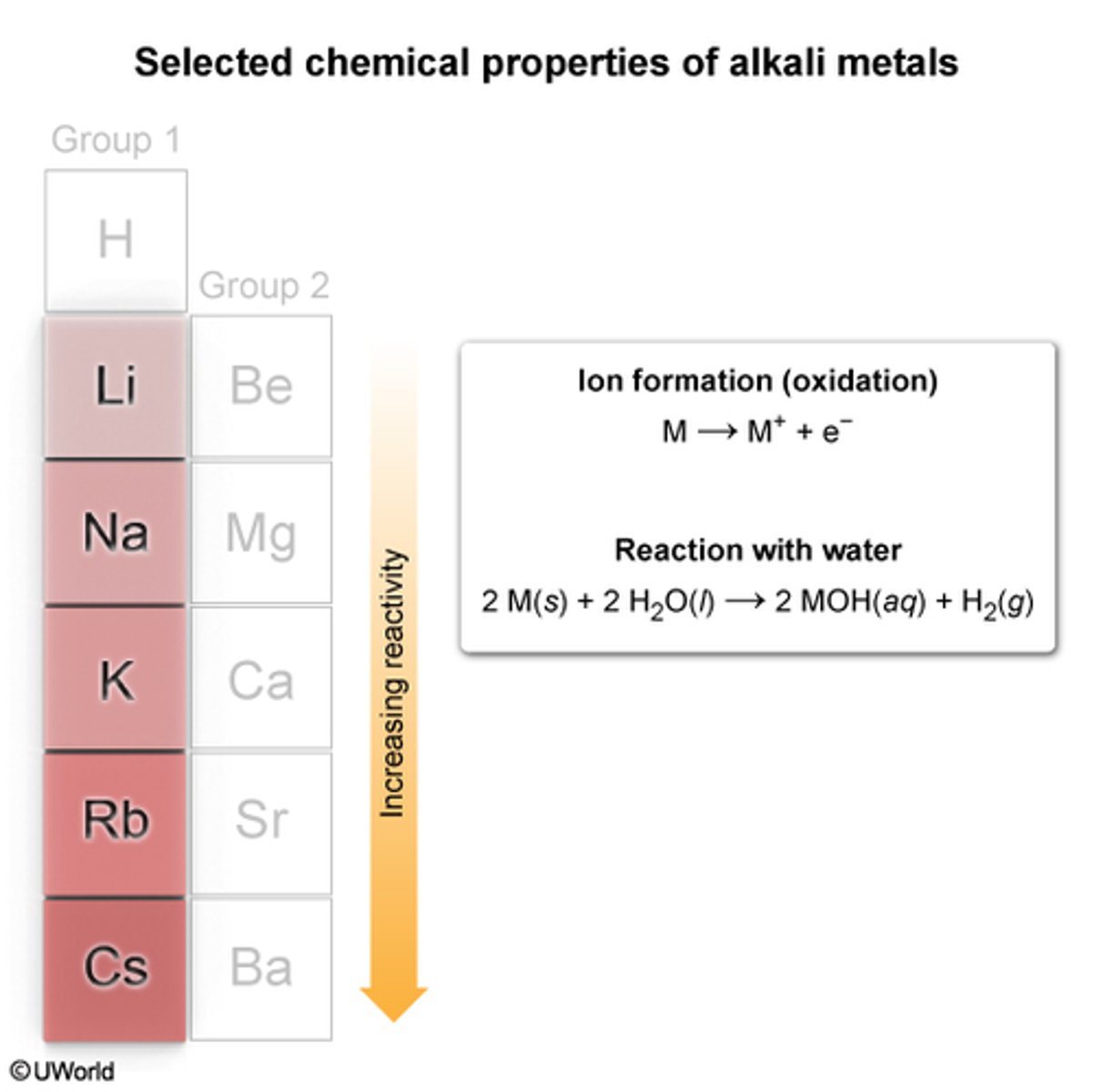
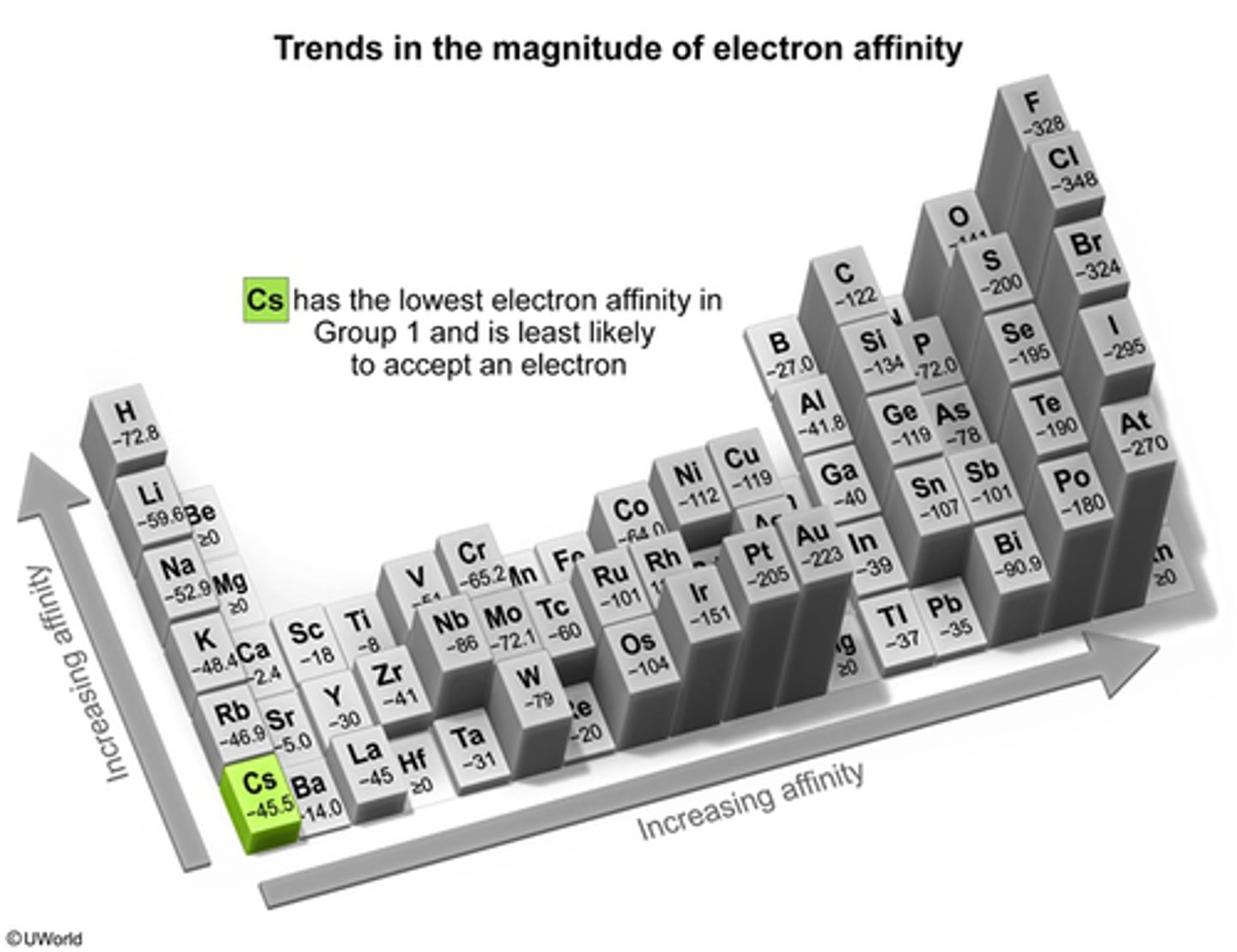
What is the Periodic Trend of Electron Affinity?
Increase all the way on the (Right) hand side and
Increase from left to right on the (Bottom)
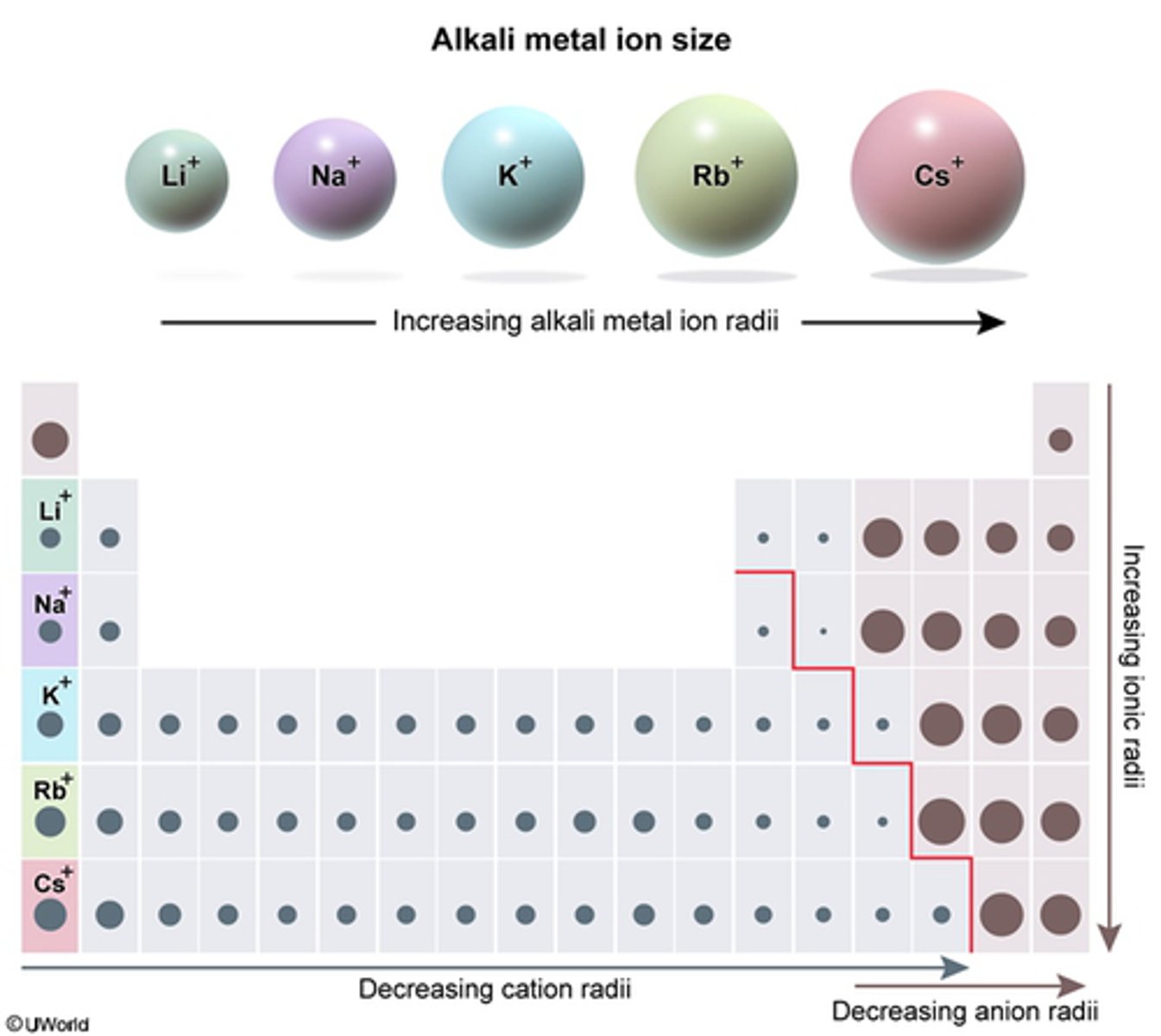
At higher concentrations, alkali metal cations with an ionic radius close to that of a potassium ion can displace potassium ions in biological systems and promote low potassium levels (hypokalemia). Which cation is most likely to displace K+ ions?
A.Li+
B.Rb+
C.Cs+
D.Fr+
This question is asking what element is most likely to displace K+ Ions
1. Look at the Atomic Radius of the element:
2. The element that is closest to K+ will be the answer:
B.Rb
What is the Periodic Trend for the Alkali Metal Ion Size?
Increasing alkali metal ion radii increases from the Left to the Right on the TOP of PT
Increases from the top to bottom on the RIGHT of PT