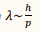1- Wave-Particle Duality
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What does classical electromagnetism predict for a "black body" radiation's intensity and wavelength relationship?
It predicts an inversely proportional relationship between wavelength (𝜆) and intensity (𝐼(𝜆))(Rayleigh-Jeans law)
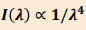
What is the formula for calculating energy quanta in Planck's model?
𝑓 = the frequency of light
ℎ = Planck’s constant
ℏ=h/2π
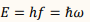
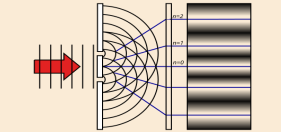
Which experiment demonstrates light's wave-particle duality?
Youngs double slit
What is the de Broglie wavelength?
Its the wavelength associated with a particle of matter
p= magnitude of the momentum of the particle.
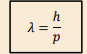
What is required for wave effects, such as diffraction, to be observable?
Wave effects, such as diffraction, require the waves to pass through an aperture roughly the size of the wavelength.
This is also true for matter.
What behavior does all matter exhibit?
All matter exhibits both particle-like and wave-like behaviour
When are quantum effects significant?
At the length scale where: