Chapter 4.2 - Transport across membranes
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
what does diffusion require
both a concentration gradient and membrane permeability
lipid permeability is determined by
molecular size, polarity, partition coefficient
partition coefficient
ratio of solubility in nonpolar solvent to that in water
the greater the lipid solubilit, the ______ the penetration
faster
____ molecules penetrate the lipid bilayer more rapidly that _____ ones (size)
small
____ molecules have poor membrane penetration (polarity)
polar
are sugars and amino acids able to penetrate the bilayer easily
no, both polar
what types of molecules is simple diffusion relevant to
small molecules that are small enough and/or nonpolar enough to cross the membrane without aid of transport proteins, o2, co2, ethanol
relationship between diffusion rate and concentration gradient for simple diffusion
linear, non saturating
relationship between diffusion rate and concentration gradient for facilitated diffusion
hyperbolic positive
facilitated diffusion
uses protein to move solutes across a cell membrane
two main classes of membrane transport proteins
channels, transporter
channels
discriminate on size and electric charge
how do channels work
when open, only ions (and some polar organic molecules) of the right size charge can pass through
bidirectional
exists only in open and closed conformation
what controls opening and closing of channels
external stimuli or conditions within the cells
transporters
undergo a series of conformational change to transfer samll solutes across the bilayer
which membrane transport protein type has a slower transfer rate
transporters
most abundant cation inside the cell and what anion balances it out
K+, balanced by a variety of negative changed anions that includ nucleic acids, proteins, and various metabolites
most abundant cation outside the cell and what anion balances it out
Na+, balanced by extracellular Cl-
what creates a membrane potential
differences in the concentration of inorganic ions across a cell membrane
is the overall charge both inside and outside completely balanced
Though overall charge might be generally balanced, tiny excesses of positive or negative chare, concentrated near the plasma membrane do occur
t or f - resting membrane potential is 0 mV
false, steady stae but not 0, anywhere between -200 to -200 mV
what is more negative, the inside or outside of the cell?
interior
passive vs active transport
passive = down a concentration gradient – no energy is required
active = against a concentration gradient – energy (ATP hydrolysis is required)
what types of proteins mediate passive diffusion, and what direction can it occur
all channels and many transporters
can go in either diection (in or out) ass long as it follows the molecules concentration gradient
what are active transporters called
pumps
what constitutes direction of neutral molecule diffusion? what about charged molecules?
neutral - only concentration gradient
charged - membrane potential and concentration gradient
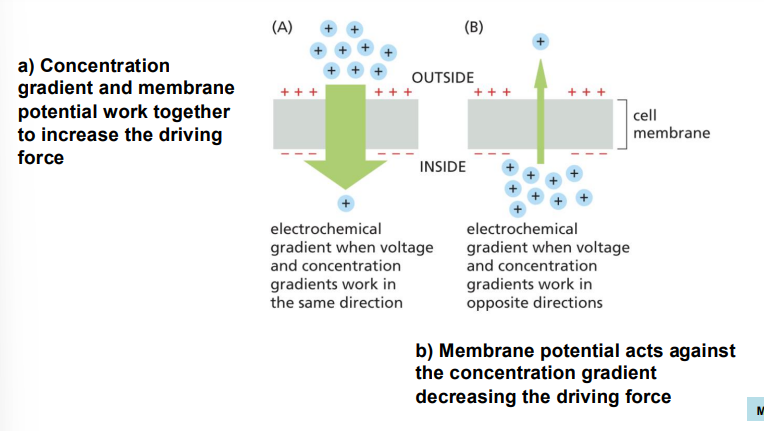
electrochemical gradient
membrane potential and concentration gradient
is the gucose transporter active or passive
passive
what determines direction of glucose transport
concentration gradient (glucose is uncharged)
uniporter and an example
transports a single molecule ar a time down its [] gradient, glucose transporter
different conformations of transporters
Open conformation accepts substrate
Occluded conformation – binding sites are not accessible from either side
Open conformation releases substrate
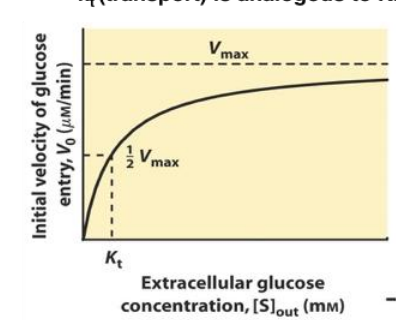
describe this plot
more glucose in the cell results in less glucose entering (v0 decreases)
kt equals to
½ vmax
what does a higher kt iindicate
transporter only starts to uptake its substrates when extracellular concentrations are high (low affinity)
what two GLUT’s are found in all mammalina tissues and what is their Kt
GLUT 1 and 3; 1mM
where is GLUT 2 found
liver and pancreatic beta cells
GLUT2 Kt
15-20 mM
GLUT4 Kt
5 mM
where is GLUT5 found
small intestine
what prevents glucose from leaving the cell
gets phosphorylated the second it enters the cell, no longer recognized by the transporter and cannot diffue back
what is the normal concentration of glucose in the blood stream
4-5 mM
what stimulates GLUT4 and what does that cause
insulin, increases the rate of glucose uptake into muscle and adipose tissue when glucose concentrations are high
t or f - GLUT1 is found in the same amount in all cells
false, present only in small amounts in nuscle and adipose tissue
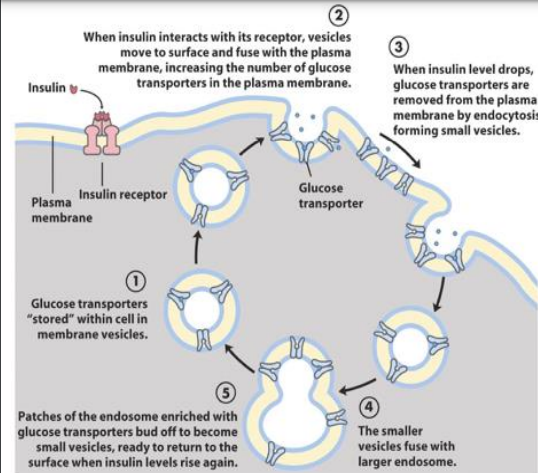
how does insulin stimulate and adipose cells to take up cells
GLUT4 stored within cell in membrane vesicles (not usually on cell surface)
insulin binds the insulin receptor on the surface of the myocyte/adipocyte
when insulin binds with its receptor, vesicles move to the surface and fuse with the plasma membrane, increasing the number of glucose transporters in the plasma membrane
when insulin levels drop, glucose transporters are removed from the plasma membrane by endocytosis, forming small vesicles
smaller vesicles fuse with larger endosome
patches of the endosome enriched with glucose transporters bud off to become small vesicles, ready to return to the surface when insulin levels rise again
what would the consequences be if insulin were absent in an individual?
glucose can bind non enzymatically to proteins
messes protein function up
causes various problems through out the body
idirect/secondary transport
where direct active ttransport set up one of the gradients
coupled transport
another way to describe indirect active transport - links the uphill transport (against its gradient) of one solute across a membrane to the downhill (with its gradient) of another
what types of proteins participate in coupled transport
antiporters and symporters
direct active transport
requires “pumps” that selectively bind and move the solute across a membrane driven by changes in the proteins conformation
what must the movement against a []gradient be linked to in direct active transport
an exergonic reaction liike ATP hydrolysis
what type of ATP driven pump do we focus on this class and why is it ccalled that
p-class pumps, directly phophorylated
4 types of ATP driven driven pumps
p-class pumps
v-class proton pumps
f-class proton pumps
ABC superfamily
example of a p-class pump in out body, v important, think of the brain
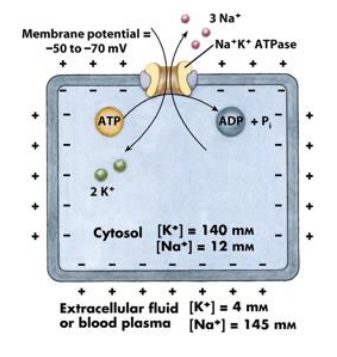
Na+/K+ ATPase
what does the Na+/K+ ATPase do
maintains the membrane potential
how does the Na+/K+ ATPase work (steps)
3 Na+ bind
pump phosphorylates itself, hydrolyzing ATP
phosphorylation triggers a conformational change and 3 Na+ is ejected
2 K+ binds
pump dephosphorylates itself
oumo returns to original conformation and 2 K+ are ejected
what do Ca 2+ pumps do
keep intracellular Ca2+ concentrations low, p class pumps, unidirectional
sarcoplasmic reticulum
specialized type of ER that forms a network of tubular sacs in the cytosol of muscle cells and serves as an intracellular store of calcium (involved in muscle contraction)
what direction does the Ca2+ pump pump
unidirectional - from cytosol to lumen of SR
what else is intracellular calcium important for
signal transduction
antiporter vs symporter
both transport 2 (/2 types of) molecules, one against its gradient and the other with its gradient
both rely on direct active transport to establish a gradient that can be used
symporter
transports both molecules in the same direction
antiporter
transports the two molecules in opposite directions
are intestinal lumen concentrations of glucose higher or lower than the blood stream
in blood the glucose levels are lower because the blood contains a lot more water, thus waters the glucose down
could glucose get into intestinal cells via a glucose uniporter
no, the glucose concentration is higher in the intestinal cells due to the action of the symporter on the apical membrane, so glucose would diffuse down its gradient (from the intestinal cell to te bloodstream or gut lumen)
is extracellular concentration of Na+ or lower than intracellular and why?
it is higher due to the action of the Na+/K+ ATPases that are present on the apical and basal membranes
how does glucose get transported from the gut lumen to the bloodstream
Na+/K+ ATPases on the apical membrane establish a Na+ gradient
symporter transports a glucose and Na+ into the intestinal cell (energy derived from the Na+ gradient)
glucose diffuses passively into the blood stream through a uniporter in the basal membrane
excess Na+ ions are removed via Na+/K+ ATPases to maintain the gradient
role of tight junctions in the process of glucose uptake from the intestine?
stopping the glucose from directly diffusing into the bloodstream which allows for tighter control on blood glucose levels
ion chennels selectivity and directionality, what do they allow
selective
bidirectional
allow diffusion in the direction of the electrochemical gradient
different types of stimuli that influence the opening and closing of ion channels
mechanically gated
ligand gated
voltage gated
mechanically gated ion channel definition and example
conformational state depends on mechanical forces (like stretch tension) that are apllied to the membrane
ex. auditory hair cells in the ear
ligand gated ion channel definition and example
conformational state depends on the binding of specific molecules (ligand) and can be open or closed upon ligand binding
ex. acetylcholine receptor
voltage gated ion channel definition and example
conformational state deoends on the difference in ionic charge on the two sides of the membrane
ex. sodium and potassium channels that depolarize and repolarize membranes
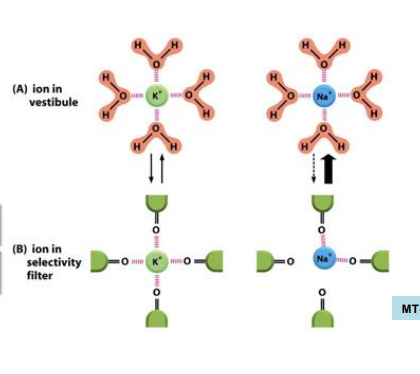
what happens when K+ enters its ion chennel
loses it water if hydration (bound water) in the selectivity filter section of the channel and become coordinated to channel carbonyl oxygens
Na+ vs K+ in ion channels and how that effects selectivity
Na+ is smaller than K+ and thus cannot coordinate as well as K+ can with the oxygen atoms in the channel, this means K+ passes through the channel more
how does water move across membranes (2)
simple diffusion - takes a long time
aquaporins (faster)
aquaporins
specialized channels for water transport
describ ethe structure of aquaporins
one face includes a series of hydrophobic amino acids that transiently form hydrogen bonds with individuals water molecules as they move single file through the channe;, so narrow that ions that are hydrated cannot pass through, only singluar water molecules
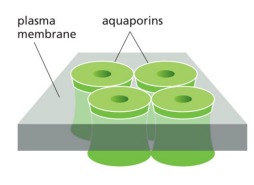
direction of water diffusion through aquaporins
from outside (high [water]/low [solute]) to inside (low [water]/ high [solute])
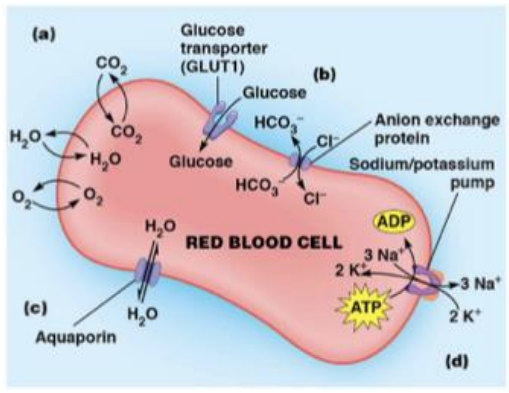
RBC examples of transport
simple diffusion - oxygen and carbon dioxide
passive diffusion - glucose uniporter
indirect actove transport - anion exchange antiporter
promary active transport - Na+/K+ ATPase
aquaporins - water channels