2.2H Formal Charges
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
Last updated 2:26 PM on 5/14/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
What are Formal Charges? What are they assigned to? What happens in covalent bonds?
electron book-keeping
assigned to specific atoms in a molecule (especially to atoms with an abnormal number of bonds
in covalent bonds: each atom donates an electron to the bond. So each atom “owns” one electron in the bond
2
New cards
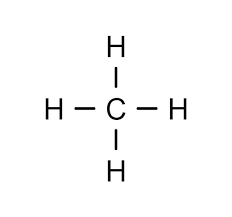
Methane has what formal charge?
no formal charge
3
New cards
What are the rules for formal charges?
As many formal charges as possible in a molecule should be zero
•A negative formal charge should be on the most electronegative atom
If there is a charge on a polyatomic ion, the sum of the formal charges should equal the charge of the ion
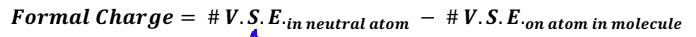
4
New cards
placholder
placholder