Energy and States of Matter
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

25 Terms
Kinetic Energy
The energy an object possesses due to its motion, which depends on its mass and velocity.
The faster a particle moves the _______________ kinetic energy it has
more
Temperature
The measure of the average kinetic energy of a substance
Potential Energy
Stored energy due to the interactions between particles or objects.
Highest kinetic and potential energy
Gas
Lowest kinetic energy and potential energy
Solids
Type of matter in the middle with potential and kinetic energy
Liquid
Thermal Energy
The temperature of the particles as well as the distance between those
The ____________ a sample of matter is, the more thermal energy it has.
larger
Atom
Building block of matter
Substance
Matter with a composition that is always the same
Element
Substance that is made up of only one type of atom and cannot be broken down
Compound
A substance made up of two or more different elements chemically joined together
True or False: 80 degree water has less kinetic energy than 20 degree water
False
True or false: Gas has more potential energy than liquids
True
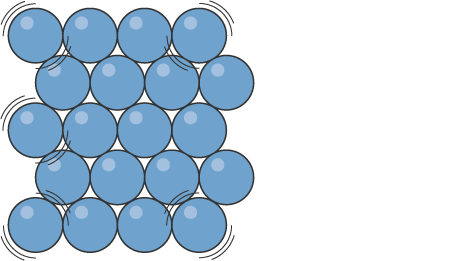
What state of matter is this?
Image of solid particles
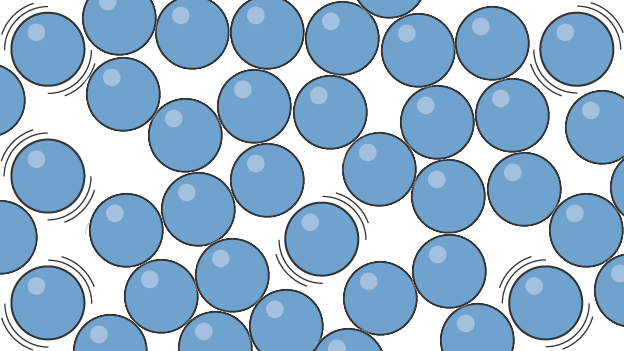
What state of matter is this?
Image of liquid particles
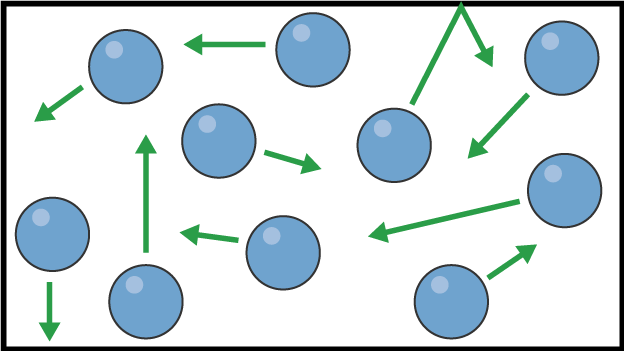
What state of matter is this?
Image of gas particles
Characteristics of solid particles
Closely packed particles that vibrate in fixed positions, maintaining a definite shape and volume.
Characteristics of liquid particles
Particles that are closely packed but can flow past one another, maintaining a definite volume but not a fixed shape.
Characteristics of gas particles
Particles that are far apart and move freely, filling the available space and not maintaining a definite shape or volume.

Image of element
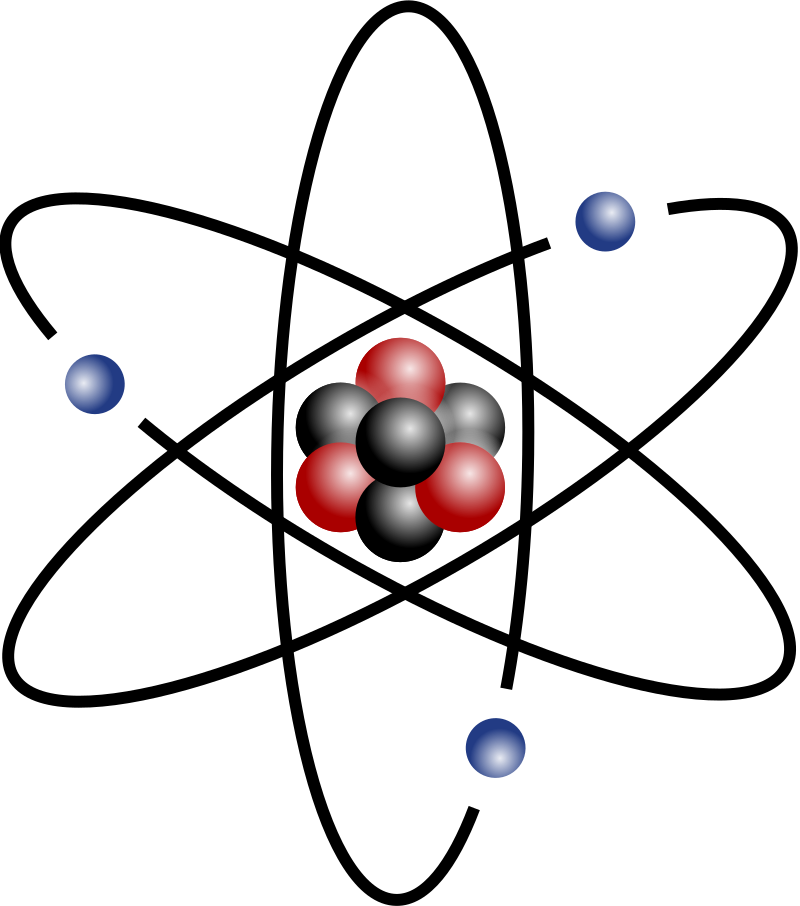
Image of an atom
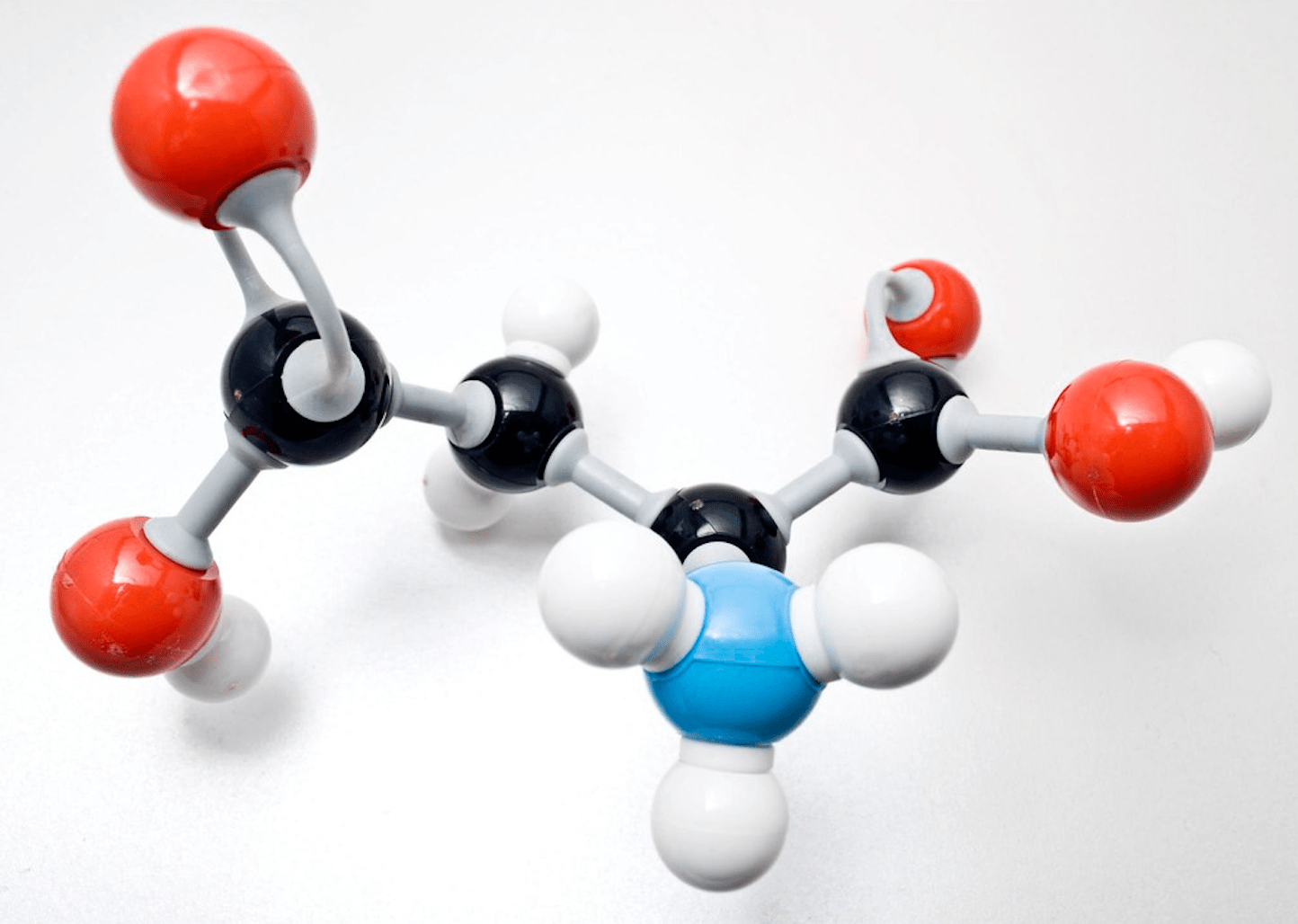
Image of a compound
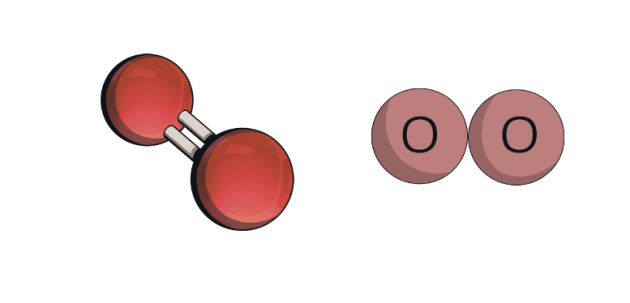
Image of a molecule