Amino Acids
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Learning Objectives
Recognize the structural features of the twenty amino acids found in proteins.
Identify nonpolar-aliphatic amino acids.
Identify aromatic amino acids.
Identify polar-uncharged amino acids
Identify basic amino acids
Identify the amino acids which contain one of the following chemical moieties; imidazole, indole, guanidinium, sulfhydryl, phenyl.
Define the following terms in the context of amino acids: Zwitterion, Amphoteric, Asymmetric carbon atom, D,L convention of amino acids, Isoelectric point (pI).
Identify the predominant ionic structure of an amino acid at a given pH.
Explain how the Henderson-Hasselbalch equation is used to determine the relative concentrations of the different ionic states of a given amino acid.
Identify the amino acid side chains involved in the following chemical forces: Disulfide bonds, Hydrogen bonds, Ionic interaction (salt linkages), Hydrophobic interactions.
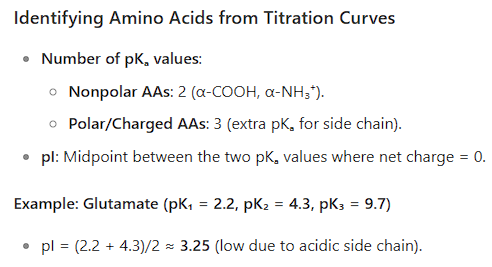
Identify amino acids from their titration curves.
Identify the pI of amino acids.
Predict the electrophoretic behavior of amino acids at various pH.
what is another name for the R group?
alkyl group
(learning objectives)
1. Structural Features & Classification of the 20 (+1) Amino Acids
Amino acids are grouped based on the chemical properties of their side chains (R groups), which determine their role in protein structure and function.
A. Nonpolar, Aliphatic Amino Acids
Feature: Hydrocarbon chains that are hydrophobic ("water-fearing"). They avoid water and cluster together inside proteins.
Amino Acids:
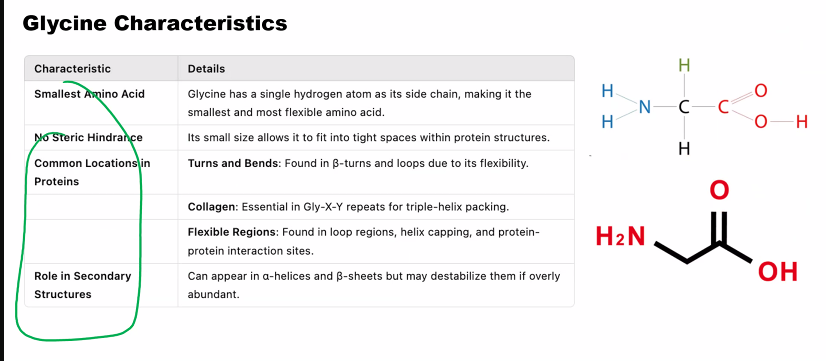
Glycine (Gly, G): Just an H atom. The smallest, gives flexibility.
Alanine (Ala, A): A methyl group (-CH₃). Simple and common.
Valine (Val, V): Branched 3-carbon chain.
Leucine (Leu, L): Branched 4-carbon chain.
Isoleucine (Ile, I): Isomer of Leucine (different branch point).
Methionine (Met, M): Contains a sulfur atom (thioether). Often the start amino acid.
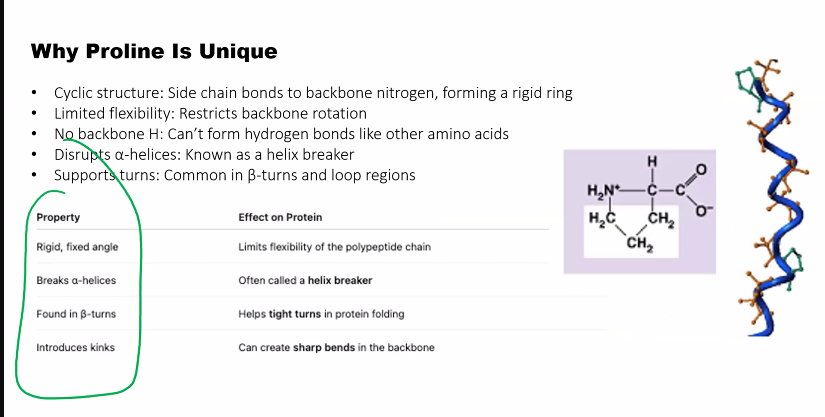
Proline (Pro, P): Unique because its side chain forms a ring with the amino group. This makes it rigid and often creates kinks in protein chains.
B. Aromatic Amino Acids
Feature: Contain a planar, hydrophobic ring structure (benzene ring) that can absorb UV light (~280 nm).
Amino Acids:
Phenylalanine (Phe, F): Benzene ring attached to an amino acid backbone.
Tyrosine (Tyr, Y): Phenol ring (has an -OH group). Can be phosphorylated.
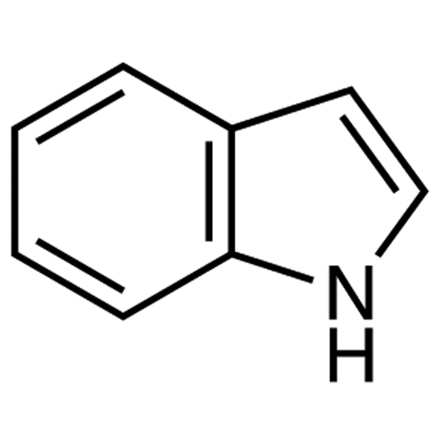
Tryptophan (Trp, W): Double-ringed indole group. The largest amino acid.
C. Polar, Uncharged Amino Acids
Feature: Side chains contain functional groups that can form hydrogen bonds with water, making them hydrophilic ("water-loving"). They are not acidic or basic at physiological pH.
Amino Acids:
Serine (Ser, S): Hydroxyl group (-OH). Can be phosphorylated.
Threonine (Thr, T): Hydroxyl group. Can be phosphorylated.
Cysteine (Cys, C): Thiol group (-SH). Can form disulfide bonds.
Asparagine (Asn, N): Amide group (-CONH₂).
Glutamine (Gln, Q): Amide group (-CONH₂).
D. Basic Amino Acids (Positively Charged at pH 7.4)
Feature: Side chains accept protons, giving them a positive charge. Hydrophilic.
Amino Acids:
Lysine (Lys, K): Terminal amino group (-NH₃⁺). pKa ~10.5.
Arginine (Arg, R): Guanidinium group. pKa ~12.5. Always positively charged in biology.
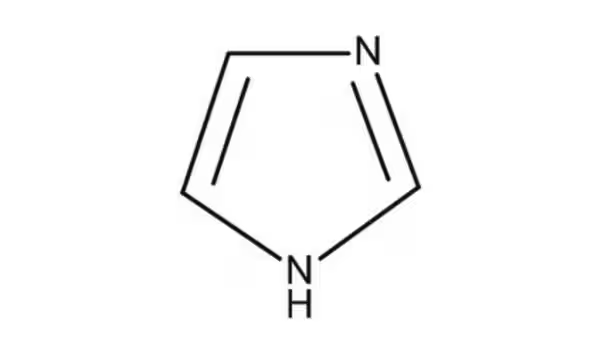
Histidine (His, H): Imidazole group. pKa ~6.0. Can be neutral or positive at physiological pH, making it a key player in enzyme catalysis.
E. Acidic Amino Acids (Negatively Charged at pH 7.4)
Feature: Side chains donate protons, giving them a negative charge. Hydrophilic.
Amino Acids:
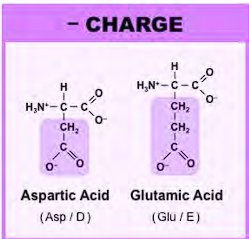
Aspartic Acid (Asp, D): Carboxylic acid group (-COOH). pKa ~3.9.
Glutamic Acid (Glu, E): Carboxylic acid group (-COOH). pKa ~4.3.
(Their amide forms, Asn and Gln, are uncharged)
(learning objectives)
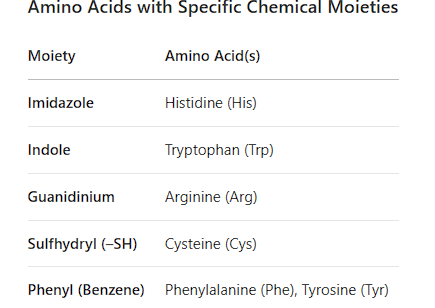
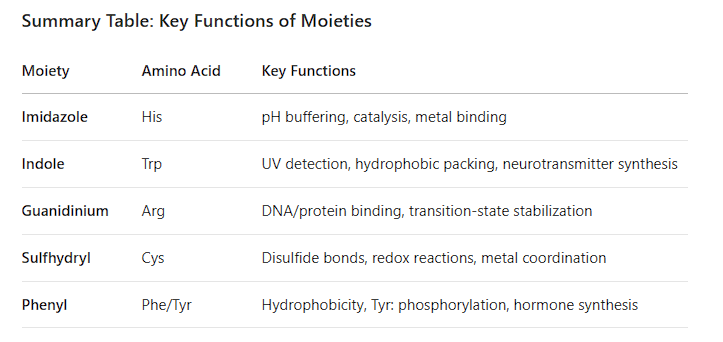
2. Amino Acids with Specific Moieties
Imidazole: Histidine (H)
Indole: Tryptophan (W)
Guanidinium: Arginine (R)
Sulfhydryl (-SH): Cysteine (C)
Phenyl: Phenylalanine (F)
imidazole (histidine)
what does an imidazole look like?
what is the imidazole (side chain of histidine) used for?
1. Imidazole (buffer)
Amino Acid: Histidine (His, H)
allows histidine to act as both a proton donor and acceptor at physiological pH, making it crucial for the catalytic activity of many enzymes.
(indole, tryptophan)
what does the indole look like?
what is the indole on tryptophan used for? (even though it’s hydrophobic)
2. Indole
Amino Acid: Tryptophan (Trp, W)
nitrogen can form hydrogen bonds (even though it’s hydrophobic)
(learning objectives)
2. Amino Acids with Specific Moieties
3. Guanidinium
Amino Acid: Arginine (Arg, R)
Explanation: The guanidinium group is a highly basic, positively charged functional group. It is planar and can form multiple hydrogen bonds and ionic interactions, often found in enzyme active sites and protein-DNA interfaces.
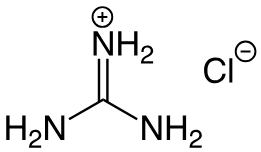
4. Sulfhydryl (Thiol)
Amino Acid: Cysteine (Cys, C)
4. Sulfhydryl (Thiol)
Amino Acid: Cysteine (Cys, C)
Explanation: The sulfhydryl (-SH) group is highly reactive. Its most important property is the ability to form disulfide bonds (-S-S-) with another cysteine, which are critical for stabilizing the three-dimensional structure of many proteins.
5. Phenyl
Amino Acids: Phenylalanine (Phe, F), Tyrosine (Tyr, Y), Tryptophan (Trp, W)
Phenylalanine (Phe, F): Contains a phenyl group (a benzene ring). It is strictly hydrophobic and non-polar.
Tyrosine (Tyr, Y): Contains a phenol group (a benzene ring with a hydroxyl group). The -OH group makes it polar and capable of forming hydrogen bonds and phosphorylation.
Tryptophan (Trp, W): As mentioned above, its indole group contains a phenyl (benzene) moiety as part of its fused ring system.
(learning objectives)
3. Key Definitions
zwitterion & pI
Isoelectric Point
how is isoelectric point calculated?
amphoteric
assymetric carbon atom
D, L Convention (which AMINO ACID is biologically active?)
Isoelectric Point
Zwitterion: A molecule with both a positive and a negative charge, resulting in a net charge of zero. Amino acids exist as zwitterions at their isoelectric point (pI).
Isoelectric Point (pI): The specific pH at which an amino acid or protein has a net charge of zero.
Isoelectric point is calculated as the average of the two relevant pKa values.
Amphoteric: The ability of a molecule to act as both an acid (donate a proton) and a base (accept a proton). All amino acids are amphoteric.
Asymmetric Carbon Atom: A carbon atom with four different substituents attached to it. The α-carbon of every amino acid (except glycine) is asymmetric, making them chiral.
D, L Convention: A system to describe the stereochemistry (handedness) of chiral molecules. biologically active aa are in the L-configuration.
(learning objectives)
how to find predominant structure at a given pH?
Ionic Structures & Henderson-Hasselbalch Equation
Predominant Structure at a Given pH:
pH < pKa: The functional group is protonated.
pH > pKa: The functional group is deprotonated.
To find the net charge, apply this rule to the α-carboxyl, α-amino, and side chain groups.
Henderson-Hasselbalch Equation:
pH = pKa + log([A⁻] / [HA])
It is used to calculate the ratio of deprotonated ([A⁻]) to protonated ([HA]) species for any ionizable group at a given pH.
At pH = pKa: [A⁻] = [HA] (ratio = 1:1).
how do you find the net charge of an amino acid?
1) find the pI (the pH at which the net charge is 0)
2) pH < pKa, pH=pKa, pH>pKa
(learning objectives)
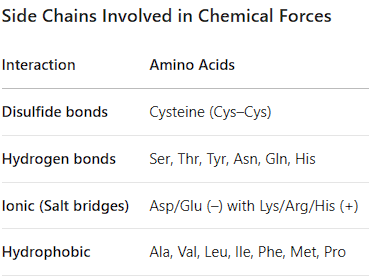
Side Chains Involved in Chemical Forces (disulfide bonds, hydrogen bonds through polar amino acids, ionic interactions, hydrophobic interactions)
Disulfide Bonds: Cysteine (C) - Covalent bond between two cysteine thiol groups used to stabilize tertiary structures.
Hydrogen Bonds: Polar side chains: Ser, Thr, Asn, Gln, Tyr, Trp, His, Arg, Cys. Also the backbone -NH and -C=O groups. (secondary structure, tertiary and quaternary structure, Function & Catalysis- Creates specific binding sites and enables enzymes to perform catalysis.
Ionic Interactions (Salt Bridges): Charged side chains: Asp(-), Glu(-) with Lys(+), Arg(+), His(+). (Stabilize Tertiary and Quaternary Structure, Provide Solubility → they are typically found on the outer surface of proteins, where they interact with water molecules, making the protein soluble in the aqueous environment of the cell, Enable Function in Active Sites: The charges in salt bridges are crucial for the function of many proteins:
Enzyme Catalysis: They can help stabilize transition states during reactions or participate directly in acid-base catalysis.
Binding Specificity: The precise arrangement of positive and negative charges in a binding pocket can ensure a protein only interacts with its specific target (e.g., a substrate, another protein, or DNA).
Key Property: Ionic salt bridges strength is highly dependent on the environment. Inside the hydrophobic core of a protein, they are very strong. On the surface, exposed to water, they are weaker because water molecules compete with the interaction.
Hydrophobic Interactions: Nonpolar side chains: Gly, Ala, Val, Leu, Ile, Pro, Met, Phe
What they are: This is not a physical attraction like a bond. It is a driving force caused by the tendency of nonpolar molecules to avoid water and cluster together.
Nonpolar Side Chains: Glycine (Gly, G), Alanine (Ala, A), Valine (Val, V), Leucine (Leu, L), Isoleucine (Ile, I), Proline (Pro, P), Methionine (Met, M), Phenylalanine (Phe, F). (Also includes Tryptophan (Trp, W), which is on your previous list for its indole ring).
What are they used for:
The Primary Driving Force of Protein Folding: This is their most important role. In a watery cellular environment, the protein chain folds to bury these hydrophobic residues away from the water, forming a tightly packed hydrophobic core. This is often called the hydrophobic effect, and it is the major reason proteins spontaneously fold into a specific 3D structure.
Stabilizing the Protein Core: The clustering of nonpolar side chains in the interior of the protein provides a great deal of stability. While there are no formal "hydrophobic bonds," the van der Waals forces between these closely packed atoms are very favorable and contribute to stability.
Membrane Proteins: Hydrophobic amino acids are crucial for proteins that span the cell membrane. They form a hydrophobic belt around the part of the protein that sits within the lipid bilayer (which is also hydrophobic), anchoring the protein in place.
(learning objectives)
6. Titration Curves & pI Identification
A titration curve plots the pH of an amino acid solution vs. the amount of base added.
Each flat region (buffer zone) corresponds to a point where the amino acid resists pH change (pH = pKa of a group).
Each steep rise corresponds to the titration of a specific proton.
The pI is the midpoint between the two pKa values that bracket the zwitterion form.
For amino acids with non-ionizable side chains (e.g., Ala): pI = (pKa₁ + pKa₂)/2
For acidic amino acids (Asp, Glu): pI = (pKa₁ + pKa_R)/2
For basic amino acids (Lys, Arg): pI = (pKa₂ + pKa_R)/2
(learning objectives)
7. Predicting Electrophoretic Behavior
Electrophoresis separates molecules based on their net charge.
At a pH < pI: The amino acid has a net positive charge. It will move toward the cathode (negative electrode).
At a pH = pI: The amino acid has a net charge of zero. It will not move.
At a pH > pI: The amino acid has a net negative charge. It will move toward the anode (positive electrode).
Example: At pH 7.4 (physiological pH):
Asp (pI ~2.98): pH > pI, so it's negative → moves to anode.
Arg (pI ~10.76): pH < pI, so it's positive → moves to cathode.
Val (pI ~6.0): pH > pI, so it's slightly negative → moves slowly to anode.
amino acid forces
(primary, secondary, tertiary, quaternary)
amino acid forces
(primary, secondary, tertiary, quaternary)
amino acid forces
what is the order from strongest to weakest?
(covalent, ionic, hydrogen bonds, hydrophobic effect, van der Waals Forces)
Covalent Bonds: Very strong, shared-electron bonds (e.g., peptide bonds, disulfide bonds).
Ionic Bonds (Salt Bridges): Electrostatic attraction between oppositely charged groups.
Hydrogen Bonds: Attraction between a hydrogen donor (N-H, O-H) and a hydrogen acceptor (O, N).
Hydrophobic Effect: The clustering of nonpolar groups to avoid water, driven by entropy (not a physical bond but a major driving force).
van der Waals Forces: Weak attractions between very close atoms.
(amino acid forces in different structures)
what is the primary structure and what are the forces involved?
what is the bond formed between?
what type of formation is the bond?
1. Primary Structure: The linear sequence of amino acids in a polypeptide chain. It's like the order of letters in a very long word.
Forces Involved:
Peptide Bonds: This is the only force involved at this level. A peptide bond is a strong, covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of the next, releasing a water molecule.
dehydration synthesis is a chemical reaction where two molecules are joined together to form a larger molecule, with the removal of a water molecule (H₂O).
(amino acid forces in different structures)
what is the secondary structure and what are the forces involved?
Secondary Structure
Definition: Local, repetitive folding patterns within a segment of the polypeptide chain. The most common are the alpha-helix (α-helix) and the beta-pleated sheet (β-sheet).
Forces Involved:
Hydrogen Bonds: This is the dominant stabilizing force. These bonds form between the backbone atoms of different amino acids.
In an α-helix, the
-C=Ogroup of one amino acid hydrogen bonds with the-N-Hgroup of the amino acid four residues away.In a β-sheet, the
-C=Oand-N-Hgroups of different strands (which can be from distant parts of the sequence) hydrogen bond with each other.
(amino acid forces in different structures)
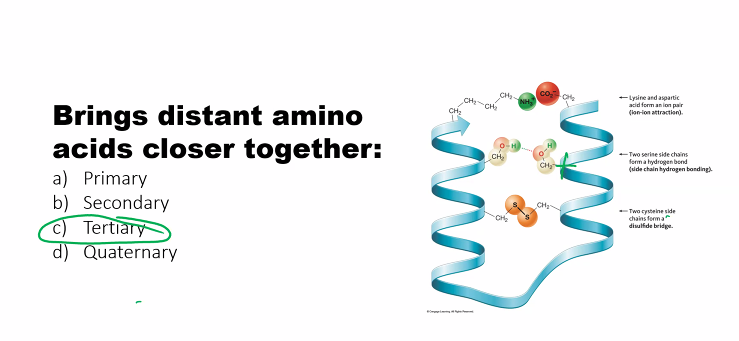
what is the tertiary structure and what are the forces involved (there are multiple, 4 new, 1 old)
what is the strongest bond in the tertiary structure?
3. Tertiary Structure
Definition: The overall three-dimensional shape of a single, complete polypeptide chain. It is the result of long-range interactions between amino acid side chains (R-groups).
Forces Involved: This is where all the forces come together.
Hydrophobic Effect: The major driving force for folding. Nonpolar side chains (Val, Leu, Ile, Phe, etc.) cluster together in the interior of the protein to avoid water, forming a stable core.
Hydrogen Bonds: Form between polar side chains (Ser, Thr, Asn, Gln, Tyr, etc.) and sometimes with the backbone.
Ionic Bonds (Salt Bridges): Occur between charged side chains: Asp⁻/Glu⁻ and Lys⁺/Arg⁺/His⁺.
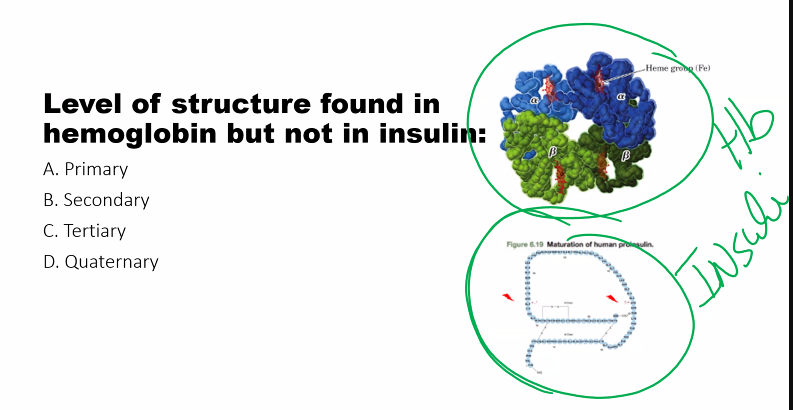
Disulfide Bonds (Covalent): Strong covalent bonds between the sulfur atoms of two cysteine residues. These are the strongest bonds stabilizing tertiary structure and are especially common in proteins secreted outside the cell (e.g., insulin).
van der Waals Forces: Provide stability through close packing of atoms in the protein's core.
(amino acid forces in different structures)
what is the quaternary structure and what are the forces involved?
even though the forces are the same, what is different?
4. Quaternary Structure
Definition: The association of multiple polypeptide chains (subunits) into a single, functional protein complex.
Forces Involved: The forces are identical to those in tertiary structure, but they occur between the side chains of different polypeptide subunits.
Hydrophobic Effect
Hydrogen Bonds
Ionic Bonds
van der Waals Forces
Note: Disulfide bonds are less common but can also occur between subunits to covalently lock the quaternary structure (e.g., in antibodies).
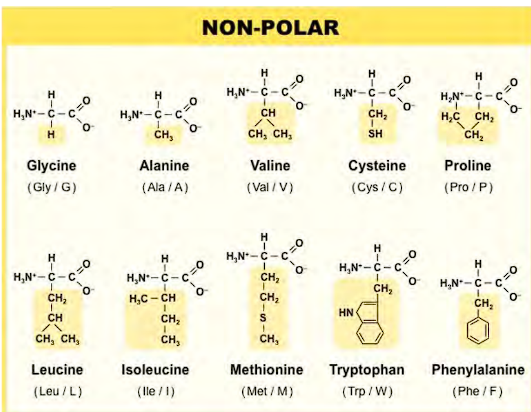
HYDROPHOBIC AMINO ACIDS
what are they?
what did you notice about the one letter code? what is the one exception to the one letter code?
all of them EXCEPT FOR TRYPTOPHAN has a one letter code that matches the first letter of their name
Both cysteine and methionine have sulfur
The single letter for phenyl-alanine is “F”, because the first three letters sound like an F
Methionine is the longer version of cysteine, & the sulfur on methionine does NOT have a hydrogen
Valine forms an upside down V
Proline forms a V, the V attaches to the amino side of the amino acid, forming the kink.
Isoleucine has the side chain in the shape of an L
Tryptophan & phenylalanine are nonpolar, TYROSINE is polar
tryptophan has a cyclopentane, HN, and a phenyl group (a phenyl is a benzene with an R group)
tryptophan has an indole group
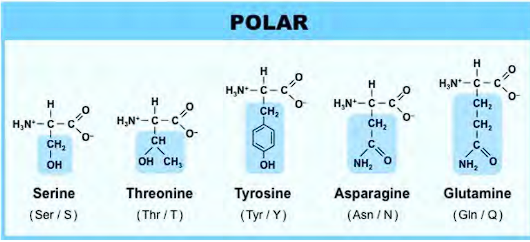
POLAR amino acids
made polar because of an OH group, oxygen and NH2 group
the one letter codes are STRAIGHTFORWARD except for Asparagine & Glutamine.
Asparagine is Asn, Glutamine is Gln.
Serine and Threonine & Tyrosine are the only polar AA with OH groups. Asparagine and Glutamine are (NH2, O).
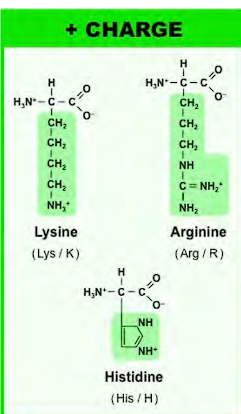
positively chard amino acids
which amino acid has the guanidium group?
why is the guanidium group important?
which amino acid has the imidazole group?
why is the imidazole group important?
lysine has four CH2, 1 NH3+
Arginine has 2 NH2, only one NH2 (the one with the double bond to carbon) has the + charge.
Arginine has the guanidium group
guanidium group is a multi-tool:
+ charge allows formation of salt bridges with - charged groups such as aspartate and glutamate (negatively charged amino acids)
binding to phosphate groups and DNA/RNA → gene regulation: transcription factors and histones are rich in guanidium to stabilize and grab onto dna backbone. ATP binding: enzymes use ATP frequently have arginine residues positioned to coordinate the ATP’s phosphate groups.
Making multiple hydrogen bonds: guanidium is an excellent donor or making hydrogen bonds to clamp down a target molecule to provide stability and & specificity.
Histidine has 2 NH, only NH with a double bond has the + charge.
Histidine looks like a golf club
Histidine has the Imidazole group
the pKa of the histidine is 6.0. this allows histidine to easily be protonated and deprotonated, which allows it to act as a buffer.
In the catalytic triad, serine (Ser) residue performs a nucleophilic attack but needs to be deprotonated, histidine residue accepts the proton from serine, facilitating its deprotonation, histidine then donates this proton to an aspartate (Asp) residue, completing the shuttle, allows serine to participate in the reaction without ever forming a unstable, high-energy alkoxide ion (
O⁻) on its own.The nitrogen atoms in the imidazole ring have lone pairs of electrons that can donate to electron-deficient metal ions. Histidine is frequently found in the active sites of metalloproteins. Hemoglobin/Myoglobin: A histidine residue (the proximal histidine) coordinates directly with the iron atom in the heme group. This binding is essential for oxygen binding and release. Carbonic Anhydrase: Histidine residues help coordinate the zinc ion, which is critical for catalyzing the reaction between CO₂ and water. Superoxide Dismutase: Histidine is part of the active site that coordinates copper and zinc ions to break down toxic superoxide radicals.
It Modulates Protein Function via pH Changes: Because its charge state changes with small shifts in pH, histidine can act as a pH sensor. Why it's Important: In proteins where histidine is strategically placed, a drop in pH (e.g., in a muscle cell during exercise or in an endosome) can cause it to become protonated. This addition of a positive charge can trigger a conformational change in the entire protein. Example: The protein hemoglobin uses this principle. As pH drops in active tissues (due to CO₂ buildup), specific histidine residues become protonated, which promotes the release of oxygen from hemoglobin—a phenomenon known as the Bohr effect.
negatively charged amino acids (ACIDS)
the one letter codes are unusual
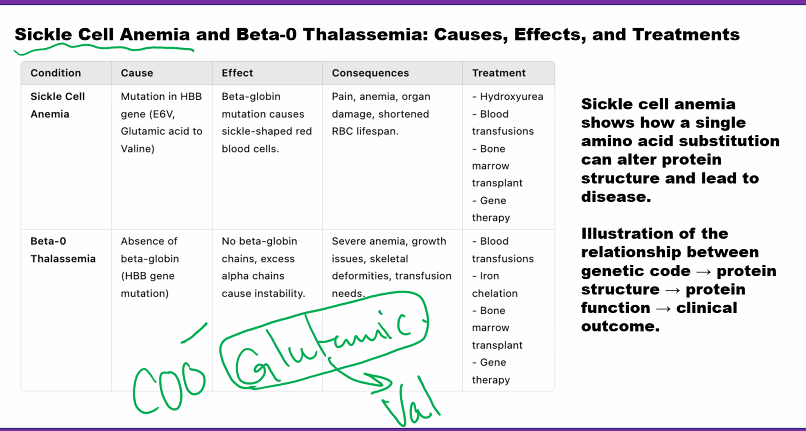
the one letter code for aspartic acid is D, the one letter code for glutamic acid is E, why? D & E because both aa combined are dicarboxylic acids (they have two carboxylic acids combined). D for aspartic acid because it comes first, E for glutamine (Edicarboxylic acid= GlutamE)
a aspartic acid has one CH2, glutamic acid has a second CH2.
the O with the negative charge is the one that gives up the proton.
Two oxygen on both (resonance)
-resonance hybrid to stabilize the negative charged form after donating the proton.
-highly acidic because the conjugate base is strong due to resonance stabilization.
-negative charge able to form salt bridges, bind metal ions, and help keep protons soluble in aqueous environment.
Structural Features of the 20 Amino Acid
Amino acids consist of:
Central (α) carbon
Amino group (–NH₂)
Carboxyl group (–COOH)
Hydrogen atom (H)
Variable side chain (R-group)
cysteine bond formation
Amino Acids with Specific Chemical Moieties
A moiety (pronounced moy-uh-tee) refers to a distinct part or functional group within a larger molecule.
what are these moieties responsible for?
1. Imidazole (Histidine, His)
Role:
pH buffering (pKₐ ~6.0, near physiological pH).
Enzyme catalysis: Acts as a proton donor/acceptor in:
Serine proteases (e.g., chymotrypsin).
Hemoglobin (stabilizes oxygen binding via proximal histidine).
Metal ion binding (e.g., zinc fingers in DNA-binding proteins).
Example:
In carbonic anhydrase, His residues coordinate Zn²⁺ to catalyze CO₂ hydration.
2. Indole (Tryptophan, Trp)
Role:
UV absorption (λₘₐₓ = 280 nm), used to measure protein concentration.
Hydrophobic core packing in proteins (large, rigid ring).
Signal transduction: Precursor for:
Serotonin (neurotransmitter).
Melatonin (sleep regulator).
Example:
In fluorescence quenching assays, Trp’s indole group reports conformational changes.
3. Guanidinium (Arginine, Arg)
Role:
Strong base (pKₐ ~12.5, always protonated at physiological pH).
Ionic interactions: Binds anions (e.g., phosphate groups in DNA).
Enzyme catalysis: Stabilizes transition states (e.g., in nitric oxide synthase).
Example:
In histones, Arg’s guanidinium group binds DNA’s phosphate backbone.
4. Sulfhydryl (–SH, Cysteine, Cys)
Role:
Disulfide bonds (Cys–Cys bridges stabilize protein structure).
Metal binding (e.g., iron-sulfur clusters in electron transport).
Redox reactions: Active site of antioxidants (e.g., glutathione).
Example:
In insulin, disulfide bonds link polypeptide chains.
5. Phenyl (Benzene Ring, Phe/Tyr)
Role:
Hydrophobic interactions: Stabilizes protein cores.
Tyrosine (Tyr):
Phosphorylation site (cell signaling, e.g., kinase substrates).
UV absorption (280 nm, weaker than Trp).
Precursor for hormones (thyroxine, dopamine).
Example:
In receptor tyrosine kinases (RTKs), Tyr phosphorylation activates signaling.
Key Definitions
Zwitterion:
A molecule with both positive (–NH₃⁺) and negative (–COO⁻) charges (neutral overall).
Formed at pH = pI (isoelectric point).
Amphoteric:
Can act as both an acid (donate H⁺) and a base (accept H⁺).
Asymmetric Carbon (Chiral Center):
All amino acids (except Glycine) have a chiral α-carbon.
D,L Convention:
L-amino acids are biologically active (proteins).
D-amino acids are rare (bacterial cell walls, some antibiotics).
Isoelectric Point (pI):
pH where an amino acid has no net charge (zwitterion form).
For neutral AAs: pI = (pK₁ + pK₂)/2.
For charged AAs (e.g., His, Asp, Lys): pI depends on side chain pKₐ.
Predominant Ionic Form at a Given pH
pH < pI: Net positive charge (because –NH₃⁺ dominates).
pH = pI: Zwitterion (neutral).
pH > pI: Net negative charge (–COO⁻ dominates).
reasoning
The Structure of an Amino Acid
A generic amino acid in its neutral form has two functional groups:
A basic amino group (-NH₂) that can accept a proton (H⁺) to become -NH₃⁺.
An acidic carboxyl group (-COOH) that can donate a proton (H⁺) to become -COO⁻.
These two groups can exist in different protonation states depending on the pH of the solution they are in.
At the pI (pH = pI):
The environment has just the right concentration of H⁺ to create the zwitterion.
The molecule has both a +1 charge (-NH₃⁺) and a -1 charge (-COO⁻), resulting in a net charge of zero.
At Low pH (pH < pI):
The solution has an excess of H⁺ protons.
Both functional groups are protonated: the amino group as -NH₃⁺ and the carboxyl group as -COOH.
The molecule has a +1 charge and no negative charge, giving it a net positive charge.
At High pH (pH > pI):
The solution has a very low concentration of H⁺ protons (it's proton-deficient).
Both functional groups are deprotonated: the amino group as -NH₂ and the carboxyl group as -COO⁻.
The molecule has a -1 charge and no positive charge, giving it a net negative charge.
A Simple Analogy: A See-Saw
Imagine the pH is the position of a see-saw, and the pI is the balancing point.
pH < pI: The see-saw is tilted to the "protonated" side (+ charge).
pH = pI: The see-saw is perfectly balanced (0 charge).
pH > pI: The see-saw is tilted to the "deprotonated" side (- charge).
Example: Alanine (pI ≈ 6.0)
At pH 2: NH₃⁺–CH–COOH (fully protonated).
At pH 6: NH₃⁺–CH–COO⁻ (zwitterion).
At pH 10: NH₂–CH–COO⁻ (fully deprotonated).
Henderson-Hasselbalch Equation
Used to calculate the protonation state of ionizable groups (e.g., –COOH, –NH₃⁺, side chains).
Example:
For –COOH (pKₐ ≈ 2): At pH 4, –COO⁻ dominates (pH > pKₐ).
For –NH₃⁺ (pKₐ ≈ 9): At pH 7, –NH₃⁺ dominates (pH < pKₐ).
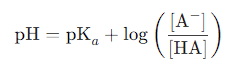
sidechains involved in chemical forces
Electrophoretic Behavior at Different pH
Electrophoretic Behavior at Different pH
pH < pI: Migrates to cathode (–) (net + charge).
pH = pI: No movement (neutral).
pH > pI: Migrates to anode (+) (net – charge).
Example: Lysine (pI ≈ 9.7)
At pH 7: Net + charge → moves to cathode.
At pH 10: Net neutral → no movement.
At pH 12: Net – charge → moves to anode.
SECTION 3.1 FUNCTIONAL PROTEINS IN HUMANS
3.2• AMINO ACID COMPOSITION OF PROTEIN
All the different types of proteins are polymers of only 21 amino acids. The com- mon amino acids are defined as the amino acids for which at least one codon existsin the genetic code.
Transcription and translation of the DNA code result in the joining together of amino acids into a specific linear sequence characteristic of a protein(Figure 3.1). Many proteins also contain derived amino acids, which are usually formed by enzymatic modification of one of the common amino acids after it hasbeen incorporated into a protein. Examples of derived amino acids are cystine (p. 79), desmosine, and isodesmosine found in elastin, hydroxyproline and hydroxylysine incollagen,-carboxyglutamate in prothrombin, and phosphoserine, phosphothreonine, and phosphotyrosine
Common Amino Acids
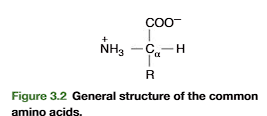
Common amino acids have the general structure depicted in Figure 3.2. They contain in common a central alpha (a)-carbon atom to which a carboxylic acid group, an amino group, and a hydrogen atom are covalently bonded. In addition, the fourth valency of the a-carbon atom is linked to a specific chemical group, designated R and called the side chain that uniquely defines each of the common amino acids. Figure 3.2 depicts the ionized form of the common amino acids in solution at pH 7. Under physiological conditions, the a-amino group is protonated and in its ammonium ion form; the carboxylic acid group is in its unprotonated or carboxylate ion form
Side Chains Define the Chemical Nature and Structures of␣-Amino Acids
Structures of the common amino acids are shown in Figure 3.3. Amino acids that con-tain alkyl group side chains include glycine, alanine, valine, leucine, and isoleucine.Glycinehas the simplest structure, with R double bond H. Alanine contains a methyl (CH3!) group.Valine has an isopropyl R group (Figure 3.4, p. 79). The leucine and isoleucine R groups are isobutyl groups that are structural isomers of each other. In leucine the branching of the isobutyl side chain occurs on the gamma (y)-carbon and in isoleucine it is branched on the beta (b)-carbon (Figure 3.4)
Phenylalanine, tyrosine, and tryptophan are aromatic amino acids. Phenylalaninecontains a benzene ring, tyrosinea phenol group, and tryptophanthe heterocyclic struc-ture, indole. In each case the aromatic moiety is attached to the -carbon through a meth-ylene (!CH2!) carbon (Figure 3.3)
Sulfur-containing amino acids are cysteine and methionine. The cysteineside-chain group is a thiolmethyl (HSCH2!), and in methioninethe side chain is a methyl ethyl thiol ether (CH3SCH2CH2!).
The two hydroxy (alcohol)-containing amino acids are serine and threonine. In serine the side chain is a hydroxymethyl (HOCH2!). In threoninean ethanol structure is con- nected to the -carbon to produce a secondary alcohol (CH3!CHOH!CH!).
Prolineis unique in that it incorporates the -amino group in its side chain and is more accurately classified as an -imino acid, since its -amine is a secondary amine with its -nitrogen having two covalent bonds to carbon. Incorporation of the -amino nitro- gen into a five-member ring constrains the rotational freedom around the !N!C! bond in proline and thereby limits proline participation to particular polypeptide chain conformations.
The amino acids described so far contain side chains that are uncharged at physiologi-cal pH. The dicarboxylic-monoamino acidscontain a carboxylic group in their side chain.Inaspartate, this group is separated by a methylene carbon (!CH2!) from the -carbon (Figure 3.5, p. 79). In glutamate(Figure 3.5), the group is separated by two methy-lene(!CH2!CH2!) carbon atoms from the -carbon (Figure 3.5). At physiological pH,these groups are unprotonated and negatively charged. The dibasic-monocarboxylicacids are lysine, arginine, and histidine (Figure 3.3). In these, the R group contains one or two nitro-gen atoms that act as a base by binding a proton. In lysinethe side chain is an N-butyl amine.Inarginine,the side chain contains a guanidino group (Figure 3.6, p. 79) separated from the -carbon by three methylene carbon atoms. Both the guanidino group of arginine and the-amino group of lysine are protonated at physiological pH (pH ⬃7) and positively charged.Inhistidinethe side chain contains a five-member heterocyclic structure, the imidazole group
what are the structures of common amino acids?
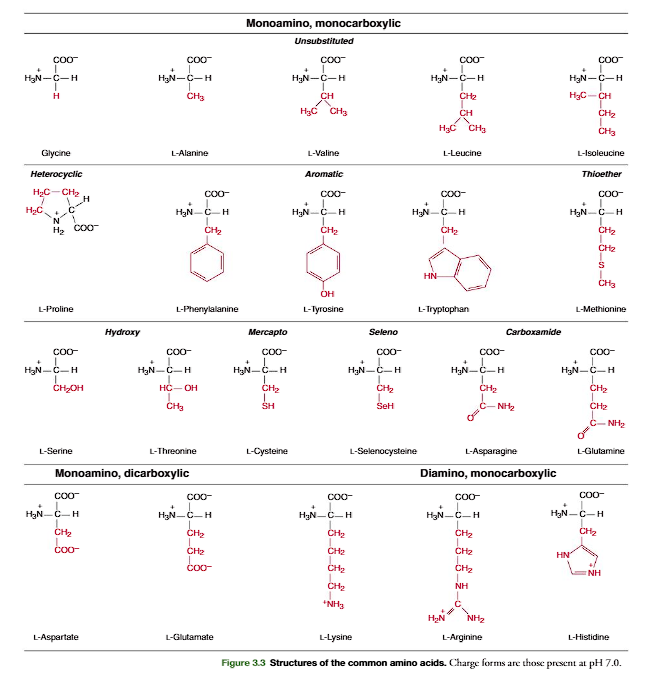
(Figure 3.6). The pKaof the imidazole group is approximately 6.0 in water; thus physi- ological solutions contain relatively high concentrations of both basic (imidazole) and acidic(imidazolium) forms of the histidine side chain.Glutamineand asparagineare structural analogs of glutamic acid and aspartic acid with their carboxylic acid side-chain groups amidated. Unique DNA codons exist for glutamine and asparagine separate from those for glutamic acid and aspartic acid. The amide side chains of glutamine and asparagine cannot be protonated and are uncharged at physiological pH
By the mid-1960s it was believed that the three-nucleotide DNA codons specifying the common amino acids found in proteins had been determined. The total number of common amino acids was 20 derived from 64 possible triplet codons with 3 codons assigned to termi-nate the translation of open reading frames (Table 6.1, p. 211). However, in 1988 a 21st common amino acid, selenocysteine(Figure 3.3), with a specific mRNA codon and tRNA that places the selenocysteine into polypeptide chains during mRNA translation was shown. The selenocysteine is structurally similar to cysteine, but with a selenium atom replacing the sulfur atom. A difficulty with the codon for selenocysteine is that it was identical to one of the termination codons that translates to a stop translation signal. Thus this codon, UGA, has two meanings. The codon codes a stop translation signal the great majority of the time, but within special RNA contexts it will code for selenocysteine incorporation into a polypeptide sequence. These instances are rare as only 25 genes have been identified within the human genome that code for proteins that incorporate selenocysteine. In addition, while selenocysteine proteins are found in all animals, the codon is not universal as it is not present in higher plants, yeast, or the majority of bacteria. In animal studies, knockout of the gene for the selenocysteine tRNA is lethal, inferring that selenocysteine is essential for life in animal species. Many of the selenoproteins have an antioxidant role in eliminating reactive oxygen species.
To represent sequences of amino acids in proteins, three-letter or one-letter abbre- viations for the common amino acids are used (Table 3.1). These abbreviations are uni-versally accepted and will be used throughout this book. The three-letter abbreviationsof aspartic acid (Asp) and glutamic acid (Glu) should not be confused with those forasparagine (Asn) and glutamine (Gln). In determination of the amino acid composi-tion of a protein by chemical procedures, one cannot easily differentiate between Asnand Asp, or between Gln and Glu, because the amide side-chain groups in Asn andGln are often hydrolyzed in the procedure and generate Asp and Glu (Section 3.9).The symbols of Asx for Asp or Asn, and Glx for Glu or Gln indicate this ambiguity in the analysis. A similar scheme is used with the one-letter abbreviations for Asp or Asn,and Glu or Gln
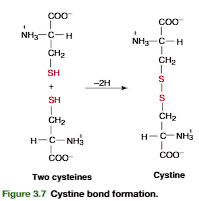
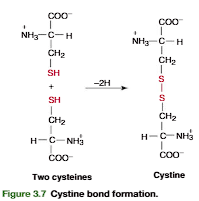
Cystine Is a Derived Amino Acid Produced by Oxidation of Two Cysteine Residues
A derived amino acid found in many proteins is cystine. It is formed by the oxidation of two cysteine thiol side chains to form a covalent disulfide bond (Figure 3.7). Within pro-teins, disulfide links of cystine formed from cysteine residues, separated from each other within a polypeptide chain (intrachain) or between two polypeptide chains (interchain), have an important role in stabilizing the folded conformation of proteins (insulin structure; see Figure 3.23, p. 91)
a-Amino Acids Have an Asymmetric Center
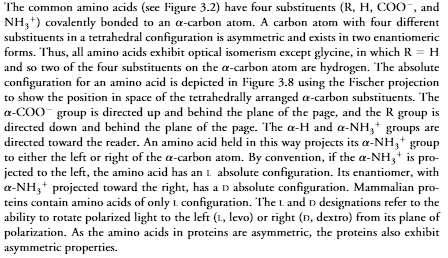
The common amino acids (see Figure 3.2) have four substituents (R, H, COO, andNH3) covalently bonded to an -carbon atom. A carbon atom with four differentsubstituents in a tetrahedral configuration is asymmetric and exists in two enantiomericforms. Thus, all amino acids exhibit optical isomerism except glycine, in which R H and so two of the four substituents on the -carbon atom are hydrogen. The absoluteconfiguration for an amino acid is depicted in Figure 3.8 using the Fischer projectionto show the position in space of the tetrahedrally arranged -carbon substituents. The-COOgroup is directed up and behind the plane of the page, and the R group isdirected down and behind the plane of the page. The -H and -NH3groups aredirected toward the reader. An amino acid held in this way projects its -NH3groupto either the left or right of the -carbon atom. By convention, if the -NH3is pro-jected to the left, the amino acid has an Labsolute configuration. Its enantiomer, with-NH3projected toward the right, has a Dabsolute configuration. Mammalian pro-teins contain amino acids of only Lconfiguration. The Land Ddesignations refer to theability to rotate polarized light to the left (L, levo) or right (D, dextro) from its plane ofpolarization. As the amino acids in proteins are asymmetric, the proteins also exhibitasymmetric properties
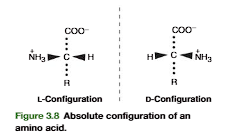
Amino Acids Are Joined into Peptides and Protein
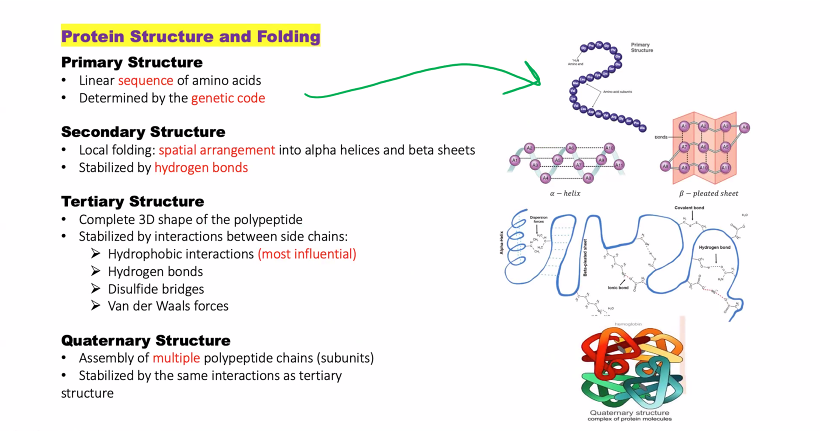
Linking of a selection of the common amino acids into polypeptide chains in cells isenzymatically catalyzed (p. 221). Chemically, this is a dehydration reaction (Figure3.9). The -carboxyl group of one amino acid forms a covalent peptide bondwith the-amino group of another amino acid by elimination of a molecule of water. The dipeptide(two amino acid residues joined by a single peptide bond) can then form a second peptide bond through its terminal carboxylic acid group and the -amino of a third amino acid, to generate a tripeptide (Figure 3.9). Repetition of this process gen-erates a polypeptideor protein of specific amino acid sequence. This is the primary structureof the protein, and it is predetermined by the nucleotide sequence of its gene. The unique primary structure enables a polypeptide chain to fold into a specific three-dimensional structure that gives the protein its chemical and physiological properties.
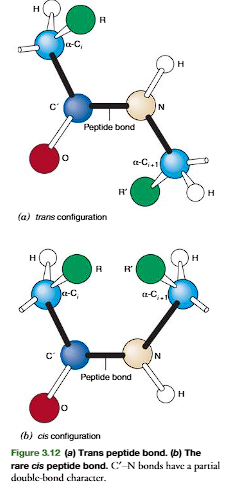
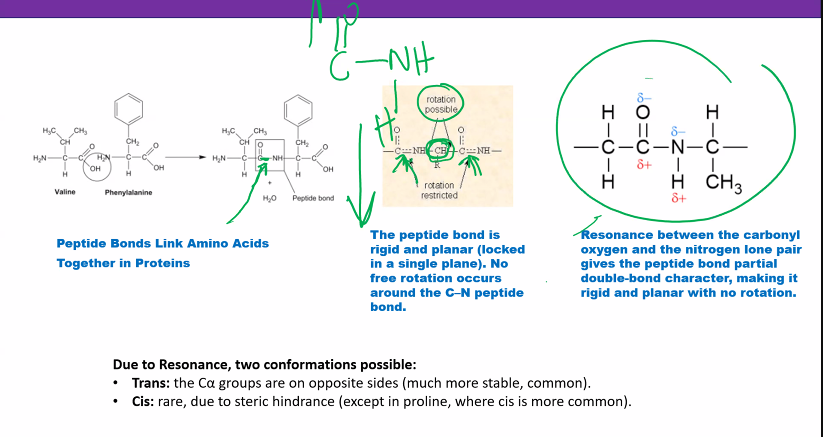
A peptide bond can be represented as two resonance isomers(Figure 3.10). Instructure I, a double bond is located between the carbonyl carbon and carbonyl oxygen. (CO), and the carbonyl carbon to nitrogen (C!N) linkage is a single bond. In structure II, the carbon to oxygen bond (C!O) is a single bond and the bond located between the carbon and nitrogen is a double bond (CN). In structure II there is a negative charge on the oxygen and a positive charge on the nitrogen. Actual peptidebonds are a resonance hybrid of these two electron isomer structures, the carbon tonitrogen bond having a 50% double-bond character. X-ray diffraction studies confirmthis and show the carbonyl carbon to nitrogen bond length (1.33 Å) is approximatelyhalf way between that of a C!N single bond (⬃1.45 Å) and a CN double bond (⬃1.25 Å)
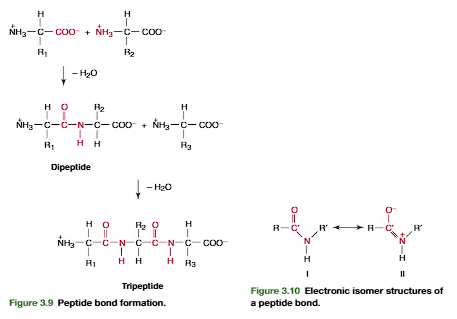
A consequence of the partial double bond character is that rotation does not occur about the carbonyl carbon to nitrogen of a peptide bond at physiological temperatures. A second consequence of the double bond character is that all the atoms attached to Cand N lie in a common plane
Thus a polypeptide chain consists of peptide-bond planes interconnected at the -carbon atoms. The -carbon interconnects the peptide bonds through single bonds that allow rotation of adjacent peptide planes with respect to each other. Each amino acid residuecontributes one -carbon, two single bonds, and a peptide bond to the polypeptide chain (Figure 3.11). The termresiduerefers to the atoms contributed by an amino acid to a polypeptide chain including those of the side chain.
The peptide bond in Figure 3.12ashows atransconfigurationof its oxygen (O) and hydrogen (H) atoms. This is the most stable configuration with the two side chainsof the adjacent residues (R and R) also being in trans. The cisconfiguration(Figure 3.12b) brings the two side-chain groups to the same side of the CN bond, which is unfavorable because of steric hindrance between the R groups. Accordingly, transpeptide bonds occur in proteins except where there are proline residues. In proline the side chainincludes the -amino group, and both the cisand transpeptide bond configurations with the proline -imino group have unfavorable interactions with the -carbon of the adjacent amino acid forming a peptide bond with the -imino. Accordingly, the peptide bond of proline in an unstructured or denatured polypeptide chain will have significantamounts of its peptide bond in both transandcisconfigurations. The configuration of the proline peptide bond in a folded protein depends on the forces on the proline bondgenerated by the three-dimensional structure of the particular protein molecule in whichthe proline is imbedded.
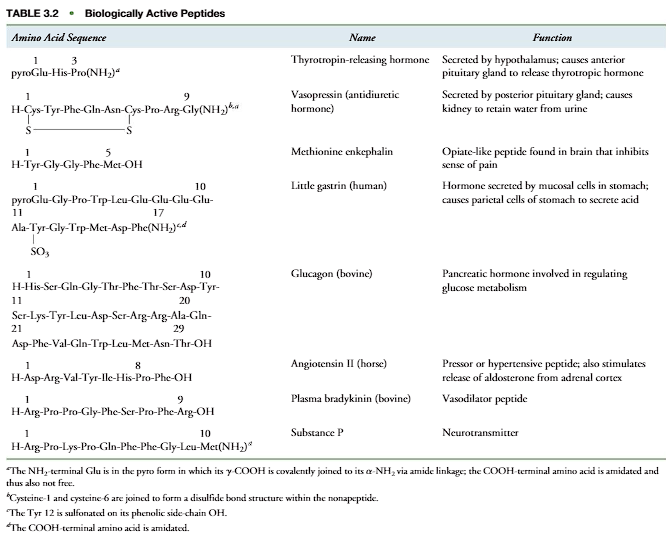
One of the largest polypeptides in humans is the muscle protein titin, which con- tains about 27,000 amino acid residues in a single polypeptide chain. Chain length alone,however, does not determine the function of a polypeptide. Many small peptides ofless than 10 amino acids perform important biochemical and physiological functions inhumans (Table 3.2). Primary structures are written in a standard convention and sequen-tially numbered from their NH2-terminal end to their COOH-terminal end, consistent with the order of addition of the amino acid to the chain during biosynthesis. Accord-ingly, for thyrotropin-releasing hormone (Table 3.2) the glutamic acid residue on theleft is the NH2-terminal amino acid of the tripeptide and amino acid residue 1 in the sequence. The proline is the COOH-terminal amino acid and is residue 3. The defineddirection of the polypeptide chain is from Glu to Pro (NH2-terminal amino acid to COOH-terminal amino acid.
peptide bond
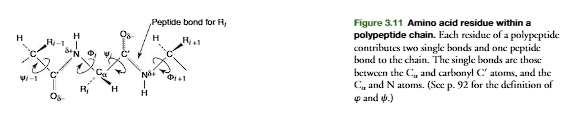
biologically active peptides
3.3•CHARGE AND CHEMICAL PROPERTIES OF AMINO ACIDS AND PROTEINS
Ionizable Groups of Amino Acids and Proteins Are Critical for Biological Function
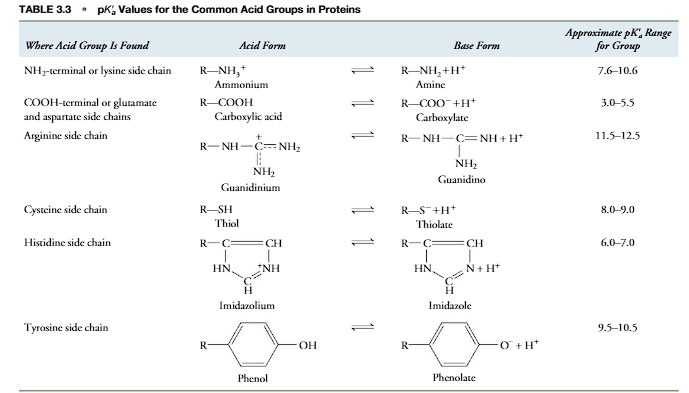
Ionizable groups common to proteins and amino acids are shown in Table 3.3. The acid forms are on the left of the equilibrium sign and the base forms on the right. In forming its conjugate base, the acid form releases a proton. In reverse, the base form associates with a proton to form the respective acid. The dissociation of an acid is characterized by an acid dissociation constant (Ka) and its pKavalue: pKalog(1/Ka). Table 3.3 shows a range of pKavalues for each acid group, as the actual pKadepends on the environment in which an acid group is placed. For example, when a positively charged ammonium group (!NH3) is placed near a negatively charged group in a protein, the negative charge stabilizes the positively charged acid form, making it more difficult to dissociate its proton. The pKaof the!NH3will have a higher value than normal for an ammonium group in the absence of a nearby negative-charge stabilization. Other factors that affect the pKainclude polarity of the environment, absence or presence of water, and the potential for hydrogen bond formation. In addition, acid groups (-COOH or -NH3) at the ends of polypeptides. typically have a lower pKavalue than the same types of acid groups in the side chains (Table 3.4). The amino acids whose R groups contain nitrogen atoms (Lys and Arg) are thebasic amino acids, since their side chains have relatively high pKavalues and function as bases at physiological pH. They are usually in their acid form and are positively charged at physiological pH. Amino acids whose side chains contain a carboxylic acid group have relatively low pKavalues that easily lose their protons and are acidic amino acids. They are predominantly in their unprotonated forms and negatively charged at physiological pH. Proteins in which the ratio (ΣLysArg)/(GluAsp) is greater than 1 are basic proteins. Proteins in which the ratio is less than 1 are acidic proteins


Ionic Form of an Amino Acid or Protein Can Be Determined at a Given pH
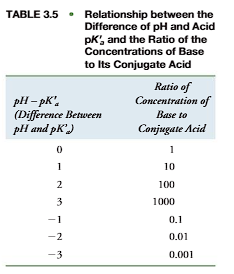
From the pKavalue for each acid group in an amino acid or protein and the Henderson– Hasselbalch equation (Figure 3.13), the ionic form of the molecule can be calculated at a given pH. This is an important equation as it shows the change in ionization state and charge of a molecule with pH. Physiological activities of a molecule differ with changes in pH and ionization state. For example, some enzymes require a histidine imidazole in its base form for catalytic activity. If the pKaof this histidine is 6.0, at pH 6.0 one-half of the enzyme molecules are in the active base (imidazole) form and one-half of the enzyme molecules are in the inactive acid (imidazolium) form. Accordingly, the enzyme exhibits 50% of its potential activity. At pH 7.0, the ratio of [imidazole]/[imidazolium] is 10:1 (Table 3.5) and the enzyme exhibits 10/(10 1) 100 91% of its maximum potential activity. Thus a change in pH has a dramatic effect on the enzyme’s activity.
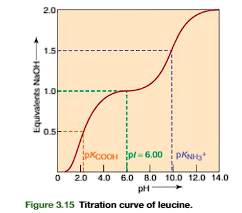
Titration of a Monoamino Monocarboxylic Acid: Determination of the Isoelectric pH
An understanding of a protein’s acid and base forms and their relation to charge ismade clearer by following the titration of the ionizable groups for a simple aminoacid. In Figure 3.14, leucine has an -COOH with pKa2.4 and an -NH3group with pKa9.6. At pH 1.0 the predominant ionic form (form I) has a charge of 1 and migrates toward the cathode in an electrical field. Addition of 0.5 equivalent ofbase titrates half the -COOH groups; that is, the ratio of [COO]/[COOH] will equal 1. The Henderson–Hasselbalch equation, with the second term on the right sideof the equation log10[(base)/(acid)]log(1) 0 at a ratio of conjugate base to acid of 1:1, shows that the pH (when the -COOH is half titrated) is directly equal to the pKa(COOH)(Figure 3.15). Addition of 1 equiv of base at pH 6.0 completely titrates the -COOH, but has no effect on the -NH3group. In the resulting form (II), the negative and positive charges cancel each other and the net charge is zero. Form II is the zwitterionform, the ionic form in which the total of positive charges equals the total of negative charges. Asthe net charge on a zwitterion molecule is zero, it will not migrate in an electric field.Further addition of 0.5 equiv of base to the zwitterion form (total base added is 1.5equiv) will half-titrate the -NH3group. At this point, the ratio of [NH2]/[NH3] 1, and the pH is equal to the value of the pKafor the -NH3group (Figure 3.15). Addition of a further 0.5 equiv of base (total of two full equivalents of base added;Figure 3.15) completely titrates the -NH3group to its base form (-NH2). The pH becomes greater than 11, and the predominant molecular species has a negative charge(form III)
![<p>An understanding of a protein’s acid and base forms and their relation to charge ismade clearer by following the titration of the ionizable groups for a simple aminoacid. In Figure 3.14, leucine has an -COOH with pKa2.4 and an -NH3group with pKa9.6. At pH 1.0 the predominant ionic form (form I) has a charge of 1 and migrates toward the cathode in an electrical field. Addition of 0.5 equivalent ofbase titrates half the -COOH groups; that is, the ratio of [COO]/[COOH] will equal 1. The Henderson–Hasselbalch equation, with the second term on the right sideof the equation log10[(base)/(acid)]log(1) 0 at a ratio of conjugate base to acid of 1:1, shows that the pH (when the -COOH is half titrated) is directly equal to the pKa(COOH)(Figure 3.15). Addition of 1 equiv of base at pH 6.0 completely titrates the -COOH, but has no effect on the -NH3group. In the resulting form (II), the negative and positive charges cancel each other and the net charge is zero. Form II is the zwitterionform, the ionic form in which the total of positive charges equals the total of negative charges. Asthe net charge on a zwitterion molecule is zero, it will not migrate in an electric field.Further addition of 0.5 equiv of base to the zwitterion form (total base added is 1.5equiv) will half-titrate the -NH3group. At this point, the ratio of [NH2]/[NH3] 1, and the pH is equal to the value of the pKafor the -NH3group (Figure 3.15). Addition of a further 0.5 equiv of base (total of two full equivalents of base added;Figure 3.15) completely titrates the -NH3group to its base form (-NH2). The pH becomes greater than 11, and the predominant molecular species has a negative charge(form III)</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d84513a9-dd63-4447-ae76-882c83d253fc.png)
It is useful to calculate the pH at which an amino acid is in its zwitterion form. This pH is the isoelectric pHfor the molecule, represented as pI. The pI value is a constant for a compound at a particular ionic strength and temperature. For simple amino acid mol-ecules, such as leucine, pI is calculated as the average of the two pKavalues that regulate the boundaries of the zwitterion form. For leucine (look at the picture)
At pH 6.0, leucine has a partial negative charge that increases at high pH to a full nega- tive charge of –1 (form III) (Figure 3.14). At pH 6, it has a partial positive charge, until at a very low pH, it has a charge of 1 (form I). The charge at any pH can be calculated from the Henderson–Hasselbalch equation or from extrapolation from the titration curve of Figure 3.15
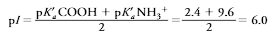
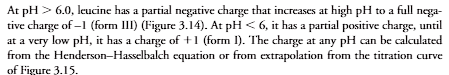
Titration of a Monoamino-Dicarboxylic Acid
A more complicated example is provided by glutamic acid. As shown in Figures 3.16 and 3.17, in glutamic acid the -COOH pKa2.2, the -COOH pKa4.3, and the -NH3 pKa9.7. The zwitterion form is generated after 1.0 equiv of base is added to (page 84).
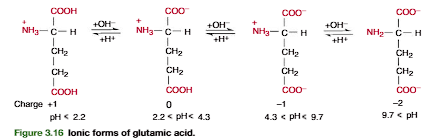
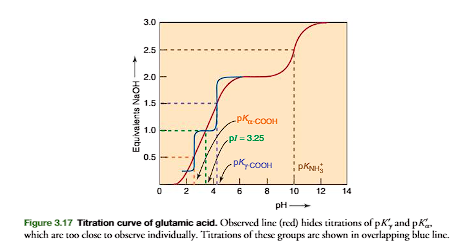

General Relationship between Charge Properties of Amino Acids and Proteins, and pH
The relationship between pH and charge for leucine and glutamate is generally true for the other amino acids. At a pH less than pI, the molecule is positively charged. At a pH greater than pI, it is negatively charged. The degree of positive or negative charge is a function of the magnitude of the difference between pH and pI. As proteins are complex polyelec-trolytes that contain many ionizable groups that regulate the zwitterion form, calculation of a protein’s isoelectric pH from its multiple acid pKavalues utilizing the Henderson– Hasselbalch relationship is difficult. Accordingly, pI values of proteins are experimentally measured by determining the pH value at which the protein does not move in an electric field. pI values for some proteins are given in Table 3.6
As with amino acids, at a pH greater than its pI, a protein has a net negative charge. At a pH less than its pI, it has a net positive charge (Figure 3.18). The magnitude of the net charge increases as the difference between pH and pI increases. An example is human plasma albumin with 585 amino acid residues including 61 glutamates, 36 aspartates, 57 lysines, 24 arginines, and 16 histidines. The titration curve is shown in Figure 3.19. Albumin’s pI 5.9, at which pH its net charge is zero. At pH 7.5 the imidazolium groups of histidines are partially titrated and there is a negative charge of 10. At pH 8.6 the net charge is approximately 20, and at pH 11 it is approximately 60. At pH 3, the approximate net charge is 60.

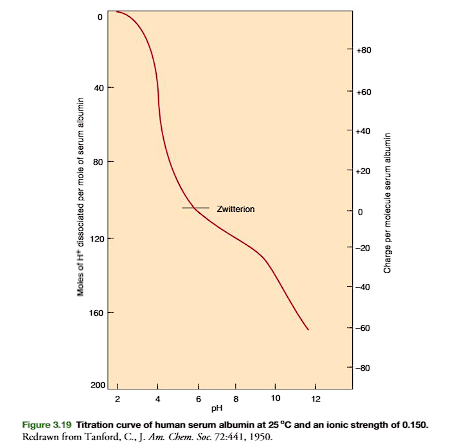
Amino Acids and Proteins Can Be Separated on the Basis of Their Differences in p
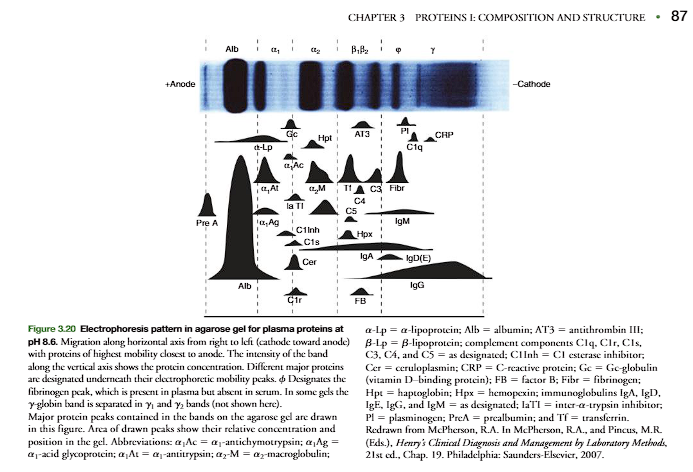
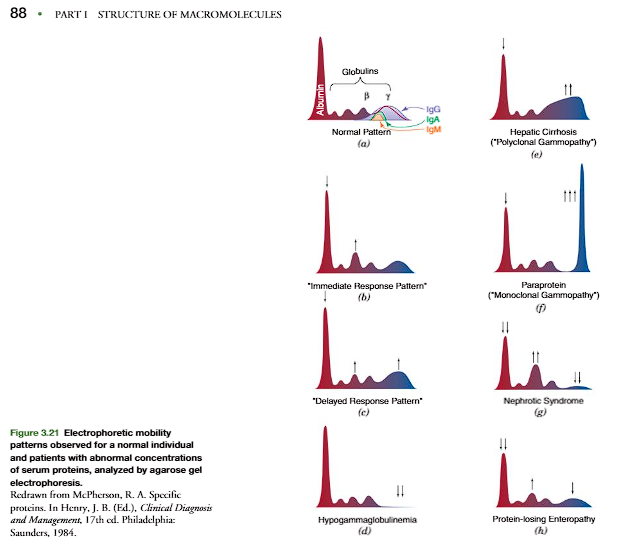
The techniques of electrophoresis, isoelectric focusing, and ion-exchange chroma-tography separate and characterize biological molecules based on their pI (p. 84). In clinical medicine, electrophoretic separation of plasma proteins has led to their classification being based on their relative electrophoretic mobility. The separationof the plasma proteins is commonly carried out at pH 8.6, which is higher than thepI values of the major proteins. Accordingly, the proteins are negatively charged andmove toward the anode at a rate dependent on their net charge. Figure 3.20 shows anelectrophoresis of plasma proteins in an agarose gel at pH 8.6 that separates the pro- teins into the five classical bands used to classify the plasma proteins. In order of their migration the major fractions are albumin, 1-,2-,-, and -globulins (Figure 3.20). Some of these bands actually represent tens to hundreds of different proteinsthat migrate similarly at pH 8.6. However, certain proteins predominate in each band,and variation in their relative amounts is characteristic of certain diseases (Figures 3.20and 3.21, and Clin. Corr. 3.1)
Amino Acid Side Chains Have Polar and Apolar Properties
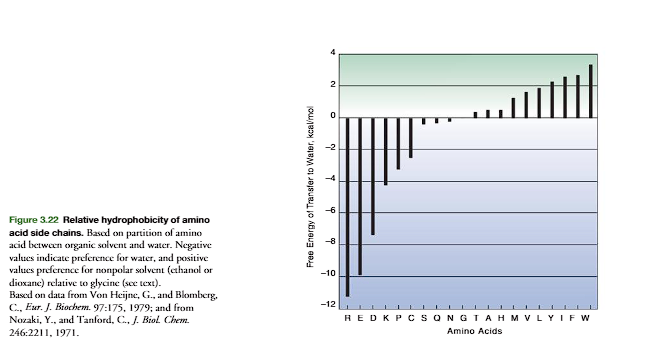
Thehydrophobicityof amino acid side chains is critical for the folding of a protein to its native structure and for stability of the folded protein. Figure 3.22 (p. 88) plots the relative hydrophobicity of the amino acids on the basis of their tendency to partition in a mixture of water and a nonpolar solvent. The scale is based on a value of zero for glycine. Side chains that preferentially dissolve in the nonpolar solvent relative to glycine show a positive () hydrophobicity value, the more positive the greater the preference for the nonpolar solvent. In folded protein structures, the majority of hydrophobic amino acids are buried away from the water solvent that interacts with the surface of a soluble protein. However, the general correlation is not perfect due to the amphoteric nature of many of the hydrophobic amino acids that place the more polar portions of their side-chain structure near the surface to interact with water. In addition, some nonpolar side chains may be on the surface. However, when on the surface, the hydrophobic groups are generally dispersed among the polar side chains. When clustering of nonpolar side chains occurs on the surface, it is usually associ-ated with a function, such as to provide a site for binding of substrate molecules through hydrophobic interactions.
Most charged side chains are on the surface of soluble globular proteins where they are stabilized by favorable energetic interactions with water. The rare positioning of a charged side chain in the interior usually implies an important functional role for that “buried” charge within the nonpolar interior in stabilizing protein conformation or participation in catalysis.Transmembrane proteins reverse the positioning of their side-chain polarity from that of water-soluble globular proteins. Within the membrane, these proteins often position hydrophobic side chains on the outside and ionic groups on the inside to provide binding interactions and to form ion channels (p. 477)
clinical correlation

Amino Acids Undergo a Variety of Chemical Reactions
Amino acids in proteins react with a variety of reagents that may be used to investigate the function of specific side chains. Some common chemical reactions are presented in Table 3.7. Reagents that modify acid side chains have been synthesized to bind to specific sites in a protein’s structure, such as the substrate-binding site. The strategy is to model the structural features of the enzyme’s natural substrate into the modifying reagent. The reagent binds to the active site like the natural substrate and reacts with a specific side chain. This identifies the modified amino acid as being located in the substrate-binding site and helps identify its role in catalysis
NEED TO WATCH SDL

A.
four aa, 3 peptide bonds
if there are 1000 aa, then there are 999 peptide bonds

the red stuff is the stuff you need to know.
hydrophobic bonds are the most influential
quaternary and tertiary bonds are the most stable.
C.

C.
the most stable molecule is the one that releases the most amount of energy.
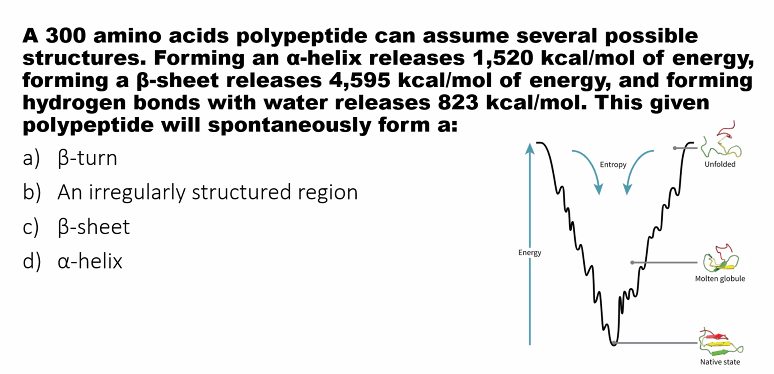
D.
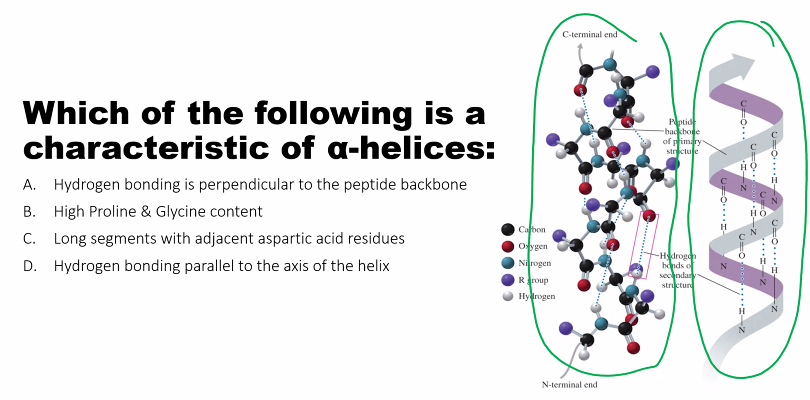
B.
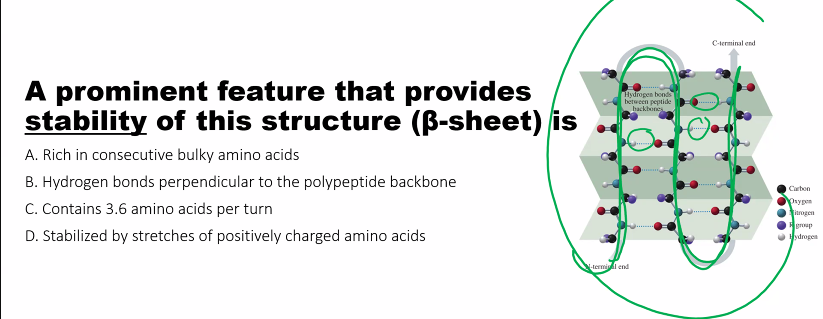
C.
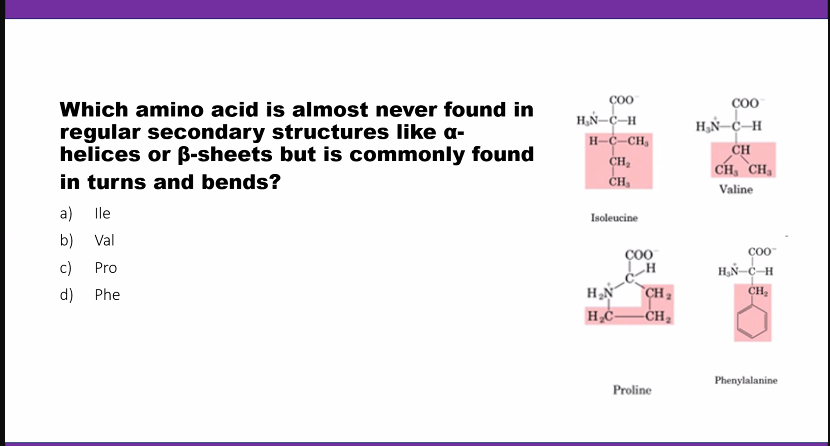
Glycine is cool because it can fit into tight places. if you lose glycine, collagen is going to suffer.
Gly-X-Y

KNOWLEDGE GAP
D.