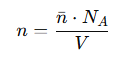Kinetic theory to ideal gas (3)
1/18
Earn XP
Description and Tags
Sem 1 first year
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
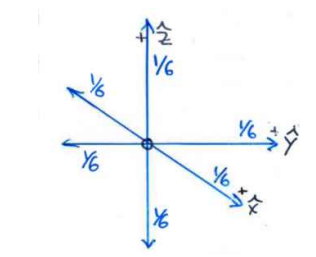
What fraction of the total number of atoms are considered to be moving along the x, y, and z directions at any instant?
1/6 of the total number of atoms.
What is the formula for the number of gas atoms per unit volume?
N= the total number of gas atoms
V= volume of container
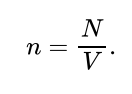
When considering the number of atoms traveling in the x-direction, what range of speeds is considered?
The range of speeds between v and v+dv.
What is the formula for the number of atoms travelling in the -z direction with speed between u and u+du per unit volume?
1/6 → accounts for the fact that only 1/6 of the total gas atoms are moving in a specific direction at any instant.
f(u)du= The fraction of atoms with speeds between u and u+du,where f(u) is the speed distribution function.
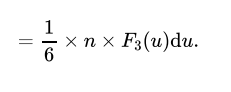
How is force related to momentum?
Force is the rate of change of momentum.
What happens to the momentum of gas atoms when they bounce off of a surface?
Their momentum is reversed, but their speed remains unchanged.
What is the formula for the number of gas atoms that strike a surface with speed between u and u+du in a small-time interval dt.
n= The number density of the gas
F3(u)du= the fraction of atoms with speeds between u and u+du that are traveling in a specific direction.
A= The area of the surface on which the atoms are striking.
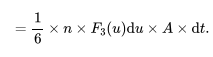
What is the formula for the number of atoms striking the surface per unit area per unit time with speeds between u and u+du?
u= The speed of the atoms
F3(u)du= the fraction of atoms with speeds between u and u+du traveling in the direction of the surface.
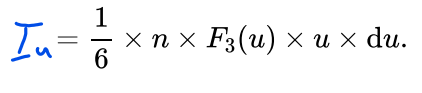
What is the formula for the mean speed in a specific direction?
F3(u)= the probability density function of the speed u in the specific direction
u= the speed of the particles.
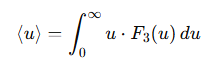
What is the formula used to calculate the total number of atoms striking the surface per unit area per unit time?
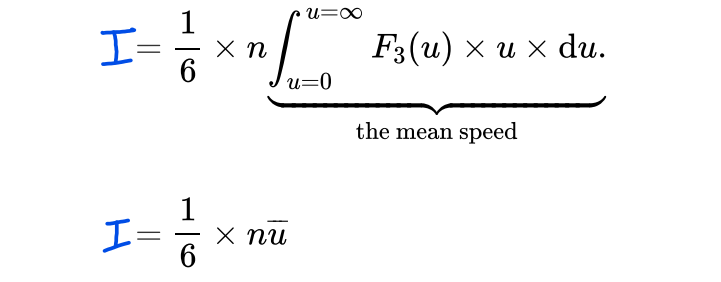
What is the definition of force in terms of momentum?
Force is the rate of change of momentum
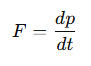
How is the change in momentum (Δp) calculated for an atom striking a surface?
m = the mass of the atom
u = speed of the atom
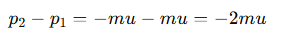
What is the force exerted by the surface on the atom during a collision?
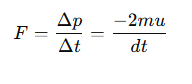
According to Newton's third law, what is the force exerted by the atom on the surface?
The force exerted is equal and opposite
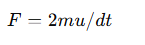
How do you calculate the infinitesimal force exerted on the surface by gas atoms of speed between u and u+du?
2mu =. The rate of change of momentum for one atom.
n= number density of gas atoms
A= area of the surface
F3(u) du= Fraction of atoms with speeds between u and u+du moving in the direction of the surface.
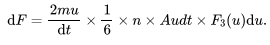
What is the formula for pressure considering all atomic speeds?
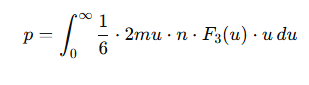
What is the relation between mean kinetic energy and temperature?
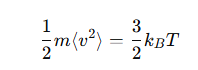
How is the pressure expressed in terms of the mean square speed?
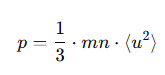
What is the number density (n) in terms of moles and Avogadro’s number?
nˉ= the number of moles
NA = Avogadro’s number